Abstract
Background The diagnostic yield of endoscopic ultrasound (EUS) guided fine-needle aspiration (FNA) is variable, and partly dependent upon rapid onsite evaluation (ROSE) by a cytopathologist. Second generation fine-needle biopsy (FNB) needles are being increasingly used to obtain core histological tissue samples.
Aims Studies comparing the diagnostic yield of EUS guided FNA versus FNB have reached conflicting conclusions. We therefore conducted a systematic review and meta-analysis to compare the diagnostic yield of FNA with FNB, and specifically evaluating the diagnostic value of ROSE while comparing the two types of needles.
Methods We searched several databases from inception to 10 April 2016 to identify studies comparing diagnostic yield of second generation FNB needles with standard FNA needles. Risk ratios (RR) were calculated for categorical outcomes of interest (diagnostic adequacy, diagnostic accuracy, and optimal quality histological cores obtained). Standard mean difference (SMD) was calculated for continuous variables (number of passes required for diagnosis). These were pooled using random effects model of meta-analysis to account for heterogeneity. Meta-regression was conducted to evaluate the effect of ROSE on various outcomes of interest.
Results Fifteen studies with a total of 1024 patients were included in the analysis. We found no significant difference in diagnostic adequacy [RR 0.98 (0.91, 1.06), (I 2 = 51 %)]. Although not statistically significant (P = 0.06), by meta-regression, in the absence of ROSE, FNB showed a relatively better diagnostic adequacy. For solid pancreatic lesions only, there was no difference in diagnostic adequacy [RR 0.96 (0.86, 1.09), (I 2 = 66 %)]. By meta-regression, in the absence of ROSE, FNB was associated with better diagnostic adequacy (P = 0.02). There was no difference in diagnostic accuracy [RR 0.99 (0.95, 1.03), (I 2 = 27 %)] or optimal quality core histological sample procurement [RR 0.97 (0.89, 1.05), (I 2 = 9.6 %)]. However, FNB established diagnosis with fewer passes [SMD 0.93 (0.45, 1.42), (I 2 = 84 %)]. The absence of ROSE was associated with a higher SMD, i. e., in the presence of an onsite pathologist, FNA required relatively fewer passes to establish the diagnosis than in the absence of an onsite pathologist.
Conclusions There is no significant difference in the diagnostic yield between FNA and FNB, when FNA is accompanied by ROSE. However, in the absence of ROSE, FNB is associated with a relatively better diagnostic adequacy in solid pancreatic lesions. Also, FNB requires fewer passes to establish the diagnosis.
Introduction
Endoscopic ultrasound (EUS) guided fine-needle aspiration (EUS-FNA) is the mainstay for tissue acquisition for evaluation of lesions adjacent to the digestive tract, including pancreas, liver, adrenals, lymph nodes, and gastrointestinal subepithelial tumors 1 2 3 4 5. Despite its widespread adoption, the diagnostic yield of FNA is highly variable, as is evident with solid pancreatic neoplasms, where reported sensitivities range from 64 % to 95 %, specificities range from 75 % to 100 %, and diagnostic accuracies range from 78 % to 95 % 6. The reported diagnostic accuracy for other lesions such as mediastinal masses and gastrointestinal tract stromal tumors (GISTs) is even lower 7 8. This variation in diagnostic utility is dependent on a number of factors, including lesion location, the availability of cytology staff for rapid onsite evaluation (ROSE), the skill and experience of the endosonographer, and the size and type of needle selected for tissue acquisition. An important limitation of EUS-FNA is that it does not provide core tissue specimens with preserved architecture, which is required for immunohistochemical staining and histologic diagnosis of conditions such as lymphoma, GIST, and autoimmune pancreatitis 9 10 11.
In an effort to overcome some of the limitations of EUS-FNA, a dedicated EUS core biopsy needle (19G Trucut needle) was developed over a decade ago. However, this first generation fine-needle biopsy (FNB) device failed to show superiority over traditional FNA 12. Moreover, the technical failure rate was high, especially when FNB was attempted with an angulated scope position – such as when working in the duodenum – due to the stiffness of the device. Consequently, more flexible second generation core biopsy needles have been developed, and are being increasingly used for tissue acquisition. These include ProCore (Cook Endoscopy) needles with a reverse-bevel for tissue acquisition and the recently approved fork-tip (SharkCore, Medtronic Corp.) needles; both are available in 19, 22, and 25 gauges. Core tissue samples obtained with these newer core biopsy needles may improve diagnostic yield, and may potentially obviate the need for ROSE. Studies comparing these second generation core biopsy needles with standard FNA needles have reached different conclusions. Studies from the United States have used ROSE routinely for FNA, but since ROSE is not uniformly available in other parts of the world, most studies conducted outside the United States have not used ROSE. We therefore conducted a systematic review and meta-analysis comparing the diagnostic performance of second generation core biopsy needles with standard FNA needles, specifically analyzing the role of ROSE in such comparisons.
Methods
This systematic review was carried out in accordance with the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 13 and meta-analysis of observational studies in epidemiology (MOOSE) 14.
Data sources and search strategy
A systematic search of the literature was conducted by an experienced medical reference librarian (R.N.) with 18 years of experience. The search strategies were developed in Ovid MEDLINE and translated to match the subject headings and keywords for Ovid EMBASE, Cochrane database, and Scopus from inception through 16 June 2016. The following MeSH, Emtree, and keyword search terms were used: “endoscopic ultrasound”, “EUS”, “fine needle aspiration”, “fine needle biopsy”, “ProCore”, “core biopsy”, “fork-tip needle”, “SharkCore”, “EUS-FNA”, and “EUS-FNB”. The search accounted for plurals and variations in spelling with the use of appropriate wildcards. Articles were selected for full text review on the basis of their title and abstract. A manual search was conducted through the bibliographies of the retrieved publications to increase the yield of potentially relevant articles. All results were downloaded into EndNote 7.5 (Thompson ISI ResearchSoft, Philadelphia, Pennsylvania, United States), a bibliographic database manager; any duplicate citation was identified and removed.
Inclusion and exclusion criteria
Two authors (M.K.I. and B.A.) searched for original articles using predetermined inclusion criteria. To meet the inclusion criteria, studies had to be randomized trials or observational studies (cohort or case – control design) and compared the second generation core biopsy needles (ProCore and SharkCore) with standard FNA needles of any gauge using EUS for sampling solid lesions. We restricted our search to studies that included patients over the age of 18 years and included at least one of the following as outcome measures: diagnostic adequacy, diagnostic accuracy, optimal core histological samples, and mean number of needle passes required to establish the diagnosis. Studies may or may not have used ROSE. Studies were excluded if they did not directly compare second generation core biopsy needles with standard FNA needles, included data on first generation 19G Trucut biopsy needles, or if data were not reported as one of the aforementioned outcomes. Studies were also excluded if they reported experimental data on animals or if data were included in a more recently published study in which case the most recent study was included. Only studies published in English in peer reviewed journals were included in the analysis. Data presented as abstracts were excluded, as there is a discrepancy between full publications and published abstracts 15 16.
Study selection and data extraction
Two reviewers (M.A.K. and M.K.I.) independently assessed the eligibility of the identified studies, collected information to assess the methodological validity of each included study, and extracted data using structured data extraction forms. Any disagreement between the reviewers was to be discussed with a third reviewer (T.H.B.), with an agreement to be reached by consensus. Extracted data included study design, country and year of study, patient demographics, location of lesion, presence or absence of onsite pathologist, follow-up period, diagnostic adequacy, diagnostic accuracy, optimal quality histological core procurement, mean number of passes required to obtain the diagnosis, and, wherever available, procedure details including needle gauges, application of suction and fanning techniques.
Quality assessment of included studies
Quality assessment was done by two reviewers (M.K.I and M.A.K.) independently using the Newcastle Ottawa Scale (NOS) for observational studies and the Cochrane tool for assessing risk of bias for randomized trials. The Newcastle Ottawa scale measures quality in the three parameters of selection, comparability, and exposure/outcome, and allocates a maximum of 4, 2, and 3 points, respectively. High-quality studies are scored greater than 7 on this scale, and moderate-quality studies, between 5 and 7. The Cochrane tool for quality assessment checks for selection bias, performance bias, detection bias, attrition bias, and reporting bias. Any discrepancy between reviewers for quality assessment was discussed with a third reviewer (I.G.) with agreement reached by consensus.
Data synthesis and statistical analysis
We assessed the following four outcomes of interest: (1) diagnostic adequacy, defined as the ability to procure cytological and/or histological samples adequate for interpretation; (2) diagnostic accuracy, defined as the ability to make a definitive diagnosis based on cytological aspirate and/or core tissue; (3) optimal core histological tissue, defined as samples with high cellularity and quality enabling appropriate core assessment in terms of tissue architecture; (4) number of passes required to establish a diagnosis.
Risk ratios (RR) were calculated for categorical outcomes of interest (diagnostic adequacy, diagnostic accuracy, and optimal core histological tissue) comparing the core biopsy needles with standard FNA needles. Standard mean differences (SMD) were calculated for continuous variables (number of passes to obtain diagnosis) comparing the two types of needles. Subgroup analyses evaluating the same variables (apart from number of passes required to establish diagnosis) for pancreatic lesions exclusively were also conducted. These outcome variables were pooled one at a time and a meta-analysis was performed using either a fixed effect model or Der-Simonian and Laird random effects model 17 depending on the presence or absence of significant heterogeneity, respectively, and corresponding forest plots constructed. Heterogeneity across the studies was assessed using the Cochran Q test and I 2 statistics. A P value of < 0.1 for the Cochran Q test was defined as indicating the presence of heterogeneity. I 2-values of 0 – 40, 30 – 60, 50 – 90, and 75 – 100 % were reflective of low, moderate, substantial, and considerable heterogeneity, respectively 18. Publication bias was assessed through funnel plots and Egger’s test for asymmetry. Meta-regression was conducted to explore heterogeneity and specifically the effect of onsite pathology was evaluated in these outcomes to explain any differences in results. When meta-regression showed a trend or significant results, scatterplots were constructed to graphically present the data. All statistical analyses were conducted using Comprehensive Meta-analysis software (version 3.0; Biostat; Englewood, New Jersey, United States).
Results
Search strategy yield, study characteristics, and quality assessment
The search strategy identified 3067 publications, of which 481 were removed as duplicates and a further 2501 were excluded as being ineligible based on title and abstract review. Backward snowballing of 85 retrieved studies did not reveal any additional study meeting our inclusion criteria. After full text review of these 85 articles, 70 studies were removed, including studies not having comparative data for standard FNA needles with second generation FNB needles, review articles, and studies not evaluating any of the four main outcome measures listed in the inclusion criteria. Consequently, 15 studies 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 with 1024 patients were included in the main analysis of which four were randomized trials 19 22 23 24 and 11 were observational studies 20 21 25 26 27 28 29 30 31 32 33. The search strategy is summarized in Fig. 1. Seven studies 20 23 25 26 27 28 33 used a crossover design in which both needles were used in all patients, while eight studies 19 21 22 24 29 30 31 32 used either a standard FNA needle or a core biopsy needle in each patient. Seven studies 19 23 24 25 28 29 30 included pancreatic lesions exclusively while one study 22 included subepithelial lesions exclusively. A total of 700 solid pancreatic lesions were included in the analysis. Rapid onsite pathology evaluation was available in all of the eight studies 19 21 25 26 28 31 32 33 conducted in the United States, whereas it was only available in one non-U.S. study 24. Two studies 32 33 used the fork-tip or SharkCore needle for FNB, while the rest of the 13 studies used the ProCore needle. Study characteristics and patient demographics are presented in Table 1 and Table 2.
Fig. 1.
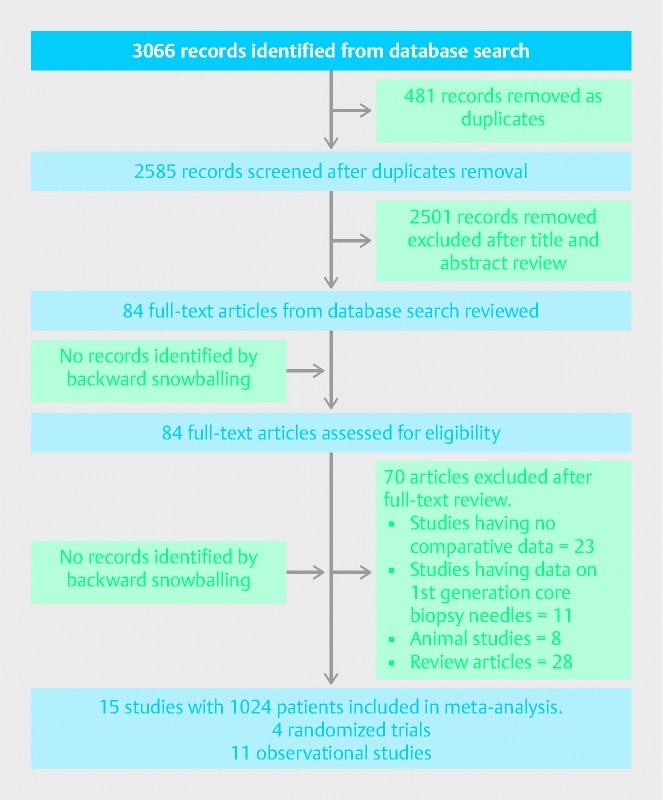
PRISMA flow chart (study selection process).
Table 1. Characteristics of included studies.
| Study, year and country | Design | Location of lesions | Inclusion criteria | Exclusion criteria | n | Males | Age, years | ROSE | Suction | Fanning | Follow-up |
| Bang, 2012 (USA) 19 | Randomized trial | Pancreas | All patients referred for solid pancreatic lesions on CT scan | Cystic pancreatic lesions, coagulopathy, lesions not seen on EUS | 56 | 31 | 65 | Yes | No | Yes | 6 months |
| Hucl, 2013 (India) 20 | Prospective | Pancreas & lymph nodes | Consecutive patients with pancreatic masses or peri-intestinal nodes | Lesion not seen on EUS | 145 | 80 | 48 | No | Yes | No | 6 months |
| Witt, 2013 (USA) 21 | Retrospective | Pancreas, gastric, mediastinal and pelvic nodes | First 18 patients undergoing EUS guided FNB for various lesions. Site matched controls undergoing EUS-FNA | NR | 36 | NR | NR | Yes | Yes | No | 3.3 months |
| Kim, 2014 (Korea) 22 | Randomized trial | Subepithelial lesions | Hypoechoic mass in submucosa and/or proper muscle layers, > 2 cm in size | Tumors not located in submucosa and/or proper muscle layers, cystic lesion, overlying vessel, platelet < 50 000, PT > 50 %, lipoma on EUS | 22 | 10 | 56.3 | No | Yes | No | NR |
| Vanbiervliet, 2014 (France) 23 | Randomized trial | Pancreas | Pancreatic mass on CT scan, dilated CBD and or dilated PD | Cystic lesions, uncorrectable coagulopathy, pregnancy | 80 | 49 | 67.1 | No | Yes | Yes | 6.4 months |
| Lee, 2014 (Korea) 24 | Randomized trial | Pancreas | Solid pancreatic mass on CT or MRI, age > 18 years | Cystic mass, INR > 1.5, platelets < 80 000 | 116 | 77 | 64.9 | Yes | Yes | No | 6 months |
| Strand, 2014 (USA) 25 | Prospective | Pancreas | Age 18 – 90 years, solid pancreatic mass on CT scan | Cystic lesion, no mass seen on EUS, uncorrectable coagulopathy | 32 | 13 | 67.7 | Yes | Yes | No | NR |
| Lin, 2014 (USA) 26 | Prospective | Pancreas, lymph nodes, gastric, mediastinal nodes, liver lesions | All patients referred for EUS guided biopsy underwent both FNA and FNB | Core biopsy not performed if cystic lesions, < 1 cm lesion, overlying vascular structures precluding biopsy | 26 | 25 | 66.8 | Yes | Yes | NR | 12 months |
| Mavrogenis, 2015 (Belgium) 27 | Prospective | Pancreas, lymph nodes | All patients > 18 years old with pancreatic lesions or lymphadenopathy referred for EUS sampling | Cystic lesion, age < 18 years, pregnancy, INR > 1.5, platelet < 50 000 | 28 | 9 | 69 (med) | No | Yes | No | 7 months |
| Berzosa, 2015 (USA) 28 | Retrospective | Pancreas | Patients with solid pancreatic lesions on CT scan undergoing EUS sampling | NR | 61 | 35 | 61 | Yes | NR | No | 6 months |
| Alatawi, 2015 (France) 29 | Prospective | Pancreas | Solid pancreatic tumors > 2 cm size on CT or MRI | Cystic lesions, patients with biliary stents | 100 | 63 | 68.4 | No | Yes | Yes | NR |
| Yang, 2015 (Korea) 30 | Retrospective | Pancreas | Solid pancreatic lesions in consecutive patients undergoing EUS | NR | 76 | 35 | 62.4 | No | Yes | Yes | 6 months |
| Dwyer, 2016 (USA) 31 | Retrospective | Pancreas, gastric and colon submucosal mass, pelvic and perirectal masses | All EUS guided biopsies of solid intraabdominal masses | NR | 58 | 32 | 63 | Yes | NR | NR | NR |
| Kandel, 2016 (USA) 32 | Retrospective | Pancreas, liver, subepithelial lesions, lymph nodes | Consecutive patients undergoing EUS-FNB were matched with random controls undergoing EUS-FNA ratio of 1:3 | NR | 156 | 84 | 66 | Yes | NR | NR | NR |
| Rodrigues-Pinto, 2016 (USA) 33 | Retrospective | Pancreas, lymph nodes, submucosal lesions | NR | NR | 33 | 15 | 65 | Yes | Yes | NR | NR |
NR, not reported; CBD, common bile duct; PD, pancreatic duct; INR, international normalized ratio; PT, prothrombin time; EUS, endoscopic ultrasound; FNB, fine-needle biopsy; FNA, fine-needle aspiration; ROSE, rapid onsite evaluation.
Table 2. Outcomes assessed in meta-analysis.
| Study | Groups | Needle size, G | Diagnostic adequacy | Diagnostic accuracy | Optimal histology cores | Mean number (SD) of passes required for diagnosis | NOS quality assessment | Cochrane tool for risk of bias | |||
| Total | Pancreas | Total | Pancreas | Total | Pancreas | ||||||
| Bang, 2012 (USA) 19 | FNAFNB | 2222 | 28/2825 /28 | 28/2825 /28 | 8/1214 /18 | 1.61 (0.88)1.28 (0.54) | High risk of performance bias, low risk for selection, detection, attrition, and reporting bias | ||||
| Hucl, 2013 (India) 20 | FNAFNB | 2222 | 127/145125 /145 | 60/6964 /69 | 112/139110 /139 | 51/6959 /69 | 96/139100 /139 | 2.47 (0.93)1.23 (0.47) | 8 | ||
| Witt, 2013 (USA) 21 | FNAFNB | 2222 | 16/1817 /18 | 17/1817 /18 | 10/138 /11 | 2.942.11 | 6 | ||||
| Kim, 2014 (Korea) 22 | FNAFNB | 2222 | 2/109/12 | 2/109/12 | 2/109/12 | 3.2 (1.3)1.8 (0.9) | High risk of performance bias, low risk for selection, detection, attrition, and reporting bias | ||||
| Vanbiervliet, 2014 (France) 23 | FNAFNB | 2222 | NRNR | 74/8072/80 | 70/8056/80 | NRNR | High risk of performance bias, low risk for selection, detection, attrition, and reporting bias | ||||
| Lee, 2014 (Korea) 24 | FNAFNB | 22 & 2522 & 25 | NRNR | 55/5857/58 | 45/5848/58 | NRNR | High risk of performance bias, low risk for selection, detection, attrition, and reporting bias | ||||
| Strand, 2014 (USA) 25 | FNAFNB | 2222 | 32/3219/27 | 30/329/27 | 7/919/27 | 2.9 (1.55)1.4 (0.67 | 4 | ||||
| Lin, 2014 (USA) 26 | FNAFNB | 2222 | NRNR | 24/2622/26 | NRNR | NRNR | 6 | ||||
| Mavrogenis, 2015 (Belgium) 27 | FNAFNB | 2225 | 22/2824/28 | 15/1916/19 | 24/2824/28 | 17/1917/19 | 24/2822/28 | 17/1915/19 | NRNR | 7 | |
| Berzosa, 2015 (USA) 28 | FNAFNB | 2522 | 50/6145/61 | 46/6142/61 | NRNR | NRNR | 6 | ||||
| Alatawi, 2015 (France) 29 | FNAFNB | 2222 | 45/5050/50 | 42/5045/50 | 3.28 (1.0)2.59 (0.49) | 8 | |||||
| Yang, 2015 (Korea) 30 | FNAFNB | 2225 | NRNR | 37/3834/38 | 23/3826/38 | NRNR | 7 | ||||
| Dwyer, 2016 (USA) 31 | FNA FNB | 22 & 2522 & 25 | 14/1838/49 | 12/1535/40 | 12/1837/49 | 10/1534/40 | NRNR | NRNR | 3.483.57 | 6 | |
| Kandel, 2016 (USA) 32 | FNAFNB | 19,22,2519,22,25 | 108/11437/39 | NRNR | 23/6713/37 | NRNR | 8 | ||||
| Rodrigues-Pinto, 2016 (USA) 33 | FNAFNB | 22, 2522, 25 | NRNR | 26/3330/33 | NRNR | NRNR | 7 | ||||
FNA, fine-needle aspiration; FNB, fine-needle biopsy; NOS, Newcastle Ottawa Scale; NR, not reported; SD, standard deviation.
Quality assessment of four randomized trials 19 22 23 24 was done using the Cochrane tool for assessing risk of bias. All of the four trials had a high risk of performance bias as none of the endoscopists was blinded to the type of needle being used. However, cytopathologists analyzing the samples were blinded to the type of needle. The risks of selection bias, detection bias, attrition bias, and reporting bias were found to be low in all of the four trials. The Newcastle Ottawa scale was used for appraising the quality of observational studies. Three studies 20 29 32 satisfied the criteria of high quality studies, seven 21 26 27 28 30 31 33 were of moderate quality, and one 25 was of low quality (Table 2).
Meta-analyses
Diagnostic adequacy
Ten studies 19 20 21 22 25 27 28 29 31 32 with a total of 694 patients evaluated the diagnostic adequacy of the two types of procurement needle. Pooled RR for diagnostic adequacy with 95 % confidence interval (CI) was 0.98 (0.91, 1.06), (Cochran Q test P = 0.01, I 2 = 51 %; Fig. 2). Sensitivity analysis was done after removing the only low quality study 25, to obtain a more robust estimate. The adjusted pooled RR with 95 %CI was 1.00 (0.94, 1.07), (Cochran Q test P = 0.14, I2 = 36 %). Six studies 19 21 25 28 31 32 had an onsite pathologist available for specimen analysis. We evaluated the effect of onsite pathology by conducting a meta-regression to further explore heterogeneity in our estimate. Although not statistically significant, the absence of onsite pathologist showed a trend of better diagnostic adequacy with FNB in comparison to FNA (Intercept coefficient: – 0.51, No onsite pathology coefficient: 1.30, P = 0.06). A scatterplot for meta-regression analyzing the effect of onsite pathology is presented in Fig. 3. This signifies that in the absence of ROSE, FNB showed a trend of better diagnostic adequacy. The funnel plot appeared symmetric and Egger’s test failed to detect any publication bias (P = 0.65, two tailed).
Fig. 2.
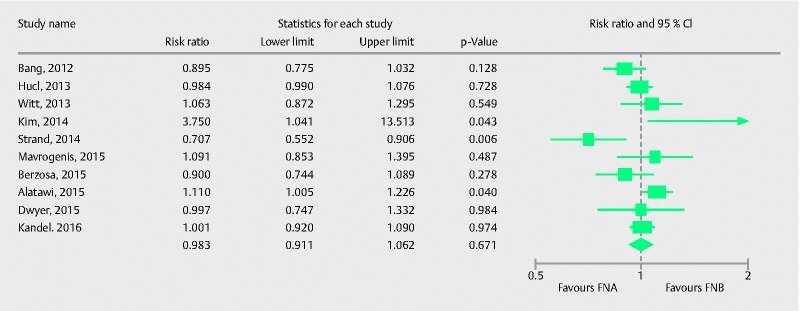
Forrest plot for diagnostic adequacy of FNA in comparison to FNB.
Fig. 3.
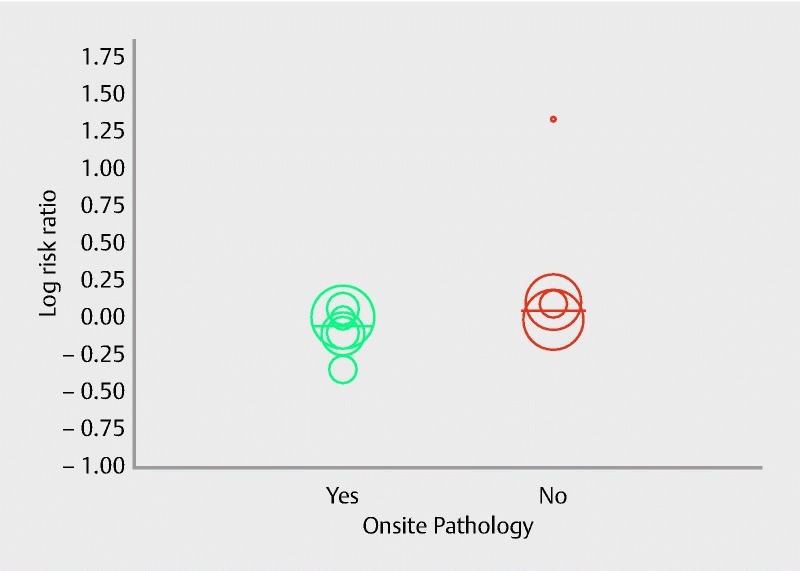
Scatterplot for meta-regression analysis for onsite pathology in diagnostic adequacy (P = 0.06).
Subgroup analysis was conducted evaluating the diagnostic adequacy in pancreatic lesions and seven studies were involved in this analysis. Pooled RR with 95 %CI for diagnostic adequacy of pancreatic lesions was 0.96 (0.86, 1.09), (Cochran Q test P = 0.004, I 2 = 66 %; Fig. 4). Sensitivity analysis was performed after removing the study by Strand et al. 25 as it appeared to be an outlier in the estimate. Pooled RR was 1.00 (0.91, 1.11), (Cochran Q test P = 0.06, I 2 = 50 %). To further explore heterogeneity, onsite pathology was evaluated as a source of heterogeneity in meta-regression analysis. The absence of onsite pathology was a significant predictor of heterogeneity and was associated with better diagnostic adequacy of core biopsy needle in comparison to fine-needle aspiration (intercept coefficient = – 0.56, No onsite pathology coefficient = 1.30, P = 0.02). A scatterplot summarizing this meta-regression is shown in Fig. 5. This signifies that, in the absence of ROSE, FNB had better diagnostic adequacy in pancreatic lesions. To summarize, in the absence of an onsite pathologist, the performance of core needle biopsy was relatively better compared to fine-needle aspiration in terms of diagnostic adequacy of pancreatic lesions.
Fig. 4.
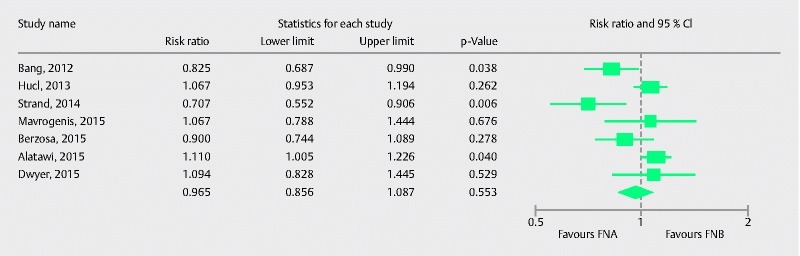
Forrest plot for diagnostic adequacy of FNA in comparison to FNB for pancreatic lesions.
Fig. 5.
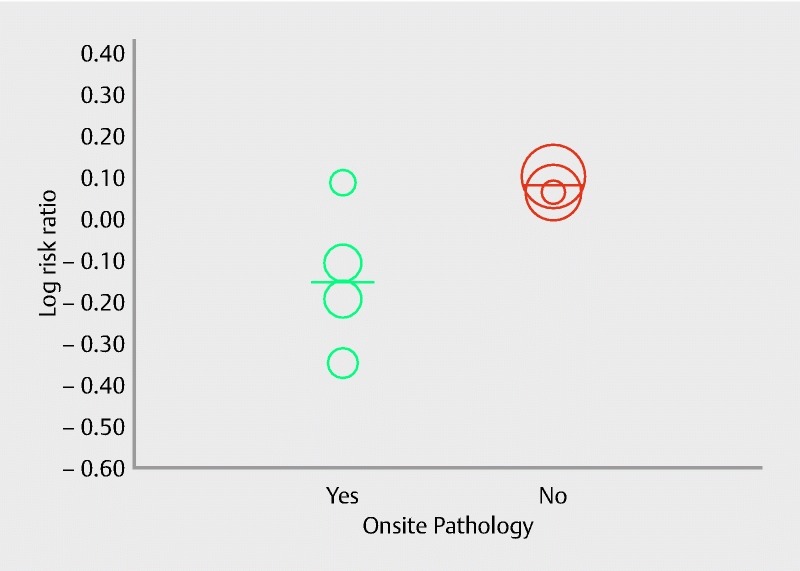
Scatterplot for meta-regression analysis for onsite pathology in diagnostic adequacy for pancreatic lesions (P = 0.02).
Diagnostic accuracy
A total of 12 studies 20 21 23 24 25 26 27 28 29 30 31 33 comprising 791 patients evaluated the diagnostic accuracy of FNB in comparison to FNA. Pooled RR with 95 %CI for diagnostic accuracy was 0.99 (0.95, 1.03), (Cochran Q test P = 0.18, I 2 = 27 %; Fig. 6). Once again the study by Strand et al. 25 appeared to be an outlier in the estimate; therefore, we conducted a sensitivity analysis after excluding it. The adjusted RR with 95 %CI was 1.00 (0.96, 1.04), (Cochran Q test P = 0.68, I 2 = 0 %). The funnel plot appeared symmetric and Egger’s test for asymmetry was negative (P = 0.93, two tailed).
Fig. 6.
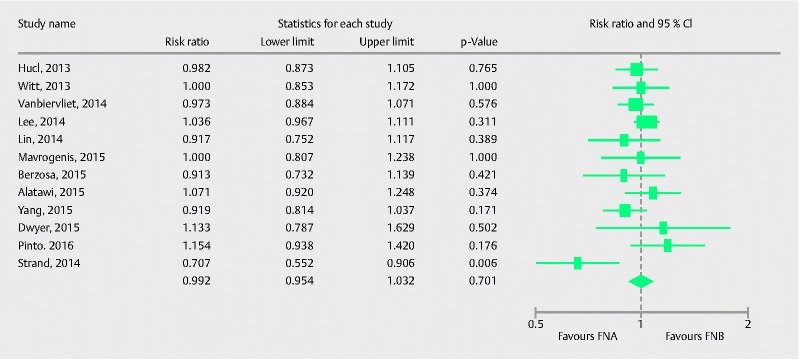
Forrest plot for diagnostic accuracy of FNA in comparison to FNB.
Nine studies evaluated the diagnostic accuracy of FNB in comparison to FNA for pancreatic lesions. Pooled RR with 95 %CI was 0.99 (0.90, 1.08), (Cochran Q test P = 0.003, I 2 = 65 %; Fig. 7). After removing Strand et al. 25, sensitivity analysis showed pooled RR 1.01 (0.96, 1.05), (Cochran Q test P = 0.28, I 2 = 19 %). On meta-regression analysis, onsite pathology was not a significant predictor of heterogeneity (intercept coefficient = – 0.43, No onsite pathology = 0.48, P = 0.56).
Fig. 7.
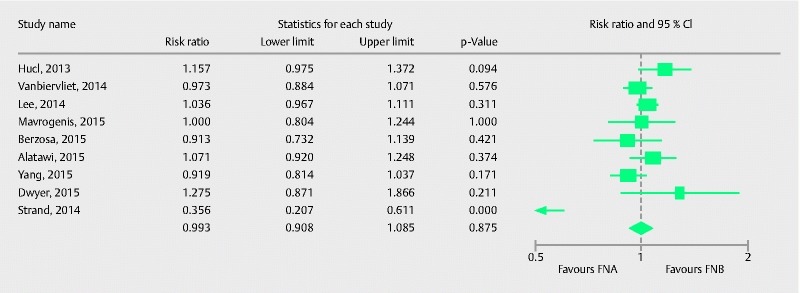
Forrest plot for diagnostic accuracy of FNA in comparison to FNB in pancreatic lesions.
Optimal quality histological core procurement
Nine studies comprising 725 patients compared the two types of needle in their ability to procure optimal histological cores. Pooled RR with 95 %CI for procurement of optimal histological cores was 0.97 (0.89, 1.05), (Cochran Q test, P = 0.35, I 2 = 9.6 %; Fig.8). The funnel plot appeared symmetric and Egger’s test did not detect any publication bias (P = 0.63, two tailed). Six studies compared optimal quality core procurement in pancreatic lesions. Pooled RR with 95 %CI was 0.95 (0.83, 1.09), (Cochran Q test P = 0.13, I2 = 35 %; Fig. 9).
Fig. 8.
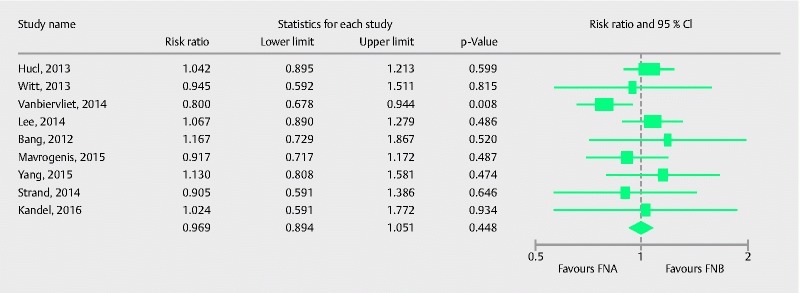
Forrest plot for optimal histological core procurement comparing FNA with FNB.
Fig. 9.
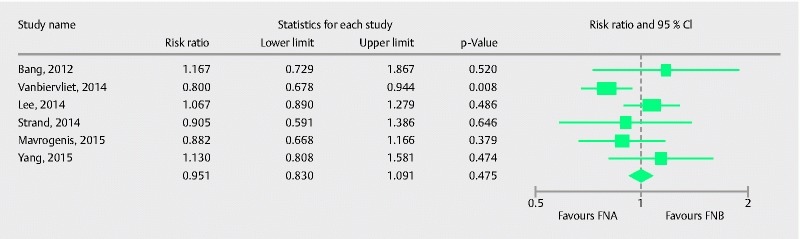
Forrest plot for optimal histological core procurement comparing FNA with FNB in pancreatic lesions.
Number of passes required to establish diagnosis
Seven studies with 449 patients provided comparative data on the mean number of passes required to establish diagnosis with each needle. Pooled standard mean difference (SMD) with 95 %CI was in favor of FNB [0.93 (0.45, 1.42), (Cochran Q test P < 0.0001, I 2 = 84 %; Fig. 10)]. Meta-regression analysis was conducted to explore heterogeneity. The absence of onsite pathology was significantly associated with a higher SMD (intercept coefficient = 0.64, No onsite pathology coefficient: 0.68, P = 0.03). Meta-regression is summarized in the scatterplot (Fig. 11). Therefore, in the presence of an onsite pathologist, FNA required relatively fewer passes to establish the diagnosis than in the absence of an onsite pathologist. In summary, core needles are superior in establishing the diagnosis with fewer passes irrespective of the presence of onsite pathology. No publication bias was detected by Egger’s test of asymmetry (P = 0.13).
Fig. 10.
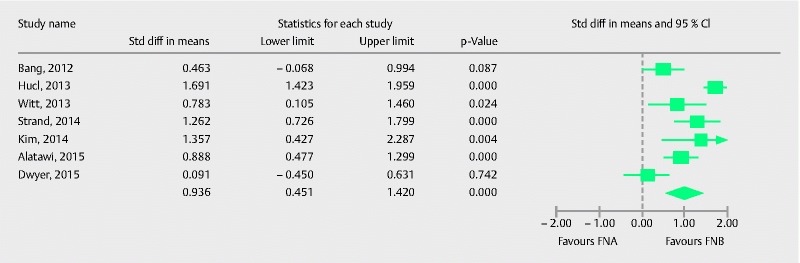
Forrest plot for number of passes required for diagnosis with FNA in comparison to FNB.
Fig. 11.
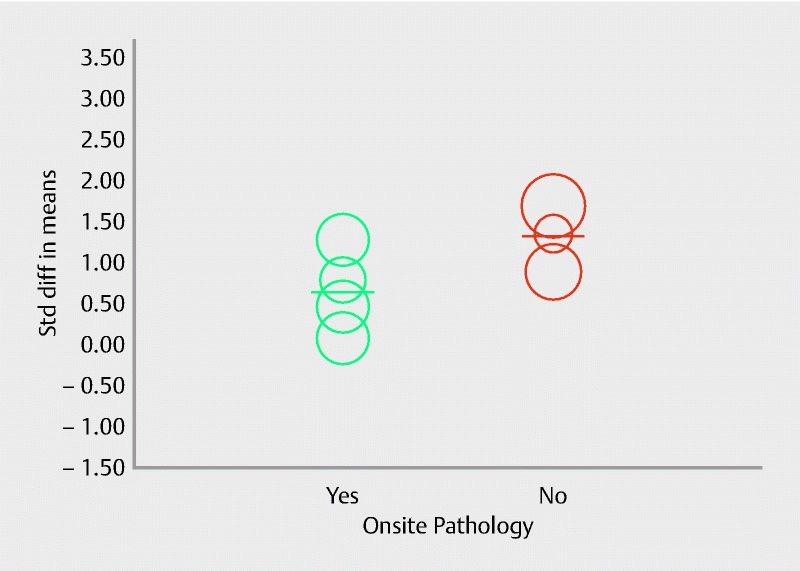
Scatterplot for meta-regression evaluating effect of onsite pathology on number of passes.
Discussion
EUS-FNA with ROSE is considered to be the gold standard for EUS-guided tissue acquisition in the United States; however, ROSE has not been uniformly incorporated in all centers in the United States and even less so worldwide. The main aim of this meta-analysis was to evaluate diagnostic performance of second generation FNB needles in comparison to FNA and to evaluate the influence of onsite cytopathology in such an estimate.
Major limitations of EUS-FNA are the relatively small amount of tissue obtained and the inability to provide core tissue for histochemical analysis, which is indispensable not only in the diagnosis of certain malignant conditions such as GISTs, and lymphoma but also in diagnosing benign conditions such as autoimmune pancreatitis 9 11. Another drawback of standard FNA is the presumed requirement of ROSE to increase diagnostic yield. As a result, the field continues to search for tissue acquisition alternatives that can allow high diagnostic accuracy without the use of ROSE. One such alternative is the development of core biopsy needles for procuring histological samples. Studies comparing the first generation Trucut biopsy needle with standard FNA failed to establish superiority of core biopsy needles to FNA 34 35. Studies comparing the second generation needles have reached conflicting results. A previous meta-analysis 36 of small numbers of studies and patient populations failed to show any difference between the diagnostic performances of FNA in comparison to second generation core biopsy needles; however, they did not specifically address the issue of onsite cytopathology, which is one of the major reasons for the development of core biopsy needles.
Onsite pathology increases the diagnostic performance of EUS-FNA 37, but because of its limited availability to tertiary care centers in the United States, this increased diagnostic performance may not be applicable to centers that do not have ROSE. A recent study by Kandel et al. 32 showed significantly better histological sample procurement with a fork-tip FNB needle (SharkCore) in comparison with a standard FNA needle (95 % versus 59 %, P = 0.01), with fewer passes in favor of FNB (2 versus 4, P = 0.001). Likewise, another observational study 33 with a crossover design demonstrated that a diagnosis of malignancy was more likely with FNB (72.7 % versus 66.7 %, P = 0.003). This study also showed that FNB samples also provided qualitative information such as degree of differentiation in malignancy, metastatic origin, and rate of proliferation in neuroendocrine tumors which were not available with samples procured from standard FNA needles.
In our meta-analysis, diagnostic adequacy was similar for both types of FNB needles for all lesions. However, the analysis was limited by moderate heterogeneity and on meta-regression. A trend towards better diagnostic adequacy with second generation core biopsy needles was seen in the absence of onsite pathology. Likewise, when these two needles were used exclusively for pancreatic lesions, we found no significant difference in diagnostic adequacy. Once again the analysis for only pancreatic lesions was limited by moderate heterogeneity and on meta-regression analysis; onsite pathology was a significant predictor of heterogeneity. The absence of onsite pathology was associated with a significant increase in diagnostic adequacy when core biopsy needles were used. We did not find any difference in diagnostic accuracy and optimal histological core procurement between FNA and core needles for all lesions, and when analyzing only pancreatic lesions. Finally, core biopsy needles required significantly fewer passes to establish the diagnosis compared to standard FNA. This analysis was limited by considerable heterogeneity and onsite pathology was found to be a significant predictor of heterogeneity. In short, standard FNA needles required relatively fewer passes in the presence of onsite pathology compared to absence of onsite pathology.
It is worth noting that while ROSE increases the diagnostic performance of FNA, it also increases direct costs and procedure duration. If similar diagnostic ability can be attained with core biopsy needles, ROSE may not be required for better diagnostic performance. Increased procedure duration translates into higher opportunity costs (costs associated with lost time while awaiting cytological interpretation feedback). Lin et al. 26 found that FNB using two needle passes had similar diagnostic accuracy as FNA. Rodrigues-Pinto et al. 33 reported higher malignancy detection with FNB than with FNA when the same number of passes was performed. According to a recent study 37 examining the cost benefit analysis of ROSE in FNA, ROSE was advantageous when per-pass adequacy was low. In our estimate, we found no difference in diagnostic adequacy and diagnostic accuracy, but core needle biopsy required fewer passes to establish a diagnosis. This points towards the fact that the performance of the core biopsy needle may be superior to standard FNA.
Strengths and limitations
To our knowledge, this is the first meta-analysis comparing the diagnostic performance of two types of second generation core biopsy needles with standard FNA needles, and the first to specifically assess the influence of ROSE. Our meticulously conducted analysis included a comprehensive search strategy, with inclusion of the largest number of relevant studies, and adds substantially to the previously accumulated evidence. Due to the relatively higher number of studies in the analysis, we were able to assess for publication bias and conduct a predetermined meta-regression based on the presence or absence of onsite pathology; however, there are several limitations to our analysis. First, our estimates of diagnostic adequacy and number of passes required for diagnosis were limited by moderate and considerable heterogeneity. We evaluated the role of onsite pathology via meta-regression and found that it was a significant predictor of heterogeneity in diagnostic adequacy of pancreatic lesions and in mean number of passes required for diagnosis. Second, we could not perform a cost-benefit analysis from the included studies as such data were not reported. Third, the included studies mostly used 22G or 25G needles and data from the 19G needle was only included in one study. Finally, we have pooled the studies using ProCore and SharkCore needles together. All of these factors may have accounted for heterogeneity in our estimate.
How does this knowledge help our endoscopy practice? This meta-analysis does not establish superiority of core biopsy needles in comparison to standard FNA needles in terms of diagnostic adequacy, diagnostic accuracy, and optimal quality core procurement. However, in the absence of onsite cytopathological assessment, core biopsy needles showed a trend toward better diagnostic adequacy in all lesions, and significantly better diagnostic adequacy for pancreatic lesions. Also, core biopsy needles required a lower number of passes to establish the diagnosis.
In summary, this analysis of the literature adds considerable weight to the conclusion that FNB without ROSE can supplant EUS-FNA with ROSE without loss of diagnostic accuracy. The evolution of endosonographic tissue acquisition from FNA to FNB seems almost inevitable, as the elimination of ROSE not only makes the procedure more economical but it can also simultaneously provide qualitatively superior histologic specimens. Development of additional core needles for this purpose is in progress.
Footnotes
Competing interests Dr. Todd H. Baron is a consultant and speaker for BSCI, Olympus, Cook, and Medtronic. All other authors have no financial disclosures or conflicts of interest to declare.
References
- 1.Siddiqui U D, Rossi F, Rosenthal L S et al. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–1097. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 2.Vander Noot M R, 3rd, Eloubeidi M A, Chen V K et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102:157–163. doi: 10.1002/cncr.20360. [DOI] [PubMed] [Google Scholar]
- 3.Chen V K, Eloubeidi M A. Endoscopic ultrasound-guided fine-needle aspiration of intramural and extraintestinal mass lesions: diagnostic accuracy, complication assessment, and impact on management. Endoscopy. 2005;37:984–989. doi: 10.1055/s-2005-870272. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda I, Tsurumi H, Omar S et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–924. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 5.DeWitt J, Alsatie M, LeBlanc J et al. Endoscopic ultrasound-guided fine-needle aspiration of left adrenal gland masses. Endoscopy. 2007;39:65–71. doi: 10.1055/s-2006-945042. [DOI] [PubMed] [Google Scholar]
- 6.Itoi T, Sofuni A, Itokawa F et al. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011;23 01:17–21. doi: 10.1111/j.1443-1661.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 7.Dumonceau J M, Polkowski M, Larghi A et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 8.Sepe P S, Moparty B, Pitman M B et al. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254–261. doi: 10.1016/j.gie.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno N, Bhatia V, Hosoda W et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742–750. doi: 10.1007/s00535-009-0062-6. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro A, Vazquez-Sequeiros E, Wiersema L M et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–491. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 11.Na H K, Lee J H, Park Y S et al. Yields and utility of endoscopic ultrasonography-guided 19-gauge Trucut biopsy versus 22-Gauge fine needle aspiration for diagnosing gastric subepithelial tumors. Clin Endosc. 2015;48:152–157. doi: 10.5946/ce.2015.48.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wittmann J, Kocjan G, Sgouros S N et al. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman D G, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D F, Berlin J A, Morton S C et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Taddio A, Pain T, Fassos F F et al. Quality of nonstructured and structured abstracts of original research articles in the British Medical Journal, the Canadian Medical Association Journal and the Journal of the American Medical Association. CMAJ. 1994;150:1611–1615. [PMC free article] [PubMed] [Google Scholar]
- 16.Scherer R W, Langenberg P, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.MR000005.pub3. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Deeks J J, Higgins J PT, Altman D G.on behalf of the Cochrane Statistical Methods Group.Chapter 9: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.1 [updated September 2008]Available at:http://handbook.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm[Accessed 10 July 2016]
- 19.Bang J Y, Hebert-Magee S, Trevino J et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–327. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hucl T, Wee E, Anuradha S et al. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy. 2013;45:792–798. doi: 10.1055/s-0033-1344217. [DOI] [PubMed] [Google Scholar]
- 21.Witt B L, Adler D G, Hilden K et al. A comparative needle study: EUS-FNA procedures using the HD ProCore(™) and EchoTip(®) 22-gauge needle types. Diagn Cytopathol. 2013;41:1069–1074. doi: 10.1002/dc.22971. [DOI] [PubMed] [Google Scholar]
- 22.Kim G H, Cho Y K, Kim E Y et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347–354. doi: 10.3109/00365521.2013.867361. [DOI] [PubMed] [Google Scholar]
- 23.Vanbiervliet G, Napoleon B, Saint Paul M C et al. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: a randomized crossover study. Endoscopy. 2014;46:1063–1070. doi: 10.1055/s-0034-1377559. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y N, Moon J H, Kim H K et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014;46:1056–1062. doi: 10.1055/s-0034-1377558. [DOI] [PubMed] [Google Scholar]
- 25.Strand D S, Jeffus S K, Sauer B G et al. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol. 2014;42:751–758. doi: 10.1002/dc.23116. [DOI] [PubMed] [Google Scholar]
- 26.Lin M, Hair C D, Green L K et al. Endoscopic ultrasound-guided fine-needle aspiration with on-site cytopathology versus core biopsy: a comparison of both techniques performed at the same endoscopic session. Endosc Int Open. 2014;2:E220–E223. doi: 10.1055/s-0034-1377611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavrogenis G, Weynand B, Sibille A et al. 25-gauge histology needle versus 22-gauge cytology needle in endoscopic ultrasonography-guided sampling of pancreatic lesions and lymphadenopathy. Endosc Int Open. 2015;3:E63–E68. doi: 10.1055/s-0034-1390889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berzosa M, Villa N, El-Serag H B et al. Comparison of endoscopic ultrasound guided 22-gauge core needle with standard 25-gauge fine-needle aspiration for diagnosing solid pancreatic lesions. Endosc Ultrasound. 2015;4:28–33. doi: 10.4103/2303-9027.151320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alatawi A, Beuvon F, Grabar S et al. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015;3:343–352. doi: 10.1177/2050640615577533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M J, Yim H, Hwang J C et al. Endoscopic ultrasound-guided sampling of solid pancreatic masses: 22-gauge aspiration versus 25-gauge biopsy needles. BMC Gastroenterol. 2015;15:122. doi: 10.1186/s12876-015-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dwyer J, Pantanowitz L, Ohori N P et al. Endoscopic ultrasound-guided FNA and ProCore biopsy in sampling pancreatic and intra-abdominal masses. Cancer Cytopathol. 2016;124:110–121. doi: 10.1002/cncy.21623. [DOI] [PubMed] [Google Scholar]
- 32.Kandel P, Tranesh G, Nassar A et al. EUS-guided fine needle biopsy using a novel fork-tip needle: a case-control study. Gastrointest Endosc. 2016;84:1034–1039. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues-Pinto E, Jalaj S, Grimm I S et al. EUS-guided fine needle biopsy with a new core needle may eliminate the need for an on-site cytopathological assessment. Gastrointest Endosc. 2016;84:1040–1046. doi: 10.1016/j.gie.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 34.Varadarajulu S, Fraig M, Schmulewitz N et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 35.Storch I, Jorda M, Thurer R et al. Advantage of EUS Trucut biopsy combined with fine-needle aspiration without immediate on-site cytopathologic examination. Gastrointest Endosc. 2006;64:505–511. doi: 10.1016/j.gie.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 36.Bang J Y, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–349. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt R L, Walker B S, Cohen M B. When is rapid on-site evaluation cost-effective for fine-needle aspiration biopsy? PLoS One. 2015;10:e0135466. doi: 10.1371/journal.pone.0135466. [DOI] [PMC free article] [PubMed] [Google Scholar]


