Abstract
Objectives
To examine whether pre-heart failure (HF) myocardial injury explains the differential mortality after HF across weight categories.
Background
Obesity is a risk factor for HF, but pre-HF obesity is associated with lower mortality after incident HF. High-sensitivity Troponin T (hs-cTnT), a sensitive marker of myocardial injury, predicts incident HF and mortality.
Methods
Stratifying 1279 individuals with incident HF hospitalizations by their pre-HF hs-cTnT levels (< and ≥ 14 ng/L), we examined the association of pre-HF body mass index (BMI) with mortality after incident HF hospitalization in the ARIC study.
Results
Mean age at HF was 74 years (53% women, 27% black). Individuals with pre-HF hs-cTnT≥14 ng/L had higher mortality after incident HF (HR 1.46, 95% CI 1.18-1.80) compared to individuals with hs-cTnT<14 ng/L in an adjusted model including BMI. Compared with normal weight, the mortality was lower in overweight (HR 0.69, 95% CI 0.48-0.98) and obese (HR 0.50, 95% CI 0.35-0.72) with hs-cTnT<14 ng/L; and in those with hs-cTnT ≥14 ng/L (overweight: HR 0.50, 95% CI 0.30-0.83; obese: HR 0.56, 95% CI 0.34-0.91) (interaction p=0.154 between BMI and hs-cTnT). The lower mortality risk in obese and overweight remained similar when log hs-cTnT was added as a continuous variable to a multivariable model and in sensitivity analyses adjusting for left ventricular hypertrophy and high-sensitivity C-reactive protein.
Conclusion
Although greater pre-existing subclinical myocardial injury was associated with higher mortality after incident HF hospitalization, it did not explain the obesity paradox in HF, which was observed irrespective of subclinical myocardial injury.
Keywords: Obesity paradox, Heart failure, Myocardial injury, Outcomes
Introduction
Obesity and overweight affect about two-thirds of the U.S. population (1). Obesity is associated with an increased risk of heart failure (HF) (2,3). Paradoxically, once HF develops, obesity is associated with better prognosis (4-7). Using the Atherosclerosis Risk In Communities (ARIC) study, we previously showed that individuals who were overweight and obese (using pre-HF body mass index, BMI) before the onset of incident HF hospitalization also had lower mortality after HF development, compared to those with normal BMI, independent of demographics and comorbidities, including cancer, smoking and diabetes (8). Our findings suggested that weight loss from advanced HF did not completely explain the survival benefit associated with higher BMI in HF patients. One potential explanation for this obesity paradox is that the HF patients who are able to preserve their weight may represent a non-catabolic subgroup with different neurohormonal, inflammatory and metabolic profiles (9). However, there may also be heterogeneity in the degree of myocardial injury in obese individuals who present with the constellation of symptoms and signs such as shortness of breath and fluid retention, leading to a diagnosis of HF.
Cardiac troponin T measured using a high-sensitivity assay (hs-cTnT) is a sensitive marker of subclinical myocardial injury (10). We have shown that hs-cTnT predicts incident HF in the ARIC study (11). Other studies of community dwelling, otherwise asymptomatic individuals have also demonstrated similar findings (12,13). Furthermore, higher levels of hs-cTnT were also associated with increased cardiac and all-cause mortality (11-13), and higher degree of myocardial dysfunction (13). We postulated that hs-cTnT measured before incident HF would be helpful in identifying overweight and obese individuals at varying risk for adverse outcomes after the development of the clinical HF syndrome. Undetectable or low levels of hs-TnT may therefore identify ‘cardiac biomarker-healthy’ obese individuals with lower degree of myocardial injury in whom symptoms of HF such as shortness of breath and edema are driven mostly by non-cardiac obesity-related mechanisms, and who would therefore have a good prognosis contributing to the obesity paradox. In contrast, ‘cardiac biomarker-unhealthy’ obese, in whom HF is driven to a greater extent by myocardial injury/dysfunction as indexed by hs-cTnT, would be expected to have a poor prognosis. We postulated that the obesity paradox will be observed in “biomarker-healthy obese” individuals but not in the “biomarker-unhealthy obese”, as stratified by levels of hs-cTnT prior to incident HF. Accordingly, we examined the risk of mortality associated with pre-HF BMI and hs-cTnT in individuals who subsequently had incident HF hospitalization in the ARIC study.
Methods
Study population
The ARIC study is an ongoing prospective cohort study of 15,792 individuals enrolled from four U.S. communities: Washington County, Maryland; Jackson, Mississippi; Forsyth County, North Carolina; and suburbs of Minneapolis, Minnesota (14). The baseline evaluation (Visit 1) of individuals aged 45-64 years, took place between 1987 and 1989. Participants were subsequently examined at three follow-up visits at approximately 3 year intervals, and after an extended interval, a fifth study visit was recently completed between 2011 and 2013. The design, recruitment and examination protocols for the ARIC study has been described in detail previously (14). The institutional review boards at each site approved all study protocols, and informed consent was provided by all study participants. The authors are solely responsible for the design, conduct and analyses of the study and the drafting, editing and preparation of the final version of the manuscript. Visit 4 (1996-1998), at which hs-cTnT was measured for all participants, was the baseline for the current analysis. Of 11,656 ARIC Visit 4 participants, 1,279 individuals who had an incident HF hospitalization at least 6 months after Visit 4 were eligible for this analysis after exclusions as detailed in Figure 1.
Figure 1. Development of the Study Cohort.
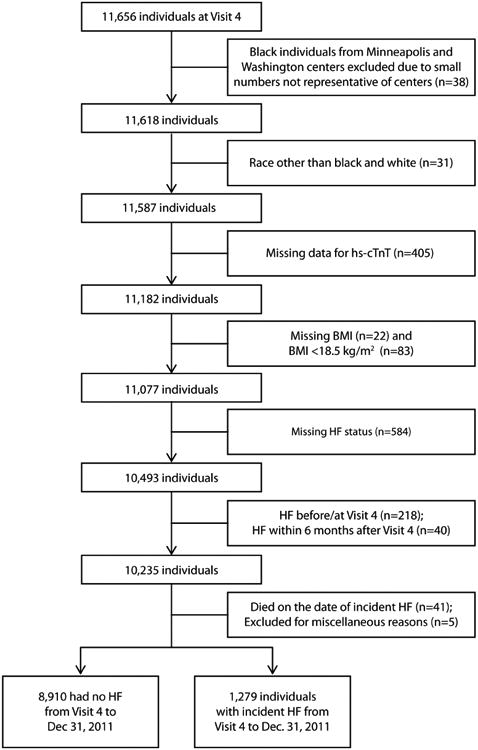
BMI = body mass index, HF = heart failure, hs-cTnT = cardiac troponin T measured using high-sensitivity assay
Measurement of hs-cTnT
The details regarding the hs-cTnT assay have been previously published (11). Briefly, hs-cTnT was measured in 2010 on blood samples collected during ARIC Visit 4 with a high-sensitivity assay, Elecsys Troponin T (Roche Diagnostics®), on an automated Cobas e411 analyzer. The 99th percentile value for the hs-cTnT measured in a healthy reference population (aged 20–70) was 14 ng/L (Roche Diagnostics®, data on file).
Baseline variables
Measurements were taken in standard scrub attire with no shoes. Weight was measured using a scale that was zeroed daily and calibrated quarterly. BMI assessment was performed at the time of hs-cTnT measurement. Pre-HF BMI was defined as the BMI measurement from ARIC Visit 4, which was at least 6 months before the date of incident HF hospitalization (8). Patients with HF were categorized by the pre-HF BMI into normal weight (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), and obese (≥30 kg/m2) groups. Patients in the underweight category (BMI <18.5 kg/m2; n=83) were excluded because of small numbers and the possible presence of other comorbidities leading to lower BMI (8).
The ascertainment of demographics and comorbidities at each study visit has been described previously (14). Age was assessed at the time of incident HF hospitalization, and sex, race and education levels were obtained at ARIC Visit 1 from interviewer-administered questionnaires. Physical activity was assessed at ARIC Visit 3 using the sport index of the Baecke Questionnaire (15). Baecke sport activity score ranges from 1-5 with a higher score indicating higher physical activity (16). Comorbidities such as hypertension, diabetes, cancer, history of coronary heart disease (CHD), stroke and transient ischemic attacks were assessed as present if the conditions were documented to be present at any pre-HF ARIC visit. All other baseline variables were obtained from Visit 4. Diabetes was defined as fasting blood glucose level ≥126 mg/dL, non-fasting blood glucose level ≥200 mg/dL, or self-reported physician diagnosis of or treatment for diabetes. Total cholesterol and high-density lipoprotein cholesterol were determined by enzymatic methods. Estimated glomerular filtration rate was computed using the Modification of Diet in Renal Disease study equation (17). Blood pressure was measured twice and the average of the two measurements was used. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure BP ≥90 mmHg measured with random-zero mercury manometers or use of antihypertensive medications. Details of ascertainment of CHD and stroke have been described previously (18-20).
Ascertainment of HF and All-Cause Mortality
ARIC investigators conduct continuous, comprehensive surveillance for all cardiovascular (CV) disease-related hospitalizations and deaths in the 4 communities. Incident HF hospitalization was determined using annual interviews with study participants regarding interim hospitalizations (response rate: 93-96%), review of discharge lists from local hospitals and survey of health department death certificate files and the national death index. In the ARIC cohort, incident HF was defined as the first occurrence of either a hospitalization that included an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) discharge diagnosis code for HF beginning with “428” (ie, 428.0 to 428.9) in any position or a death certificate ICD-9 code beginning with “428” (HF) or ICD-10 code “I50” (HF or I50.0 to I50.9) in any position (8,21). Deaths were confirmed by review of hospital discharge records for inpatient deaths and death certificates for deaths outside the hospital.
Statistical Analysis
The outcome of interest was all-cause mortality in individuals who had incident HF hospitalization. For this study only incident HF ascertained ≥ 6 months after ARIC Visit 4 until December 31, 2011 was included because this was the last date for which follow up was available at the time the study was designed. The last HF hospitalization occurred on December 29, 2011. Eligible study participants with incident HF hospitalization were followed up for death up to 5 years after incident HF hospitalization or until December 31, 2011. Categorical variables were compared between groups using Chi-square tests and continuous variables were compared using the analysis of variance test (ANOVA). To accommodate for the wide range of follow-up duration, we calculated survivor function in the overall population and within BMI and hs-cTnT categories using survival estimate from Cox proportional hazard model. The survivor function estimates the proportion of patients surviving at any time point. We assessed the associations between pre-HF BMI categories (normal weight, overweight and obese) and all-cause mortality after HF in a model adjusted for age, gender, race, education level, health insurance, systolic blood pressure, physical activity, total cholesterol, current smoking, current alcohol use, estimated glomerular filtration rate, diabetes mellitus, hypertension, history of CHD, stroke/transient ischemic attack and cancer using Cox proportional hazards model (Model 1). Subsequently, we added log-transformed hs-cTnT as a continuous variable to the model above. We selected covariates a priori based on previous knowledge of potentially important variables (8). We also examined hazard ratios (HRs) for all-cause mortality by pre-HF BMI and hs-cTnT, with hs-cTnT as two (< or ≥14 ng/L) categories. This cutoff for hs-cTnT was chosen because hs-cTnT=14 ng/L corresponds to the 99th percentile in a healthy reference population as provided by the assay manufacturer, and has been used in prior analyses (11,20,22). We examined the interaction between BMI and hs-cTnT categories and between BMI category and hs-cTnT as a continuous variable (log-transformed).. We also assessed the hazard ratios for death in individuals with pre-HF hs-cTnT ≥14 ng/L compared to hs-cTnT<14 using Model 1 plus BMI (treated as ordinal variable, i.e., normal weight, overweight and obese). The proportional hazards assumption was examined using time-dependent covariates and likelihood ratio statistics. When the proportionality assumption was violated, a time-dependent term, the product of BMI/hs-cTnT group and log time was added to the model (8). In addition, we performed some sensitivity analyses. We developed additional models where we further adjusted Model 1 with left ventricular hypertrophy as determined by Cornell voltage electrocardiographic criteria (data available for 976 out of the 1279 individuals in the study cohort) and with high-sensitivity C-reactive protein (data available in 1176 individuals). All presented tests were 2-tailed and a p value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.3 (Cary, NC) and STATA version 12 (College Station, TX).
Results
Baseline characteristics
The final study cohort consisted of 1,279 individuals with incident HF hospitalization whose BMI and hs-cTnT were measured at least 6 months before incident HF. The pre-HF BMI and hs-cTnT were both measured at a median of 8.3 (25th, 75th percentile 5.0, 11.4) years before the HF diagnosis. The mean age was 74 years at the time of incident HF hospitalization, 53% of the participants with HF were women, 27% were black, 17% had normal weight, 35% were overweight and 48% were obese.
Compared to individuals with lower pre-HF hs-cTnT levels, those with higher hs-cTnT levels developed HF at a slightly younger age, were more often men, had more comorbidities such as hypertension, diabetes, history of myocardial infarction, CHD, left ventricular hypertrophy and cancer, had lower levels of estimated glomerular filtration rate, total and high-density lipoprotein cholesterol, but higher levels of triglycerides, high-sensitivity C-reactive protein and NT-proBNP, and had a shorter time to development of HF; however, fewer of them were smokers (Table 1). There was no difference in hemoglobin levels, heart rate and physical activity by hs-cTnT categories. The mean BMI was similar in both hs-cTnT groups. Baseline characteristics by pre-HF BMI groups are described in Supplemental Table 1. Briefly, individuals in higher BMI categories developed HF at a relatively younger age, were more likely to be black, more often had diabetes and hypertension, were less likely to be current smokers and alcohol users, had higher levels of pre-HF high-sensitivity C-reactive protein but lower levels of pre-HF NT-proBNP, but had no difference in the time to incident HF and in the prevalence of pre-existing CHD and history of MI.
Table 1. Baseline characteristics of participants with incident HF hospitalization by pre-HF hs-cTnT levels.
| Variable | hs-cTnT <14 ng/L [N=1000] | hs-cTnT ≥14 ng/L [N=279] | P-value |
|---|---|---|---|
|
| |||
| Median hs-cTnT (ng/L) | 6 (3, 9) | 20 (16, 27) | - |
|
| |||
| Age at incident HF (years) | 74.1 (6.6) | 73.1 (6.2) | 0.033 |
|
| |||
| Male (%) | 402 (40.2) | 204 (73.1) | <0.001 |
|
| |||
| Black (%) | 263 (26.3) | 88 (31.5) | 0.083 |
|
| |||
| Less than high school (%) | 310 (31.0) | 90 (32.4) | |
| High school graduate (%) | 392 (39.2) | 97 (34.9) | 0.398 |
| Greater than high school (%) | 297 (29.7) | 91 (32.7) | |
|
| |||
| BMI (kg/m2) | 30.5 (6.35) | 30.9 (5.79) | 0.311 |
|
| |||
| Normal weight | 187 (18.7) | 37 (13.3) | |
| Overweight | 347 (34.7) | 98 (35.1) | 0.089 |
| Obese | 466 (46.6) | 144 (51.6) | |
|
| |||
| Health Insurance (%) | 785 (79.2) | 211 (76.7) | 0.373 |
|
| |||
| Diabetes mellitus (%) | 301 (30.1) | 146 (52.3) | <0.001 |
|
| |||
| Hypertension (%) | 717 (71.7) | 229 (82.1) | <0.001 |
|
| |||
| LVH by electrocardiogram (%); n=976 | 45 (5.9) | 24 (11.4) | 0.006 |
|
| |||
| Myocardial infarction (%) | 64 (6.4) | 41 (14.7) | <0.001 |
|
| |||
| Coronary heart disease (%) | 148 (15.0) | 75 (27.1) | <0.001 |
|
| |||
| Stroke/TIA (%) | 139 (13.9) | 43 (15.4) | 0.523 |
|
| |||
| Cancer (%) | 133 (13.4) | 50 (18.3) | 0.041 |
|
| |||
| Current smoking (%) | 203 (20.4) | 37 (13.5) | 0.009 |
|
| |||
| Current alcohol use (%) | 415 (41.8) | 101 (36.7) | 0.133 |
|
| |||
| Total serum cholesterol (mg/dL) | 202.4 (38.33) | 193.8 (48.01) | 0.002 |
|
| |||
| High-density lipoprotein cholesterol (mg/dL) | 48.2 (16.22) | 41.9 (13.34) | <0.001 |
|
| |||
| Triglycerides (mg/dL) | 130 (95, 185) | 136 (103, 204) | 0.036 |
|
| |||
| hs-CRP (mg/L); n=1176 | 3.67 (1.53, 7.33) | 4.41 (1.67, 8.62) | 0.029 |
|
| |||
| Hemoglobin (g/dL) | 13.8 (1.32) | 13.8 (1.69) | 0.955 |
|
| |||
| NT-proBNP (pg/mL) | 103 (50, 200) | 177 (70, 483) | <0.001 |
|
| |||
| Systolic blood pressure (mmHg) | 133.4 (20.19) | 136.2 (23.21) | 0.053 |
|
| |||
| Diastolic blood pressure (mmHg) | 70.4 (11.23) | 71.2 (12.95) | 0.334 |
|
| |||
| Heart rate (beats/min) | 67.3 (10.10) | 68.0 (11.17) | 0.286 |
|
| |||
| eGFR (ml/min/1.73m2) | 84.0 (17.31) | 73.3 (24.83) | <0.001 |
|
| |||
| Baecke sport activity score | 2.44 (0.768) | 2.48 (0.820) | 0.432 |
|
| |||
| Time to HF (years) | 8.6 (3.83) | 6.5 (3.56) | <0.001 |
Data expressed as mean (standard deviation) or median (25th, 75th percentile) for continuous variables and as number (percentage) for categorical variables.
Time to HF is time between ARIC Visit 4 and incident HF hospitalization.
Pre-HF hs-cTnT was measured at a median of 8.3 (25th, 75th percentile 5.0, 11.4) years before the HF diagnosis.
BMI for normal weight = 18.5 to <25 kg/m2, overweight = 25 to <30 kg/m2 and obese ≥ 30 kg/m2.
BMI = body mass index, hs-cTnT = high-sensitivity cardiac troponin T, eGFR = estimated glomerular filtration rate, HF = heart failure, hs-CRP = high-sensitivity C reactive protein, LVH = left ventricular hypertrophy, NT-proBNP = N-terminal pro-B-type natriuretic peptide, TIA = transient ischemic attacks.
Mortality
A total of 579 among 1279 individuals (45.3%) who developed HF died within 5 years after incident HF hospitalization [survival estimate 0.48 (0.45-0.51)]. Overall, overweight and obese groups had better survival over 5 years after HF (Supplemental Figure and Supplemental Table 2) compared with normal weight group. Similarly, individuals with hs-cTnT <14 ng/L had better survival than those with hs-cTnT ≥14 ng/L (Supplemental Table 2).
After incident HF hospitalization, overweight and obese individuals had better survival compared with normal-weight people in groups with either hs-cTnT <14 or ≥14 ng/L (Figure 2 and Supplemental Table 2). In individuals with hs-cTnT≥14 ng/L, the Kaplan-Meier survival curves for obese and overweight groups overlapped eventually, however, the normal weight group curve was separated throughout follow-up.
Figure 2.
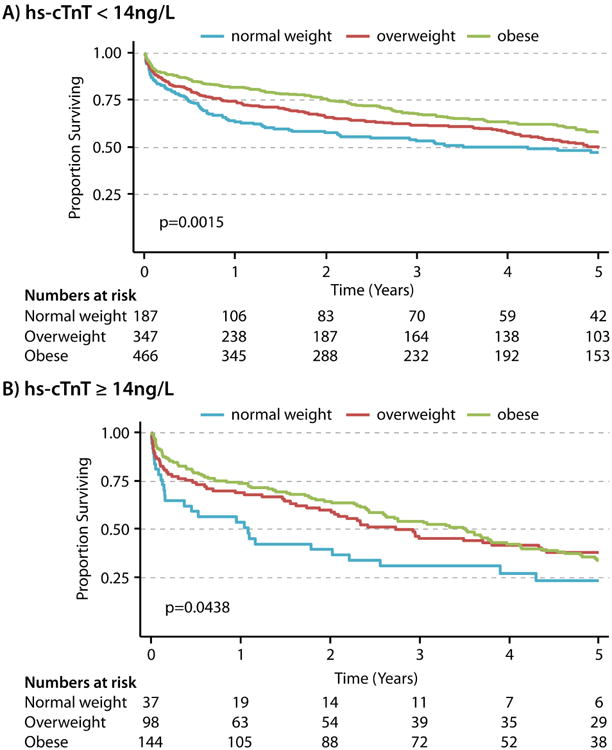
A. Kaplan-Meier Survival Curves by BMI groups in individuals with pre-HF hs-cTnT <14ng/L
B. Kaplan-Meier Survival Curves by BMI groups in individuals with pre-HF hs-cTnT ≥14ng/L HF = heart failure, hs-cTnT = cardiac troponin T measured using high-sensitivity assay
After adjusting for demographics and CV risk factors/disease, overweight and obese HF individuals had lower mortality compared to normal weight HF cohort members in both the groups with hs-cTnT<14 and with hs-cTnT≥14 ng/L (Table 2; p= 0.154 for interaction between BMI and hs-cTnT). For example, obese individuals with hs-cTnT<14 ng/L had 50% lower relative mortality risk than those with normal weight and hs-cTnT<14 ng/L [HR 0.50 (95% CI: 0.35-0.72)]; and obese individuals with hs-cTnT≥14 ng/L had 44% lower relative mortality risk than those with normal weight and hs-cTnT≥14 ng/L [HR 0.56 (0.34-0.91)]. When all the 6 possible groups resulting from BMI and hs-cTnT categories were compared with normal weight and hs-cTnT<14 ng/L as the reference, the highest mortality was observed in normal weight and hs-cTnT≥14 ng/L (HR 1.50), and the lowest mortality was seen in obese and hs-cTnT<14 ng/L (HR 0.50) (Figure 3). In the current data, we again observed that pre-morbidly overweight and obese individuals who subsequently developed HF had reduced mortality risk after HF compared with normal weight individuals after adjusting for demographics and CV risk factors/disease (Model 1, Supplemental Table 3). Results were similar when we further adjusted the model with hs-cTnT (log-transformed) as a continuous variable (Model 2, Supplemental Table 3; p= 0.635 for interaction between BMI and log hs-cTnT) with no appreciable difference in the hazard ratios between models, which did or did not include hs-cTnT. In additional analyses, individuals with hs-cTnT≥14 ng/L had greater risk for death (1.46, 1.18-1.80) when compared to individuals with hs-cTnT<14 in Model 1 plus BMI categories.
Table 2. Hazard ratios for all-cause mortality after incident HF hospitalization by pre-HF BMI and pre-HF hs-cTnT.
| BMI Category [Died/HF=%] [579/1279=45.2%] | hs-cTnT <14 ng/L* [405/1000=40.5%] Adjusted HR (95% CI) | hs-cTnT ≥14 ng/L [174/279=62.4%] Adjusted HR (95% CI) |
|---|---|---|
| Normal weight [115/224=51.3%] | [88/187=47.1%] Reference=1 | [27/37=73.0%] Reference=1 |
| Overweight [208/445=46.7%] | [151/347=43.5%] 0.69 (0.48-0.98) | [57/98=58.2%] 0.50 (0.30-0.83) |
| Obese [256/610=41.9%] | [166/466=35.6%] 0.50 (0.35-0.72) | [90/144=62.5%] 0.56 (0.34-0.91) |
For each hs-cTnT category reference is the normal weight group. BMI for normal weight = 18.5 to <25 kg/m2, overweight = 25 to <30 25 kg/m2 and obese≥ 30 25 kg/m2. Model adjusted for age, gender, race, education level, health insurance, systolic blood pressure, physical activity, total cholesterol, current smoking, current alcohol use, eGFR, diabetes mellitus, hypertension, history of coronary heart disease, TIA/stroke and cancer.
Proportional hazard assumption was violated in the hs-cTnT <14 stratum; time-dependent term of group*log (time in years) was included in adjusted models in this group.
Figure 3. Hazard ratios for all-cause mortality by pre-HF BMI and hs-cTnT.
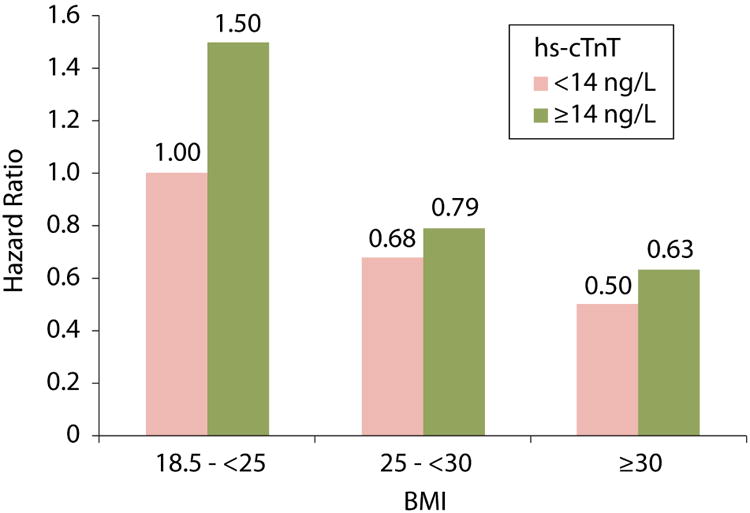
Hazard ratios for each group based on reference group of normal weight with pre-HF hs-cTnT levels <14 ng/ml.
The model was adjusted for age, gender, race, education level, health insurance, systolic blood pressure, physical activity, total cholesterol, current smoking, current alcohol use, eGFR, diabetes mellitus, hypertension, history of coronary heart disease, TIA/stroke and cancer.
Results of sensitivity analyses were similar to those of the main analyses (Supplemental Tables 4-5). When the models examining risk of mortality based on BMI categories within the higher and lower troponin groups were further adjusted for either left ventricular hypertrophy on electrocardiogram (n= 976) or high-sensitivity C-reactive protein (n=1176), the survival benefit for overweight/obese compared to normal weight individuals was still observed within the two troponin groups.
Discussion
In this study, we found that despite an increased risk for mortality with greater pre-existing subclinical myocardial injury, as assessed by hs-cTnT, the mortality risk was lower in overweight or obese individuals compared with normal weight individuals, regardless of preexisting subclinical myocardial injury. Furthermore, individuals with normal pre-HF weight and more advanced pre-HF subclinical myocardial injury (i.e., hs-cTnT≥14 ng/L) had the highest mortality after HF development.
Association of pre-HF hs-cTnT and Heart Failure
Using data from the ARIC cohort, it was recently shown that higher BMI was independently associated with a linear increase in myocardial injury as assessed using hs-cTnT (22). Furthermore, individuals with highest hs-cTnT who were also severely obese had a HR of 9.2 for incident HF hospitalization compared to those with normal weight and undetectable hs-cTnT (22). Other studies have also demonstrated that obesity is independently associated with an increased risk of incident HF (2,3). Our results are in keeping with these observations by demonstrating that overweight and obese individuals with HF have higher level of subclinical myocardial injury several years before the development of HF (Supplemental Table 1).
Association of pre-HF hs-cTnT and Mortality in Heart Failure
Prior studies have demonstrated that increasing myocardial injury as indexed by hs-cTnT was associated with higher cardiac and all-cause mortality (11-13), and higher degree of myocardial dysfunction (13). In the current analysis, we have further demonstrated that higher myocardial injury several years before incident HF hospitalization is also associated with higher post-HF mortality. Among individuals with incident HF hospitalization, those with pre-HF hs-cTnT≥14 ng/L had 46% relative increase in mortality risk compared to those with hs-cTnT<14 ng/L in a model adjusting for BMI and other baseline variables.
Pre-HF Myocardial Injury and the Obesity Paradox
Once HF develops, obese individuals have lower mortality risk compared to normal weight individuals, i.e., the obesity paradox (4-8). Obesity and overweight measured before the onset of HF (pre-morbid BMI) have also been associated with lower mortality risk compared to normal weight individuals (8), suggesting that weight loss/cachexia after HF does not completely explain the lower mortality risk seen in obese HF patients, as was replicated in the current study. We had hypothesized that the obesity paradox may only be observed in individuals with lesser myocardial injury before HF where non-cardiac factors may contribute to the volume overload presenting as the HF syndrome in obese individuals (biomarker healthy obese); but the paradox would not be seen in a more homogenous group with higher pre-HF myocardial injury. However, our analysis found that the risk for death after HF decreased in overweight and obese compared to normal weight individuals regardless of pre-existing sub-clinical myocardial injury in models stratified by hs-cTnT, as well as in models with hs-cTnT as a continuous variable. These results remained the same in sensitivty analyses which adjusted for pre-HF biomarkers of inflammation (high-sensitivity C-reactive protein) and for ventricular structure (left ventricular hypertrophy by electrocardiogram). Taken together, our findings suggest that the obesity paradox in HF may not be explained by pre-HF “biomarker-healthy” or “biomarker-unhealthy” obesity, using the biomarkers examined in this study. Previously, higher levels of tumor necrosis factor alpha, adiponectin and higher systolic pulmonary arterial pressure have been associated with lower BMI compared to those with higher BMI in HF patients (23). Future studies will need to explore whether these and other cardiac and non-cardiac factors associated with obesity such as presence of greater metabolic reserve, attenuated neurohormonal activation, or other biomarkers, such as higher levels of soluble tumor necrosis factor receptors available to bind harmful circulating tumor necrosis factor, or just a lead time bias with earlier presentation of clinical HF, contribute to the obesity paradox as noted in HF as well as in other chronic conditions (24-27).
Clinical Implications
Although differential levels of pre-HF hs-cTnT do not explain the obesity paradox, the higher incidence of HF and higher mortality after HF that is associated with higher levels of troponin measured several years before incident HF, suggests that this biomarker could be used to target individuals for aggressive preventive strategies to decrease the incidence of HF and mortality.
Study Strengths and Potential Limitations
The findings of this study should be interpreted in the context of several strengths and limitations. The study utilized a well-characterized cohort of 1279 individuals with incident HF hospitalizations from the ARIC study, which has rigorous data collection and surveillance process. Both hs-cTnT levels and BMI were measured remotely prior to the HF diagnosis and were therefore not affected by overt myocardial dysfunction or clinically manifest HF/volume overload, which could otherwise confound the levels of hs-cTnT and BMI, respectively. On the other hand, measurement of their values too remotely may not be an accurate proxy of recent myocardial injury. Although only hospitalized incident HF cases were included in the current analysis because of lack of consistent data on outpatient HF through the study period, the sensitivity and positive predictive value of ICD-9 code 428.x for HF classified by subsequent medical record review by the ARIC criteria were 0.95 and 0.77, respectively for combined acute decompensated HF and chronic HF (in comparison to 0.83 and 0.78, respectively, by Framingham criteria) (21). Furthermore, there is a possibility of differential misclassification because HF diagnosis in obese patients may be less specific than in normal-weight patients. We were also not able to account for the treatment after the onset of HF. In addition, HF types (preserved or reduced ejection fraction) were not assessed for the entire study period. Finally, despite rigorous adjustment, residual confounding could still be possible because of non-randomized nature of the study.
Conclusions
The presence of pre-existing sub-clinical myocardial injury (i.e., before incident HF) could not explain the lower mortality risk after incident HF observed in obese and overweight individuals compared with normal weight individuals. Future research is needed to explore other factors to explain the observed obesity paradox in HF even using the pre-HF obesity status.
Perspectives
Competency in medical knowledge
Although greater subclinical myocardial injury (assessed by high-sensitivity cardiac troponin-T) before heart failure increases risk of death after heart failure diagnosis, individuals who were obese and overweight prior to heart failure had lower risk of death compared with normal weight individuals after development of heart failure, irrespective of the presence or absence of pre-heart failure subclinical myocardial injury.
Translational Outlook
Future studies should evaluate other cardiac and non-cardiac factors including neurohormonal activation, metabolic reserve and other biomarkers to explain the reasons for the obesity paradox in heart failure.
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions. Reagents for high-sensitivity troponin T were donated by Roche Diagnostics®.
Dr. Pokharel is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837.
Abbreviations
- ARIC
Atherosclerosis Risk In Communities
- BMI
body mass index
- CHD
coronary heart disease
- hs-cTnT
cardiac troponin T measured using high-sensitivity assay
- CV
cardiovascular
- HF
heart failure
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
Footnotes
- Drs. Ballantyne, Hoogeveen and Nambi- Research Grant: Significant; Roche to help with measurement of assays. Other: Modest; Biomarkers to Improve Prediction of Heart Failure Risk: patent no. 61721475. Filed by Roche, Baylor College of Medicine along with 4 investigators including Drs. Ballantyne, Hoogeveen and Nambi.
- Dr. Ballantyne – consultant, Roche.
- Dr. Selvin- advisory board, Roche.
- Others: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. The New England journal of medicine. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Loehr LR, Rosamond WD, Poole C, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circulation Heart failure. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lissin LW, Gauri AJ, Froelicher VF, Ghayoumi A, Myers J, Giacommini J. The prognostic value of body mass index and standard exercise testing in male veterans with congestive heart failure. Journal of cardiac failure. 2002;8:206–15. doi: 10.1054/jcaf.2002.126812. [DOI] [PubMed] [Google Scholar]
- 5.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. Journal of the American College of Cardiology. 2001;38:789–95. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 6.Shah R, Gayat E, Januzzi JL, Jr, et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. Journal of the American College of Cardiology. 2014;63:778–85. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 7.Bozkurt B, Deswal A. Obesity as a prognostic factor in chronic symptomatic heart failure. American heart journal. 2005;150:1233–9. doi: 10.1016/j.ahj.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Khalid U, Ather S, Bavishi C, et al. Pre-Morbid Body Mass Index and Mortality After Incident Heart Failure: The ARIC Study. Journal of the American College of Cardiology. 2014;64:2743–9. doi: 10.1016/j.jacc.2014.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 10.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 11.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. Jama. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. Jama. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. The American journal of clinical nutrition. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 16.Meyer AM, Evenson KR, Couper DJ, Stevens J, Pereria MA, Heiss G. Television, physical activity, diet, and body weight status: the ARIC cohort. The international journal of behavioral nutrition and physical activity. 2008;5:68. doi: 10.1186/1479-5868-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke; a journal of cerebral circulation. 2002;33:2718–21. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- 19.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. Journal of clinical epidemiology. 1996;49:223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 20.Pokharel Y, Sun W, de Lemos JA, et al. High-sensitivity troponin T and cardiovascular events in systolic blood pressure categories: atherosclerosis risk in communities study. Hypertension. 2015;65:78–84. doi: 10.1161/HYPERTENSIONAHA.114.04206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circulation Heart failure. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndumele CE, Coresh J, Lazo M, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–7. doi: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takiguchi M, Yoshihisa A, Miura S, et al. Impact of body mass index on mortality in heart failure patients. European journal of clinical investigation. 2014;44:1197–205. doi: 10.1111/eci.12354. [DOI] [PubMed] [Google Scholar]
- 24.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–70. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. Jama. 2012;308:581–90. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clinic proceedings. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


