Abstract
Study Design
cross-sectional study
Objective
The aim of this study was to determine whether low back pain (subacute and chronic) is related to differences in brain volume.
Summary of Background Data
Inconsistent findings have been reported about the effect of chronic low back pain on brain volume, and the effect of subacute low back pain on brain volume has not been sufficiently investigated.
Methods
130 participants were included (23 subacute and 68 chronic low back pain; 39 healthy controls). The main outcome measure was whole and regional brain volume. Clinical outcome measures included pain duration, pain intensity, fear avoidance belief questionnaire, Oswestry disability index, and Beck’s depression inventory.
Results
Decrease in brain volume in several regions was observed in chronic low back pain when compared to health subjects; however after correcting for multiple comparisons, no significant differences were detected between any of the 3 groups in whole-brain volume. Regionally, we detected less gray matter volume in 2 voxels in the middle frontal gyrus in chronic low back pain participants compared to healthy controls. None of the clinical outcome measures were correlated with brain volume measurements.
Conclusion
Low back pain (subacute or chronic) is not related to significant differences in brain volume after correction for multiple comparisons. The effect size was too small to detect possible subtle changes unless much larger sample sizes are examined, or it is possible that low back pain does not affect brain volume.
Keywords: low back pain, chronic, subacute, neuroimaging, voxel-based morphometry, volumetric measurements, brain, structure
Introduction
Low back pain (LBP) is one of the most common pain conditions affecting millions of people worldwide(1, 2), and can be a major cause of disability(3, 4), depression (5–7), and loss of work(8, 9). Consequently, its economic impacts are tremendous with an annual cost in the US exceeding $100 billion(10). Furthermore almost 85% of patients have no specific patho-anatomical diagnosis but rather have idiopathic or “nonspecific” LBP(11). The mismatch between radiographic findings of spine images and clinical symptoms(12, 13) makes proper diagnosis and understanding of LBP difficult. Regardless of its underlying cause, “pain” as a nociceptive experience is processed in certain regions in the brain(14, 15). Brain imaging methods can be used to determine the relationship between pain and brain function and structure.
Pain is subjective and idiosyncratic. In general, the pain experience incorporates two main components: sensory-discriminative and affective-emotional components. These components are processed in different brain regions, yet are integrated and influenced by each other(16). Although recent evidence suggests that people with LBP have altered brain neurochemistry(17, 18) and function(19, 20), similar structural brain differences have not been established.
Smaller brain volumes have been reported in such neurodegenerative diseases as multiple sclerosis(21–23), Alzheimer’s disease(24–26), and schizophrenia(27–29), and also in chronic pain conditions like fibromyalgia(30–32), complex regional-pain syndrome (33, 34), and chronic LBP(35, 36). To date, only a few structural brain imaging studies in people with chronic LBP(35–42) have been completed. Findings from these studies were inconsistent, with some reporting smaller volumes in participants with chronic LBP compared to healthy controls, and others reporting no differences in brain volume. Importantly, the sample sizes in these studies were modest, and many that reported significance differences in brain volume did not correct for multiple comparisons(37, 40, 41), drawing into question the significance of the observation. Moreover, only one study has addressed subacute LBP in terms of brain structure and whether such potential differences exist during earlier stages of the disease(43) is unclear. The clinical significance of possible volumetric differences in LBP is also unclear.
The main aims of this study were to determine whether there are: 1) whole-brain volumetric differences in participants with subacute and chronic LBP compared to healthy controls; 2) regional brain differences in participants with subacute and chronic LBP compared to healthy controls; and 3) relationships between clinical outcome measures and brain volumes in participants with subacute and chronic LBP. We hypothesized that participants with chronic LBP would have smaller whole-brain volumes as compared to subacute and healthy controls, and participants with subacute LBP would have smaller whole-brain volumes compared to healthy controls. Secondly, we hypothesized that we would find smaller brain volumes within sensory and affective pain processing regions in participants with LBP. Finally, we hypothesized a negative correlation between normalized whole-brain volumes and clinical outcome measures such as pain intensity, pain duration, depression, or fear avoidance.
Materials and Methods
A total of 130 participants were included in this study: subacute (<6 months) LBP (n=23, 57% female), chronic (>6 months) LBP (n=68, 71% female), and healthy controls (n=39 participants, 44% female). Inclusion criteria for the LBP participants were: 1) male/female between 21 and 70 years, 2) having pain for less than 6 months (subacute group) and more than 6 moths (chronic group), and 3) being able to read and understand English. Exclusion criteria were: 1) spinal cord compression or spine surgery within the past year, 2) known injuries or arthritis to the hip, knee or ankle joints, 3) neurologic condition (including head trauma, stroke, or Alzheimer’s disease), 4) psychiatric or cardiovascular disease, tumor, or infection, 5) use of drugs or alcohol abuse, 6) pregnancy, and 7) MRI exclusion criteria (such as metallic object implants not compatible with MRI, epilepsy, or claustrophobia). The healthy controls self-reported no history of LBP within the last year. Participants were recruited through broadcast e-mails to university staff and employees, and word-of-mouth. The study was approved by the Human Subjects Committee at the University of Kansas Medical Center, and all participants provided informed consent prior to taking part in the study.
High-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) brain images were collected at 3-Tesla (matrix=256×256; 208 slices; voxels=1.0 mm × 1 mm × 0.97 mm; TE=3.05 ms; and TR=2300 ms on Allegra and Skyra scanners, Siemens Medical Solutions, Germany). Standard preprocessing was performed for all images using VBM8 toolbox(44) through Statistical Parametric Mapping software SPM8 (Welcome Department of Cognitive Neurology, London, UK) operating under MATLAB (Mathworks, Sherborn, MA, USA). Preprocessing included spatial normalization of all acquired images into the same stereotactic space, to account for head size differences between participants. DARTEL segmentation into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF), and Gaussian spatial smoothing (8 mm full-width at half-maximum) as determined by previous studies was performed. Image quality and sample homogeneity were verified through visual inspection using the voxel-based morphometry (VBM8) tools(44). We used volumetric outputs from VBM8 stream to calculate individual normalized whole-brain volume, which is the sum of GM volume and WM volume divided by total intracranial volume. Further, we used VBM analysis to generate smoothed, modulated, warped statistical brain maps of the probability of difference in brain volume between groups of participants(44).
For region-of-interest (ROI) analysis we used the Wake-Forest PickAtlas(45, 46) to create masks of pain-related brain regions(16). Four ROI masks were created; a sensory mask, which included the primary somatosensory cortex and the posterior insula; a cortical affective mask which included the cingulate, orbitofrontal, and medial prefrontal cortices and the anterior insula; a subcortical affective mask which included nucleus accumbens, amygdala, caudate, and hippocampus; and a mask of the thalamus.
The clinical outcome measures were collected only from participants with LBP (subacute and chronic) and included pain duration, pain intensity, fear avoidance, disability, and depression. Average pain intensity for previous week was measured with the Numeric Rating Pain Scale (NRS)(47). The NRS is a 0–10 scale with 0=no pain and 10=worst pain imaginable. Fear of movement was measured by the Fear Avoidance Belief Questionnaire (FABQ), which quantifies the subjective impact of work and physical activity on pain(48). Disability was measured by the Oswestry Disability Index (ODI(49)), which quantifies individual disability due to LBP. ODI scores greater than 60% indicate severe disability(50–52). Finally, depression symptoms were measured using the Beck Depression Inventory (BDI–II), which has been validated in multiple studies(53).
To investigate difference in age between the groups, we conducted an analysis-of-variance (ANOVA) test, followed by Tukey’s post-hoc testing using SPSS 22.0 software (IBM Corp. Released 2013. IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY: IBM Corp). Then, we conducted Chi-square testing to investigate differences in sex and scanner between groups. Age, sex, and scanner were then used as covariates in each of the brain volume analyses listed below.
Normalized Whole-Brain Volumes
To determine whether there were overall brain volume differences between the three groups, we conducted a univariate one-way ANOVA test using SPSS for the normalized whole-brain volumes as the dependent variable, and group (subacute, chronic, healthy) as the independent variable.
Voxel-Based Analysis (whole-brain and ROI)
We examined GM volume differences between the groups using SPM8. We conducted two-sample t-tests between each pair of groups (healthy-subacute, healthy-chronic, subacute-chronic) over the whole brain and then within the four regional masks, correcting for multiple comparisons in each test.
Correlation Analysis
We conducted partial correlations between the normalized whole-brain volumes and each clinical outcome measure in the subacute and chronic LBP groups separately using SPSS software, while controlling for age, sex, and scanner in each test.
Results
The ANOVA test revealed a significant age difference between the groups (F(2,127)=3.99, p=0.021, η2=0.06), with the chronic group being significantly older than the subacute group (p=0.025, Mdifference =−8.39, std. error=3.18) and no difference between the healthy and subacute (p=0.527, Mdifference=3.74, std. error=3.46) or healthy and chronic groups (p=0.189, Mdifference =−4.64, std. error=2.65). The Chi-square test showed that the ratio of males/females was different across the groups ((2)=7.67, p=0.022) with a greater proportion of females in the chronic LBP group. The ratio of participants scanned on the two scanners was not significantly different across groups ((2)=5.40, p=0.067) with more participants scanned on the Allegra scanner in all 3 groups (healthy 84.6%, subacute 60.8%, and chronic 66.2%). Therefore, throughout this study we included age, sex, and scanner as covariates in our analyses.
Demographic and clinical data are presented in Table 1. There was no statistical difference between both LBP groups in any of the outcome measures except for pain duration and disability scores. Participants in the chronic LBP group had experienced pain longer than the subacute LBP group (t(86)=−5.63, p<0.001) and showed greater levels of disability than subacute LBP group (t(87)=−2.47, p=0.016).
Table 1.
Demographic and clinical outcome measures:
| Characteristic | sLBP | cLBP | HC | Statistic | p |
|---|---|---|---|---|---|
| Sex (Female/Male)† |
13/10 | 48/20 | 17/22 | χ2 =7.67 | 0.022* |
| Age‡ | 36±11 | 45±12 | 40±16 | F=3.99 | 0.021* |
| Pain Duration§ | 3.16±2.17 | 98.58±81.18 | - | t=−5.63 | <0.001** |
| Pain Intensity§ | 4.18±2.19 | 4.28±1.89 | - | t=−0.21 | 0.834 |
| FABQ-w§ | 13.63±13.28 | 12.55±12.01 | - | t=0.36 | 0.723 |
| FABQ-p§ | 11.77±6.22 | 13.81±5.08 | - | t=−1.54 | 0.127 |
| ODI§ | 19±14.97% | 29.88±18.79% | - | t=−2.47 | 0.016* |
| BDI§ | 8.45±7.88 | 10.36±9.91 | - | t=−0.82 | 0.414 |
Age is measured in years, pain duration is measured in months, pain intensity is measured using a 0–10 pain scale, FABQ-w: Fear-avoidance belief questionnaire – work component, FABQ-p: Fear-avoidance belief questionnaire – physical component, ODI: Oswestery disability index, BDI: Beck depression inventory.
Chi-square
One-way ANOVA
Independent 2-sample t-test
Normalized Whole-Brain Volumes
There was no overall difference in normalized whole-brain volume between groups after controlling for age, sex, and scanner (F(2,124)=1.63, p=0.20, η2=0.03). Figure 1 presents the mean and standard deviation of the normalized whole-brain volumes for each group. Additionally we determined the effect size using G-Power software(54, 55). Through calculating the means and standard deviations of the normalized whole-brain volumes for each of our groups we detected an effect size of 0.07, which is considered a small effect size. The sample size required to detect such small effect size (0.07) at a power of 80% would require 1717 participants.
Figure 1. normalized whole-brain volumes for each group.
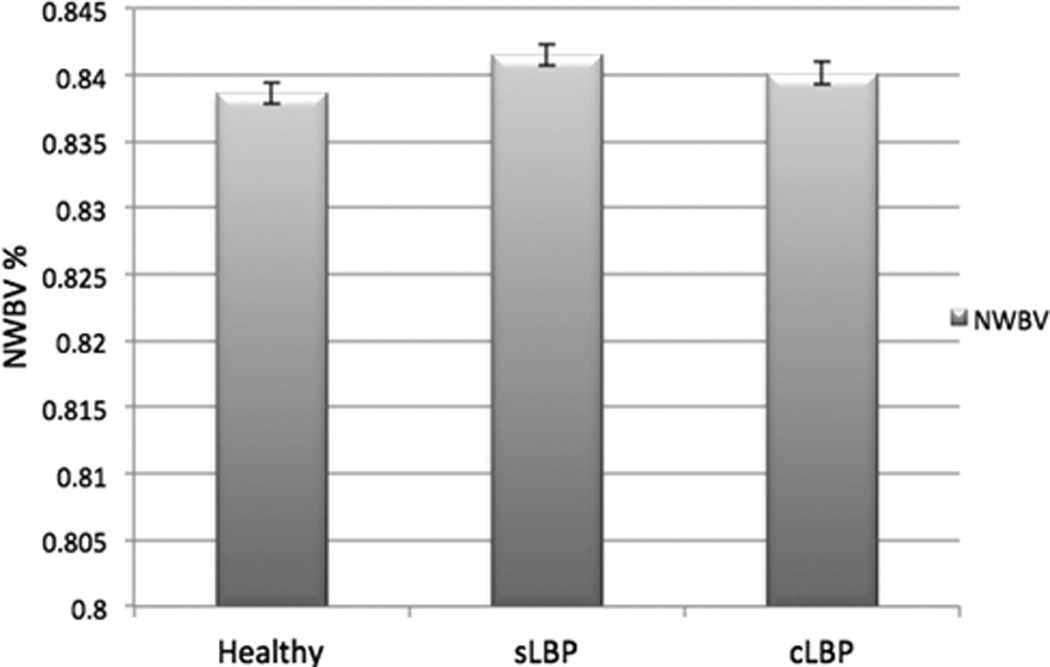
HC: Healthy controls, sLBP: subacute low back pain group, cLBP: chronic low back pain group, NWBV: normalized whole-brain volume
Voxel-Based Analysis (whole-brain and ROI)
Following corrections for multiple comparisons (family-wise error corrected p<0.05), we found no differences between any inter-group comparisons on the whole-brain level. All comparisons tested both contrasts of each set (for example, subacute>healthy and healthy>subacute). However, to verify whether previously reported trends were also observed in this large sample, we repeated the comparisons using uncorrected p<0.001 and a threshold of 100 contiguous voxels. At this less stringent threshold we observed evidence of volume differences in regions of middle frontal gyrus, superior frontal gyrus, parahippocampal gyrus, and cerebellum (see Supplementary Table 1), presenting findings similar to previous studies.
The ROI analysis of the cortical affective mask indicated that the chronic LBP group have less GM volume in 2 voxels (6.75 mm3) within the middle frontal gyrus (MNI-coordinates: −34/51/15) compared to healthy controls (corrected p<0.05; Figure 2). No other ROI comparisons showed any differences in GM volume.
Figure 2. Cortical affective mask and regions of gray matter volume loss within that mask in chronic LBP participants.
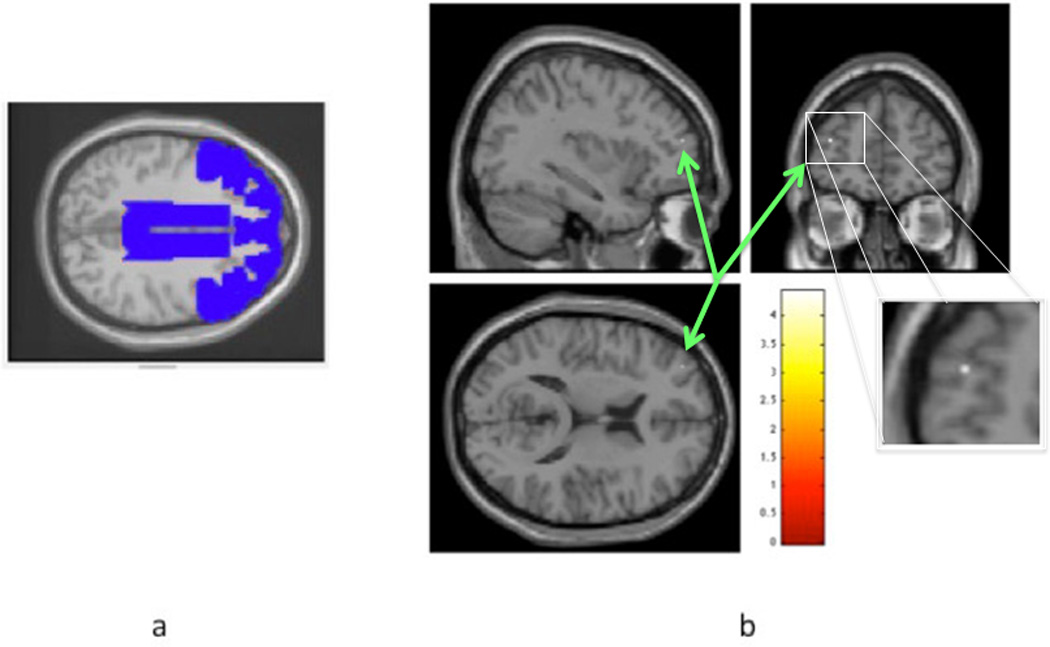
a represents cortical affective mask with cingulate, orbitofrontal, and medial prefrontal cortices in blue drawing; Fig 2b is a statistical parametric map representing scale of t-scores for the contrast healthy >cLBP; arrows indicate voxels with less gray matter volume in the cLBP group compared to healthy controls; pcorrected< 0.05
Correlation Analysis
The clinical outcome measures were not correlated with the normalized whole-brain volumes in either subacute or chronic LBP groups after controlling for age, sex, and scanner (all r<0.18 Table 2).
Table 2.
Correlation of clinical outcome measures and normalized whole-brain volume:
| Characteristic | Statistic | NWBV | p |
|---|---|---|---|
| Pain Duration | Partial correlation | 0.179 | 0.109 |
| Pain Intensity | Partial correlation | 0.098 | 0.382 |
| FABQ-w | Partial correlation | 0.068 | 0.546 |
| FABQ-p | Partial correlation | 0.167 | 0.136 |
| ODI | Partial correlation | 0.091 | 0.418 |
| BDI | Partial correlation | −0.059 | 0.600 |
FABQ-w: Fear-avoidance belief questionnaire – work component, FABQ-p: Fear-avoidance belief questionnaire – physical component, ODI: Oswestery disability index, BDI: Beck depression inventory, NWBV: normalized whole-bran volume.
All correlations are partial correlations after controlling for age, sex, and scanner. The number of participants is 84 for all the outcome measures including participants from both the subacute and chronic LBP groups.
Discussion
Our results are consistent with previous reports that found no difference in whole-brain volumes in chronic LBP(37, 38, 41, 42), and suggest that chronic LBP is not associated with robust differences in brain structure and volume. Consistent with this theoretical argument, we also found no difference in brain volume in participants in the earlier (subacute) stages of the disease. Additionally, when examining sensory and affective pain-related ROIs we found evidence of lower middle frontal gyral (cortical affective mask) volume in 2 voxels in participants with chronic LBP compared to healthy controls. These results suggest that any structural brain differences associated with persistent LBP must be subtle and would require a large sample size (about 1700 subjects) to detect. This finding is consistent with Dolman et al. who reported needing 1616 participants(38).
We did not find any correlation between clinical measures and normalized whole-brain volume. Although the broader pain literature generally suggests a correlation between clinical outcome measures and brain volume(31, 56, 57), studies specifically examining LBP reported no correlations between such outcomes and brain volume even in the presence of brain volume differences(36, 40). Such findings question the clinical relevance of the differences in brain volume reported in previous studies.
Several considerations can explain our findings of no difference in brain volume. We employed rigorous methods to avoid type 1 errors to correct for multiple comparisons as recommended by the creators of VBM(44). Previous studies either did not correct for multiple comparisons(37, 40, 41) or used a different analysis method (such as permutation testing(35)). Another difference is related to the methodology and subject recruitment. We used two-sample t-tests, unlike some of the methodology used by other researchers.
The study by Dolman et al. concluded that controlling for the main covariates (such as age and pain levels) could reduce - or even potentially eliminate - the previously reported findings of differences in brain volume(38). It is well known that aging is associated with decreases in brain volume(58). This loss is not homogeneously distributed across the brain, with some regions demonstrating more decline in GM volume with aging than others, including pain regions(59). This might explain our failure to detect volume differences after controlling for age effects. Our finding of decrease in GM volume in 2 voxels in the middle frontal gyrus of chronic LBP group represents an average of <0.001% annual loss, which is clinically nonsignificant, as 0.05% annual GM loss is associated with normal aging. Finally, our cohort represents subjects with minimum to no fear of movement and depression. Subjects with greater fear avoidance behavior, depression, or disability may experience brain volume loss.
Several theoretical models have been proposed as mechanisms for brain volume changes in chronic LBP, however these models account for both theoretical decreases and increases in brain volume, making interpretation of brain volumes from MR images difficult. Increased levels of glutamate have been reported in chronic pain condtions(60–64). Prolonged exposure to high levels of glutamate is neurotoxic, and this neurotoxicity could result in loss of neurons via neurodegeneration or neuronal apoptosis(65). Conversely, some have argued that increased glutamate might lead to tissue scarring and therefore increasing cortical thickness(38). In addition to neurochemical hypotheses, some researchers credit volumetric differences to changes in lifestyle, since chronic pain leads to decreased mobility and activity(66). Exercise has been shown to assist in increasing brain volume(67, 68), suggesting that less mobility might be related to decreased brain volume. More research is needed to confirm or refute these theories.
Although we used a large sample size and stringent data analysis methods available, we acknowledge some limitations. First, there was a significant difference in age between groups. This was anticipated since our LBP groups are defined by duration of their pain, and hence we expected the chronic group to have older participants than those in the subacute group. Second, there was a significant difference in sex proportion within our sample. This was also anticipated since chronic pain is more prevalent in females(69). Finally, although we collected data on two scanners, all acquisition parameters were identical. Since we are comparing calculated volumes that are based on careful scanner calibrations completed during routine quality assurance procedures, this is unlikely to contribute to false findings. Nonetheless, we added each of these factors as covariates in our analyses. Finally, we had no clinical data on our healthy controls (such as depression or disability scores).
Our results suggest that brain volume is not severely affected by LBP, with other factors (such as age) having a larger impact on brain volume. Nonetheless, the brain cyto-architecture might be affected by pain. Such differences require other methods of detection such as spectroscopy or fMRI (17, 19, 20, 70–72) (18, 73)) to detect changes in brain neurochemistry and function in people with chronic LBP.
Supplementary Material
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s).
This work was supported in part by the American Physical Therapy Association Foundation and the Orthopedic Section Pilot Grant, a KL2 award from Frontiers: Heartland Institute for Clinical & Translational Research (CTSA; TR000119), and KUMC Clinical Pilot and School of Health Profession Grants to author Neena Sharma. The Hoglund Brain Imaging Center is supported by a generous donation gift from Forrest and Sally Hoglund and funding from the National Institutes of Health (P30 HD002528, P30 AG 035982, S10 RR 29577, and UL1 TR000001). K01 Award: author Robyn Honea is supported by NIH grant K01AG035043.
Relevant financial activities outside the submitted work:grants.
References
- 1.Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neuroscience letters. 1997;224(1):5–8. doi: 10.1016/s0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- 2.Von Korff M, Dunn KM. Chronic pain reconsidered. Pain. 2008;138(2):267–276. doi: 10.1016/j.pain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma S, Pal BP. Correlation Between Pain, Fear of Falling and Disability in Low Back Pain. Annals of rehabilitation medicine. 2015;39(5):816–820. doi: 10.5535/arm.2015.39.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker BF, Muller R, Grant WD. Low back pain in Australian adults: prevalence and associated disability. Journal of manipulative and physiological therapeutics. 2004;27(4):238–244. doi: 10.1016/j.jmpt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27(5):E109–E120. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Ramond A, Bouton C, Richard I, Roquelaure Y, Baufreton C, Legrand E, et al. Psychosocial risk factors for chronic low back pain in primary care--a systematic review. Family practice. 2011;28(1):12–21. doi: 10.1093/fampra/cmq072. [DOI] [PubMed] [Google Scholar]
- 7.Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain: establishing priorities in treatment. Spine. 2000;25(20):2566–2571. doi: 10.1097/00007632-200010150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen TH, Randolph DC. Nonspecific low back pain and return to work. American family physician. 2007;76(10):1497–1502. [PubMed] [Google Scholar]
- 9.Rizzo JA, Abbott TA, 3rd, Berger ML. The labor productivity effects of chronic backache in the United States. Medical care. 1998;36(10):1471–1488. doi: 10.1097/00005650-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. The Journal of bone and joint surgery American volume. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Weinstein JN. Low back pain. The New England journal of medicine. 2001;344(5):363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 12.Berg L, Hellum C, Gjertsen O, Neckelmann G, Johnsen LG, Storheim K, et al. Do more MRI findings imply worse disability or more intense low back pain? A cross-sectional study of candidates for lumbar disc prosthesis. Skeletal radiology. 2013;42(11):1593–1602. doi: 10.1007/s00256-013-1700-x. [DOI] [PubMed] [Google Scholar]
- 13.Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis and rheumatism. 2013;65(12):3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borsook D, Sava S, Becerra L. The pain imaging revolution: advancing pain into the 21st century. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2010;16(2):171–185. doi: 10.1177/1073858409349902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89(1):7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 16.Sharma NK, Brooks WM, Popescu AE, Vandillen L, George SZ, McCarson KE, et al. Neurochemical analysis of primary motor cortex in chronic low back pain. Brain sciences. 2012;2(3):319–331. doi: 10.3390/brainsci2030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis and rheumatism. 2004;50(2):613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 18.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neuroscience letters. 2010;485(1):26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheriyan J, Kim S, Wolansky LJ, Cook SD, Cadavid D. Impact of inflammation on brain volume in multiple sclerosis. Archives of neurology. 2012;69(1):82–88. doi: 10.1001/archneurol.2011.674. [DOI] [PubMed] [Google Scholar]
- 20.Koenig KA, Sakaie KE, Lowe MJ, Lin J, Stone L, Bermel RA, et al. Hippocampal volume is related to cognitive decline and fornicial diffusion measures in multiple sclerosis. Magnetic resonance imaging. 2014;32(4):354–358. doi: 10.1016/j.mri.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radue EW, Barkhof F, Kappos L, Sprenger T, Haring DA, de Vera A, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology. 2015;84(8):784–793. doi: 10.1212/WNL.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon BA, Blazey T, Benzinger TL, Head D. Effects of aging and Alzheimer's disease along the longitudinal axis of the hippocampus. Journal of Alzheimer's disease : JAD. 2013;37(1):41–50. doi: 10.3233/JAD-130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim I, Horacek J, Bartos A, Hajek M, Ripova D, Brunovsky M, et al. Combination of voxel based morphometry and diffusion tensor imaging in patients with Alzheimer's disease. Neuro endocrinology letters. 2009;30(1):39–45. [PubMed] [Google Scholar]
- 24.Karas GB, Scheltens P, Rombouts SA, Visser PJ, van Schijndel RA, Fox NC, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. NeuroImage. 2004;23(2):708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Archives of general psychiatry. 2002;59(11):1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 26.Kasparek T, Prikryl R, Schwarz D, Kucerova H, Marecek R, Mikl M, et al. Gray matter morphology and the level of functioning in one-year follow-up of first-episode schizophrenia patients. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33(8):1438–1446. doi: 10.1016/j.pnpbp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia research. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Piedra C, Guzman MA, Buela-Casal G, Catena A. The impact of fibromyalgia symptoms on brain morphometry. Brain imaging and behavior. 2015 doi: 10.1007/s11682-015-9485-2. [DOI] [PubMed] [Google Scholar]
- 29.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCrae CS, O'Shea AM, Boissoneault J, Vatthauer KE, Robinson ME, Staud R, et al. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. Journal of pain research. 2015;8:47–52. doi: 10.2147/JPR.S71959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barad MJ, Ueno T, Younger J, Chatterjee N, Mackey S. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. The journal of pain : official journal of the American Pain Society. 2014;15(2):197–203. doi: 10.1016/j.jpain.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PloS one. 2011;6(10):e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckalew N, Haut MW, Morrow L, Weiner D. Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain medicine (Malden, Mass) 2008;9(2):240–248. doi: 10.1111/j.1526-4637.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 36.Dolman AJ, Loggia ML, Edwards RR, Gollub RL, Kong J, Napadow V, et al. Phenotype matters: the absence of a positive association between cortical thinning and chronic low back pain when controlling for salient clinical variables. The Clinical journal of pain. 2014;30(10):839–845. doi: 10.1097/AJP.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritz HC, McAuley JH, Wittfeld K, Hegenscheid K, Schmidt CO, Langner S, et al. Chronic Back Pain Is Associated With Decreased Prefrontal and Anterior Insular Gray Matter: Results From a Population-Based Cohort Study. The journal of pain : official journal of the American Pain Society. 2016;17(1):111–118. doi: 10.1016/j.jpain.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Ivo R, Nicklas A, Dargel J, Sobottke R, Delank KS, Eysel P, et al. Brain structural and psychometric alterations in chronic low back pain. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2013;22(9):1958–1964. doi: 10.1007/s00586-013-2692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125(1–2):89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cerebral cortex (New York, NY : 1991) 2014;24(4):1037–1044. doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nature neuroscience. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 43.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 44.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Chapman JR, Norvell DC, Hermsmeyer JT, Bransford RJ, DeVine J, McGirt MJ, et al. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine. 2011;36(21 Suppl):S54–S68. doi: 10.1097/BRS.0b013e31822ef74d. [DOI] [PubMed] [Google Scholar]
- 46.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 47.Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 48.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. discussion 52. [DOI] [PubMed] [Google Scholar]
- 49.Joshi VD, Raiturker PP, Kulkarni AA. Validity and reliability of English and Marathi Oswestry Disability Index (version 2.1a) in Indian population. Spine. 2013;38(11):E662–E668. doi: 10.1097/BRS.0b013e31828a34c3. [DOI] [PubMed] [Google Scholar]
- 50.Mohan V, Prashanth GS, Meravanigi G, Rajagopalan N, Yerramshetty J. Adaptation of the Oswestry Disability Index to Kannada Language and Evaluation of Its Validity and Reliability. Spine. 2015 doi: 10.1097/BRS.0000000000001368. [DOI] [PubMed] [Google Scholar]
- 51.Wang YP, Gorenstein C. Assessment of depression in medical patients: a systematic review of the utility of the Beck Depression Inventory-II. Clinics (Sao Paulo, Brazil) 2013;68(9):1274–1287. doi: 10.6061/clinics/2013(09)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 53.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 54.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138(5):1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia : an international journal of headache. 2008;28(6):598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 56.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 57.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fayed N, Andres E, Viguera L, Modrego PJ, Garcia-Campayo J. Higher glutamate+glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Academic radiology. 2014;21(9):1211–1217. doi: 10.1016/j.acra.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Gerstner GE, Gracely RH, Deebajah A, Ichesco E, Quintero A, Clauw DJ, et al. Posterior insular molecular changes in myofascial pain. Journal of dental research. 2012;91(5):485–490. doi: 10.1177/0022034512443366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis and rheumatism. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mullins PG, Rowland LM, Jung RE, Sibbitt WL., Jr A novel technique to study the brain's response to pain: proton magnetic resonance spectroscopy. NeuroImage. 2005;26(2):642–646. doi: 10.1016/j.neuroimage.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Valdes M, Collado A, Bargallo N, Vazquez M, Rami L, Gomez E, et al. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis and rheumatism. 2010;62(6):1829–1836. doi: 10.1002/art.27430. [DOI] [PubMed] [Google Scholar]
- 63.Rothstein JD. Excitotoxicity hypothesis. Neurology. 1996;47(4 Suppl 2):S19–S25. doi: 10.1212/wnl.47.4_suppl_2.19s. discussion S6. [DOI] [PubMed] [Google Scholar]
- 64.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gondoh Y, Sensui H, Kinomura S, Fukuda H, Fujimoto T, Masud M, et al. Effects of aerobic exercise training on brain structure and psychological well-being in young adults. The Journal of sports medicine and physical fitness. 2009;49(2):129–135. [PubMed] [Google Scholar]
- 68.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature reviews Neuroscience. 2012;13(12):859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 69.Boendermaker B, Meier ML, Luechinger R, Humphreys BK, Hotz-Boendermaker S. The cortical and cerebellar representation of the lumbar spine. Human brain mapping. 2014;35(8):3962–3971. doi: 10.1002/hbm.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gussew A, Rzanny R, Gullmar D, Scholle HC, Reichenbach JR. 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. NeuroImage. 2011;54(2):1315–1323. doi: 10.1016/j.neuroimage.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi Y, Kurata J, Sekiguchi M, Kokubun M, Akaishizawa T, Chiba Y, et al. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: an FMRI study. Spine. 2009;34(22):2431–2436. doi: 10.1097/BRS.0b013e3181b1fb76. [DOI] [PubMed] [Google Scholar]
- 72.Sharma NK, McCarson K, Van Dillen L, Lentz A, Khan T, Cirstea CM. Primary somatosensory cortex in chronic low back pain - a H-MRS study. Journal of pain research. 2011;4:143–150. doi: 10.2147/JPR.S19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


