Summary
Background
Allogeneic mesenchymal stem cells (MSCs) are a promising cell source for treating musculoskeletal injuries in horses. Controversy exists, however, over whether major histocompatibility complex (MHC)‐mismatched MSCs are recognised by the recipient immune system and targeted for death by a cytotoxic antibody response.
Objectives
To determine if cytotoxic anti‐MHC antibodies generated in vivo following MHC‐mismatched MSC injections are capable of initiating complement‐dependent cytotoxicity of MSCs.
Study design
Experimental controlled study.
Methods
Antisera previously collected at Days 0, 7, 14 and 21 post‐injection from 4 horses injected with donor MHC‐mismatched equine leucocyte antigen (ELA)‐A2 haplotype MSCs and one control horse injected with donor MHC‐matched ELA‐A2 MSCs were utilised in this study. Antisera were incubated with ELA‐A2 MSCs before adding complement in microcytotoxicity assays and cell death was analysed via eosin dye exclusion. ELA‐A2 peripheral blood leucocytes (PBLs) were used in the assays as a positive control.
Results
Antisera from all 4 horses injected with MHC‐mismatched MSCs contained antibodies that caused the death of ELA‐A2 haplotype MSCs in the microcytotoxicity assays. In 2 of the 4 horses, antibodies were present as early as Day 7 post‐injection. MSC death was consistently equivalent to that of ELA‐A2 haplotype PBL death at all time points and antisera dilutions. Antisera from the control horse that was injected with MHC‐matched MSCs did not contain cytotoxic ELA‐A2 antibodies at any of the time points examined.
Main limitations
This study examined MSC death in vitro only and utilized antisera from a small number of horses.
Conclusions
The cytotoxic antibody response induced in recipient horses following injection with donor MHC‐mismatched MSCs is capable of killing donor MSCs in vitro. These results suggest that the use of allogeneic MHC‐mismatched MSCs must be cautioned against, not only for potential adverse events, but also for reduced therapeutic efficacy due to targeted MSC death.
Keywords: horse, mesenchymal stem cell, allogeneic, major histocompatibility complex‐mismatched, antibody response, complement‐dependent cytotoxicity
Introduction
Initial studies evaluating the efficacy of equine mesenchymal stem cells (MSCs) from mature horses for the treatment of musculoskeletal injuries used autologous cells 1, 2, 3, 4, 5, 6, 7, but more recently, numerous studies have been performed to evaluate the safety of allogeneic, mature equine MSCs in the hope of replacing autologous cells with allogeneic cells 8, 9, 10, 11, 12, 13, 14, 15, 16, 17. Allogeneic MSCs would allow for the immediate treatment of acute injuries with cells of known quality and sufficient quantity and eliminate the need for either bone marrow aspirate or adipose biopsy from the patient. Controversy exists, however, over the immunogenicity of these MSCs in vivo, probably due to variability in the way the term allogeneic is defined and how the immune response is examined.
While all previous studies used the term allogeneic to describe MSCs from a different animal of the same species, most do not clarify whether or not the MSCs were major histocompatibility complex (MHC)‐matched or mismatched 10, 11, 12, 13, 14, 15, 16. This information is critical as previous studies have shown that MHC‐mismatched MSCs induce both recipient humoral and memory T cell responses in vivo 8, 9, 18, 19. Currently, only one equine study has evaluated MSC recipients for an antibody response 9: injection of recipient horses with MHC‐mismatched donor MSCs resulted in a detectable antibody against the donor MHC haplotype in all 6 recipients as determined by complement‐dependent cytotoxicity of donor peripheral blood leucocytes (PBLs). Although none of the recipients in that study had a detectable systemic inflammatory response, the antibody response findings led to major concerns over the safety of MSC injections following recipient immune priming and the viability and efficacy of donor MSCs once transplanted.
There is evidence from other species that donor allogeneic MHC‐mismatched MSCs are targeted for destruction by the recipient immune system and do not persist as long as allogeneic MHC‐matched or autologous MSCs 20, 21. Even though most adult MSCs are used for their paracrine signalling effects rather than for expected differentiation or engraftment into host tissue, they still need to persist throughout the inflammatory phase and into the remodelling phase for maximal therapeutic benefit. The purpose of this study was to determine if recipient antisera containing antibodies against the MHC haplotype of the donor would cause the death of donor MSCs and at what time points following MSC injection. Our hypothesis was that MSCs would be targeted for death by cytotoxic antibodies with similar cytotoxicity as PBLs.
Materials and methods
A schematic of the study design and methods is shown in Figure 1.
Figure 1.
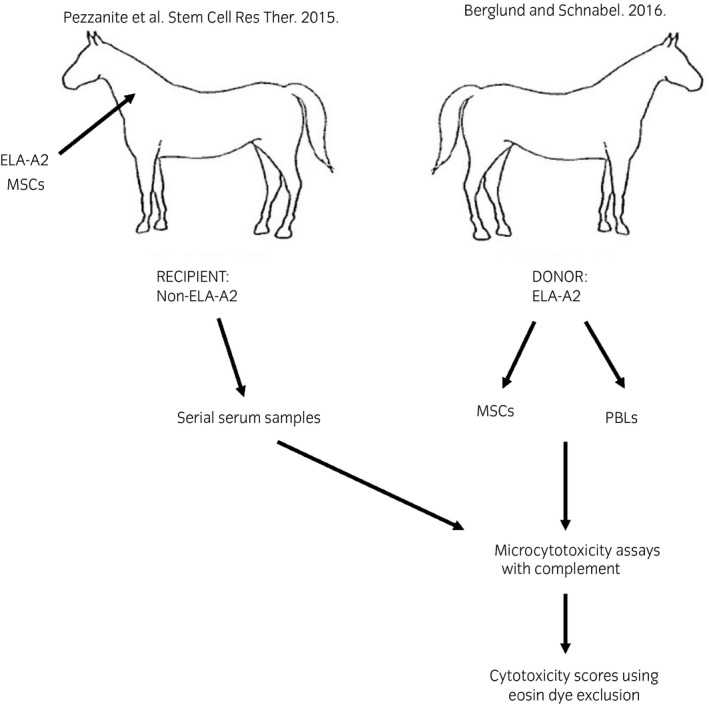
Schematic of study design. Antisera obtained in the study by Pezzanite et al.9, was incubated with mesenchymal stem cells (MSCs) and peripheral blood leucocyte (PBL) target cells collected from equine leucocyte antigen (ELA)‐A2 donor horses and complement in microcytotoxicity assays. Eosin dye exclusion was used to estimate percent cytotoxicity of target cells.
Antisera against the equine leucocyte antigen (ELA)‐A2 haplotype
This study used serum samples obtained from a previous experiment conducted by the corresponding author and colleagues at Cornell University that is described above in the introduction 9. Briefly, bone marrow‐derived MSCs from female donor horses of the ELA‐A2 haplotype were injected intradermally in the neck of MHC‐mismatched recipient horses identified as non‐ELA‐A2 haplotype by microsatellite typing. In addition, one ELA‐A2 haplotype homozygote horse was the recipient of female donor ELA‐A2 MSCs as a MHC‐matched (negative) control. Blood samples from experimental and control horses were collected preinjection, at time of injection, and every 48 h for 4 weeks following injection. Each sample was processed to collect the serum, which was then aliquoted and frozen at −20°C for later use. First, aliquots were used in microcytotoxicity assays to determine cytotoxic antibody titres for each recipient horse against donor ELA‐A2 PBLs as previously described 9. Next, antisera from the 4 recipients that were found in those assays to have strong antibody responses as determined by donor ELA‐A2 PBL death were selected to be used in this study to determine if those same antibody responses would cause the death of ELA‐A2 MSCs in vitro. Sera from the control ELA‐A2 recipient that was confirmed to have no anti‐ELA antibody response in the previous study was used again as a negative control in this study. In addition, antisera from the strongest responder in the previous study that was found to be cross‐reactive against the ELA‐A3 haplotype was used against ELA‐A3 MSCs.
Mesenchymal stem cell culture
Frozen passage 4 bone marrow‐derived MSCs from 2 female horses of the ELA‐A2 haplotype and one male horse of the ELA‐A3 haplotype were thawed and expanded in culture over 5 days. All MSCs had been previously validated by a panel of positive (MHC I, CD44, CD29, CD90) and negative (CD11a/CD18, CD45RB) markers 8. MSCs were plated at a density of 1 × 104 cells/cm2 and cultured for 24 h in standard media containing low glucose (10 g/l) DMEMa, 10% fetal bovine serumb, 2 mol/l L‐glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml), and 1 ng/ml basic fibroblast growth factorc. Media was then changed so that the serum component consisted of 5% fetal bovine serum and 5% equine serum of the same ELA haplotype as the MSCs for the next 48 h followed by another media change so that the serum component consisted solely of 10% equine serum for the 48 h just prior to the microcytotoxicity assay. MSCs were lifted from tissue culture plates using Accumaxd cell‐dissociation solution, washed three times with phosphate‐buffered saline (PBS), and counted using a Cellometer Auto 2000 cell counter and ViaStain AOPI Staining Solutione. MSCs were diluted to 1 × 109 live cells/l in PBS and used immediately in the microcytotoxicity assays.
Peripheral blood leucocyte isolation
Blood was collected via jugular venipuncture from one ELA‐A2 haplotype horse and one ELA‐A3 haplotype horse into sterile blood collection tubes containing 158 units of lithium heparinf. Plasma was allowed to separate in each tube for 20 min at room temperature. PBLs were then isolated from the plasma via carbonyl irong granulocyte depletion and Ficoll–Paque Plush gradient centrifugation. Isolated PBLs were counted using the Cellometer cell counter and ViaStain AOPI staining solution and diluted in PBS to 3 × 109 live cells/l to be used immediately in the microcytoxicity assays.
Microcytotoxicity assays
The standard two‐stage microcytotoxicity dye exclusion test was used to detect cytotoxic antibodies as previously described 22, 23, but with MSCs as target cells in addition to traditional PBLs. Briefly, PBLs and MSCs were tested against diluted antisera (neat, 1:2, and 1:16) from the four known MHC‐mismatched recipients with the strongest immune responses from the previous study as described above and the one MHC‐matched (control) recipient. One microliter of diluted antisera and 1 μl of PBL or MSC suspension was incubated for 30 min at room temperature under oil in wells of Terasaki platesi. Five microliters of rabbit complementj was then added and plates incubated for an additional hour at room temperature. The wells were then stained with 2 μl of 5% eosin dye and fixed with 5 μl of 10% buffered formalin (pH between 7.2 and 7.4). All experiments were run in duplicate and target cell death assessed by the two authors. Results are expressed as the average cytotoxicity score by microscopic evaluation of the percentage of dead cells using a modified National Institute of Heath cytotoxicity scoring system as shown: score 1: <10%, score 2: 10–19%, 4: 20–49%, 6: 50–80%, 8: 81–100%. Scores of 6 or greater are considered positive for alloantibodies in sera. Images of wells were taken at 10× using an IX83 inverted microscope and cellSens software.k
Data analysis
Microcytotoxicity scores for MHC‐mismatched PBLs and MSCs at each dilution of antisera were compared over the antisera collection times of 0, 7, 14 and 21 days using 2‐way repeated measures ANOVAs and Holm–Sidak all pairwise multiple comparison procedures. All analyses were performed using SigmaPlot Version 13l and significance set at P<0.05.
For each dilution and collection time, the cytotoxicity scores of the two lines of ELA‐A2 MSC target cells were compared using multiple t tests and the Holm–Sidak method. All analyses were performed using Prism Version 7 and significance set at P≤0.05.
Results
Cell viability prior to microcytotoxicity assay
Peripheral blood leucocyte viability was >95% after carbonyl iron granulocyte depletion and Ficoll–Paque Plus gradient centrifugation isolation. MSC viability following culture expansion and enzymatic dissociation from tissue culture plates was >92%.
Microcytoxicity assays
The antisera from the 4 horses that received a single injection of MHC‐mismatched MSCs and the one control horse that received MHC‐matched MSCs were tested in microcytotoxicity assays against PBLs from one ELA‐A2 haplotype horse and MSC target cells from 2 ELA‐A2 haplotype horses. Eosin dye exclusion was used to estimate the cytotoxicity score of the antisera following incubation of the antisera with target cells and rabbit complement. Target cells that appeared round and refractile with a clear centre were estimated to be alive, while flat, uniformly dark cells were counted as dead (Fig 2).
Figure 2.
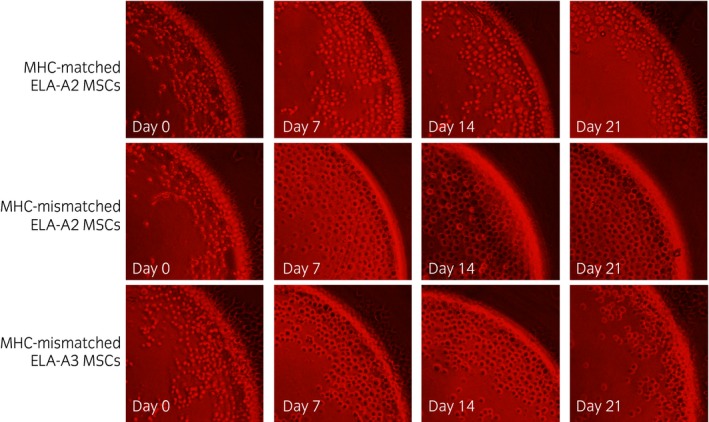
10× images from Terasaki plate wells used for microcytotoxicity assays containing equine leucocyte antigen (ELA)‐A2 mesenchymal stem cells (MSCs) or ELA‐A3 MSCs and neat antisera collected on Days 0, 7, 14, or 21 post‐injection with either major histocompatibility complex (MHC)‐matched or MHC‐mismatched MSCs. Live cells appear round with a clear centre. Dead cells appear flat with a dark centre. Cell death was estimated to be <10% for MHC‐matched wells on all days and for MHC‐mismatched wells on Day 0 as shown in this figure. Cell death was estimated to be >80% for all MHC‐mismatched wells on Days 7–21 as shown in this figure.
Incubation of antisera from the control horse with target cells did not result in significant cell death (<20% cell death) at any time point or at any dilution (Fig 3) indicating the absence of ELA‐A2 antibodies. None of the experimental horses had significant levels of pre‐existing ELA‐A2 antibodies prior to injection with ELA‐A2 MSCs as shown by the lack of significant target cell death following incubation with Day 0 antisera. By Day 7, 2 of the 4 experimental horses had cytotoxic ELA‐A2 antibodies present at concentrations capable of killing at least 50% of PBL and MSC target cells at the neat antisera concentration. By Day 14, all 4 of the recipient horses had >50% cell death of PBL and MSC target cells for neat antisera. Similar results were seen for antisera from Day 21. A comparable time‐dependent trend was seen with 1:2 and 1:16 diluted antisera, but with reduced cytotoxicity. There was a significant time‐dependent effect on cytotoxicity score from Day 0 to Day 14 and 21 for both PBL and MSC target cells at all dilutions as well as Day 7 compared with Day 14 and 21 cytotoxicity scores. There was a large amount of variation in cytotoxicity of antisera between horses at Day 7, but the median cytotoxicity scores for PBLs and MSCs were not significant (P = 0.061) compared with Day 0 scores. There was no significant difference between cytotoxicity of PBLs and MSCs at any time point (neat P = 0.9; 1:2 P = 0.3; 1:16 P = 0.3) and no significant difference between cytotoxicity of the 2 lines of ELA‐A2 MSC target cells used at any time point or dilution.
Figure 3.
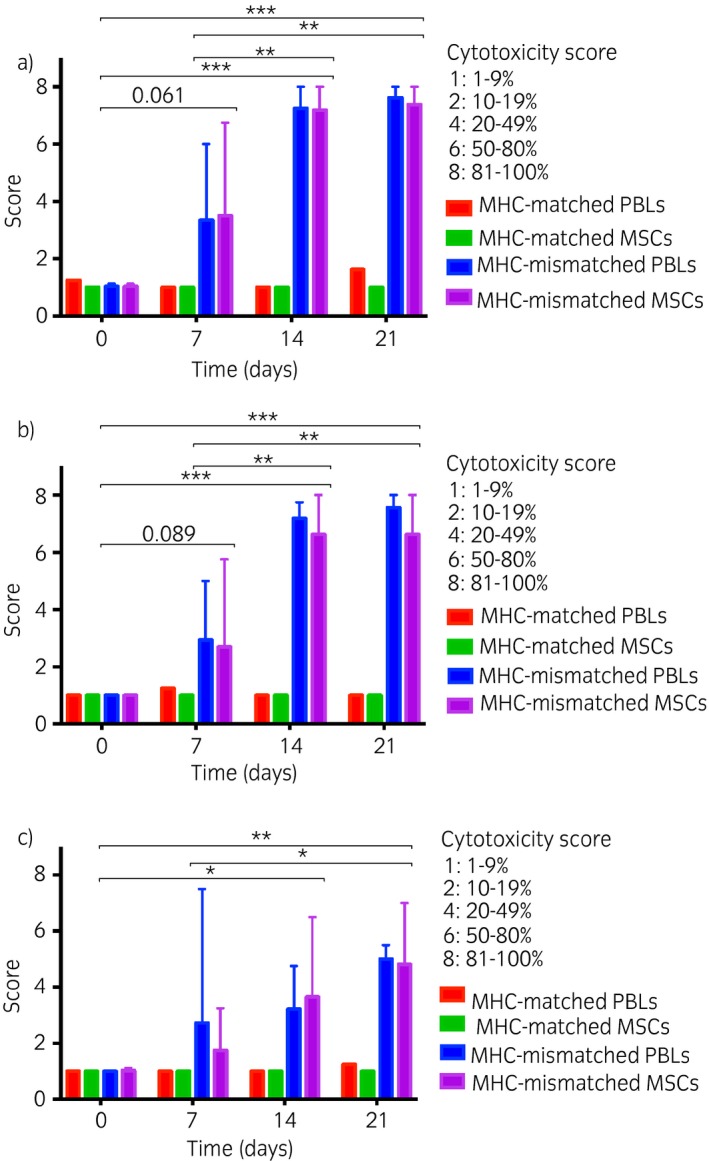
Cytotoxicity scores of peripheral blood leucocytes (PBLs) and mesenchymal stem cells (MSCs) from the equine leucocyte antigen (ELA)‐A2 microcytotoxicity assays. A standard two‐stage microcytotoxicity assay was used to compare the cytotoxicity of ELA‐A2 haplotype PBLs and MSCs following incubation with antisera. Mean cytotoxicity scores and range for ELA‐A2 haplotype PBLs and MSC target cells following microcytotoxicity assay with n = 4 major histocompatibility complex (MHC)‐mismatched antisera neat a), 1:2 b), and 1:16 c) dilutions. *P<0.05, **P<0.01, ***P<0.001 using repeated measures ANOVA and Holm–Sidak all pairwise multiple comparison procedures. Scores from the microcytotoxicity assay with MHC‐matched antisera are included as reference. Error bars indicate the range of scores above the mean.
Antisera from the one non‐ELA‐A2 experimental horse determined to have developed anti‐ELA‐A3 antibodies in the previous study was also tested against PBL and MSC target cells from an ELA‐A3 haplotype horse (Fig 2). Incubation with Day 0 antisera did not result in significant cell death of PBLs or MSCs demonstrating a lack of pre‐existing ELA‐A3 antibodies prior to MSC injection. The cytotoxicity of the ELA‐A3 target cells followed a similar time‐dependent effect as the ELA‐A2 target cells (Fig 4). Incubation with Day 7 and 14 neat antisera resulted in >50% cell death of both PBL and MSC target cells and >80% cell death of both PBL and MSC target cells following incubation with Day 21 neat antisera with similar trends seen for antisera diluted 1:2 and 1:16.
Figure 4.
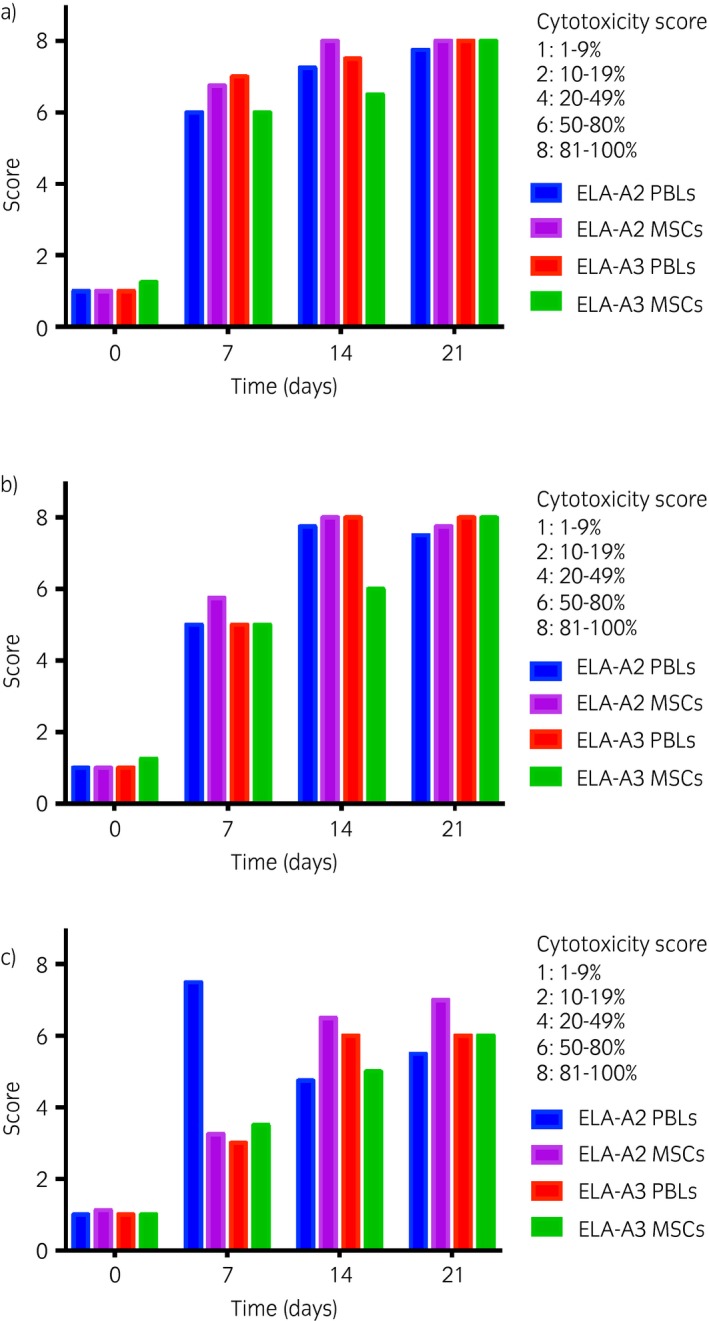
Cytotoxicity scores of peripheral blood leucocytes (PBLs) and mesenchymal stem cells (MSCs) from the equine leucocyte antigen (ELA)‐A3 microcytotoxicity assays. A standard two‐stage microcytotoxicity assay was used to compare the cytotoxicity of ELA‐A3 haplotype PBLs and MSCs following incubation with antisera. Mean cytotoxicity scores for ELA‐A3 haploytpe PBLs and MSC target cells following microcytotoxicity assay with n = 1 major histocompatibility complex (MHC)‐mismatched antisera neat a), 1:2 b), and 1:16 c) dilutions. Scores from the microcytotoxicity assay with the same MHC‐mismatched antisera and ELA‐A2 target cells from Figure 3 are included as reference.
Discussion
In a previous study, it was demonstrated that cytotoxic anti‐MHC antibodies were produced following injection of MHC‐mismatched MSCs 9. The aim of the current study was to determine if these anti‐MHC antibodies could initiate complement‐dependent cytotoxicity of equine MSCs, as they had for PBLs. Our data demonstrates that incubation of ELA‐A2 haplotype PBL and MSC target cells with antisera containing anti‐ELA‐A2 antibodies and complement resulted in target cell death. There was no significant difference between the cytotoxicity of PBLs and MSCs indicating that the immunosuppressive properties of MSCs are not capable of protecting the cells from a targeted humoral immune response against allogeneic MHC molecules and that any cell expressing allogeneic MHC surface molecules can potentially be killed by a targeted humoral immune response. All of the horses tested in the current study were strongly positive for alloantibodies by Day 14 despite having no significant amounts of pre‐existing alloantibodies. In human transplant studies, the presence of complement‐binding donor‐specific anti‐HLA antibodies post‐transplantation correlates with rejection and poor graft outcome even with immunosuppressive therapy 24. This evidence from the human literature supports that the alloantibodies induced post‐transplantation in horses are likely to contribute to targeted destruction of MSCs in vivo, similar to cells in solid organ grafts.
Importantly, anti‐ELA‐A2 antibodies were present in the serum as early as Day 7 in 2 of the horses indicating that memory T and B cells may exist in some horses that are capable of inducing a cytotoxic humoral immune response against MSCs. Even if a case has no known history of a sensitising event such as pregnancy or a blood transfusion, it is possible for animals to develop anti‐MHC I antibodies due to cross‐reactive epitopes on microorganisms, allergens, or ingested proteins 25, 26, 27. Any animal could therefore be primed and quickly mount an antibody response against MSCs even after a single injection. Repeated injections of MSCs after the initial sensitisation would result in accelerated rejection of the cells further limiting their beneficial effects and increasing the potential for adverse events. It is currently unknown how the site of transplantation or the cell dosage affect the outcome or kinetics of an alloantibody response and should be investigated in future studies.
In addition to a targeted humoral immune response, there is evidence in the literature that cell‐mediated alloimmune responses can limit the persistence of MSCs in vivo 18, 19, 20, 21. While it is possible that injection of donor MSCs into less vascularised tissues like tendons and joints may result in different responses than intradermal or intravascular administration, during injury even tendons and joints are infiltrated with immune cells 28, 29 that can contribute to allorecognition and subsequent immune rejection of MSCs. Inflammatory cytokines present in injured tissue such as interferon‐γ are known to upregulate MHC expression on equine MSCs 8, which may make MSCs more likely to be recognised and rejected by a cell‐mediated response. The health status, genetic background, immune cell repertoire and other individual variables of the recipient may all affect the strength and specificity of an alloimmune response. To complete these gaps in knowledge, any future allogeneic MSC clinical trial in horses should include analysis of both the cell‐mediated and humoral immune response following MSC therapy in addition to MHC haplotyping donors and recipients.
MSC therapies in horses can improve the outcome of potentially career‐ and life‐ending musculoskeletal injuries. While previous studies in horses have found that repeat injections of allogeneic MSCs do not cause overt clinical symptoms of transplant rejection 15, 16, these studies did not thoroughly investigate whether a cell‐mediated or humoral immune response was induced or whether this limited the persistence of the cells in vivo. As the likely therapeutic benefits of MSCs appear to be largely due to the secretion of paracrine factors that promote healing of healthy tissue 30, 31, it is necessary for the cells to persist throughout the initial inflammatory and healing period. Targeted destruction of allogeneic, MHC‐mismatched MSCs shortly after transplant would therefore limit their therapeutic potential. Until the in vivo immune response against allogeneic MSCs is better understood and strategies are developed to prevent rejection, allogeneic MSC therapy should be strongly cautioned against. Further investigation into cell‐mediated and humoral immune responses against MHC‐mismatched, allogeneic MSCs in vitro and in vivo are necessary to determine if safe and efficacious allogeneic MSC therapy is an obtainable goal.
Authors’ declaration of interests
No competing interests have been declared.
Ethical animal research
The Institutional Animal Care and Use Committee of North Carolina State University approved the use of horses in this study.
Sources of funding
This work was supported by National Institutes of Health grant #1K08AR060875 (L.V.S.) and the Morris Animal Foundation grant #D16EQ‐405 (A.K.B.).
Authorship
All authors contributed to the study conception and design, study execution, data analysis and interpretation, and preparation of the manuscript. All authors approved of the final version of the manuscript.
Acknowledgements
The assistance of Dr Anthony Blikslager with the statistical analyses is acknowledged and greatly appreciated. The authors would like to thank Drs Douglas Antczak and Lisa Fortier for their continued mentorship and permission to use the antisera samples in this study as well as Drs Lynn Pezzanite and Margaret Brosnahan for their technical advice. The authors would also like to thank Mr Donald Miller and Mrs Julie Long for their technical assistance and the NCSU Laboratory Animal Resources staff for their help with animal care and handling.
Manufacturers' addresses
Mediatech, Manassas, Virginia, USA.
Atlanta Biologicals, Lawrenceville, Georgia, USA.
Corning Inc., Corning, New York, USA.
EDM, Millipore Temecula, California, USA.
Nexcelom, Lawrence, Massachusetts, USA.
BD, Franklin Lakes, New Jersey, USA.
Sigma‐Aldrich, St Louis, Missouri, USA.
GE Healthcare, Pittsburgh, Pennsylvania, USA.
Greiner, Bio‐One Monroe, North Carolina, USA.
Invitrogen, Waltham, Massachusetts, USA.
Olympus Corporation, Center Valley, Pennsylvania, USA.
Systat Software Inc., San Jose, California, USA.
References
- 1. Schnabel, L.V. , Lynch, M.E. , van der Meulen, M.C. , Yeager, A.E. , Kornatowski, M.A. and Nixon, A.J. (2009) Mesenchymal stem cells and insulin‐like growth factor‐I gene‐enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 27, 1392–1398. [DOI] [PubMed] [Google Scholar]
- 2. Crovace, A. , Lacitignola, L. , Rossi, G. and Francioso, E. (2010) Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase‐induced tendinitis of equine superficial digital flexor tendon. Vet. Med. Int. 2010, 250978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conze, P. , van Schie, H.T. , van Weeren, R. , Staszyk, C. , Conrad, S. , Skutella, T. , Hopster, K. , Rohn, K. , Stadler, P. and Geburek, F. (2014) Effect of autologous adipose tissue‐derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen. Med. 9, 743–757. [DOI] [PubMed] [Google Scholar]
- 4. Ferris, D.J. , Frisbie, D.D. , Kisiday, J.D. , McIlwraith, C.W. , Hague, B.A. , Major, M.D. , Schneider, R.K. , Zubrod, C.J. , Kawcak, C.E. and Goodrich, L.R. (2014) Clinical outcome after intra‐articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet. Surg. 43, 255–265. [DOI] [PubMed] [Google Scholar]
- 5. Pacini, S. , Spinabella, S. , Trombi, L. , Fazzi, R. , Galimberti, S. , Dini, F. , Carlucci, F. and Petrini, M. (2007) Suspension of bone marrow‐derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 13, 2949–2955. [DOI] [PubMed] [Google Scholar]
- 6. Wilke, M.M. , Nydam, D.V. and Nixon, A.J. (2007) Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J. Orthop. Res. 25, 913–925. [DOI] [PubMed] [Google Scholar]
- 7. Caniglia, C.J. , Schramme, M.C. and Smith, R.K. (2012) The effect of intralesional injection of bone marrow derived mesenchymal stem cells and bone marrow supernatant on collagen fibril size in a surgical model of equine superficial digital flexor tendonitis. Equine Vet. J. 44, 587–593. [DOI] [PubMed] [Google Scholar]
- 8. Schnabel, L.V. , Pezzanite, L.M. , Antczak, D.F. , Felippe, M.J. and Fortier, L.A. (2014) Equine bone marrow‐derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res. Ther. 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pezzanite, L.M. , Fortier, L.A. , Antczak, D.F. , Cassano, J.M. , Brosnahan, M.M. , Miller, D. and Schnabel, L.V. (2015) Equine allogeneic bone marrow‐derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res. Ther. 6, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrade, D.D. , Lame, M.W. , Kent, M.S. , Clark, K.C. , Walker, N.J. and Borjesson, D.L. (2012) Comparative analysis of the immunomodulatory properties of equine adult‐derived mesenchymal stem cells. Cell. Med. 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paterson, Y.Z. , Rash, N. , Garvican, E.R. , Paillot, R. and Guest, D.J. (2014) Equine mesenchymal stromal cells and embryo‐derived stem cells are immune privileged in vitro. Stem Cell Res. Ther. 5, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pigott, J.H. , Ishihara, A. , Wellman, M.L. , Russell, D.S. and Bertone, A.L. (2013) Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra‐articular injection in horses. Vet. Comp. Orthop. Traumatol. 26, 453–460. [DOI] [PubMed] [Google Scholar]
- 13. Pigott, J.H. , Ishihara, A. , Wellman, M.L. , Russell, D.S. and Bertone, A.L. (2013) Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra‐articular injection in horses. Vet. Immunol. Immunopathol. 156, 99–106. [DOI] [PubMed] [Google Scholar]
- 14. Ricco, S. , Renzi, S. , Del Bue, M. , Conti, V. , Merli, E. , Ramoni, R. , Lucarelli, E. , Gnudi, G. , Ferrari, M. and Grolli, S. (2013) Allogeneic adipose tissue‐derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int. J. Immunopathol. Pharmacol. 26, 61–68. [DOI] [PubMed] [Google Scholar]
- 15. Ardanaz, N. , Vazquez, F.J. , Romero, A. , Remacha, A.R. , Barrachina, L. , Sanz, A. , Ranera, B. , Vitoria, A. , Albareda, J. , Prades, M. , Zaragoza, P. , Martin‐Burriel, I. and Rodellar, C. (2016) Inflammatory response to the administration of mesenchymal stem cells in an equine experimental model: effect of autologous, and single and repeat doses of pooled allogeneic cells in healthy joints. BMC Vet. Res. 12, 1–2 65‐016‐0692‐x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kol, A. , Wood, J.A. , Carrade Holt, D.D. , Gillette, J.A. , Bohannon‐Worsley, L.K. , Puchalski, S.M. , Walker, N.J. , Clark, K.C. , Watson, J.L. and Borjesson, D.L. (2015) Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res. Ther. 6, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guest, D.J. , Smith, M.R. and Allen, W.R. (2010) Equine embryonic stem‐like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendon. Equine Vet. J. 42, 636–642. [DOI] [PubMed] [Google Scholar]
- 18. Nauta, A.J. , Westerhuis, G. , Kruisselbrink, A.B. , Lurvink, E.G. , Willemze, R. and Fibbe, W.E. (2006) Donor‐derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badillo, A.T. , Beggs, K.J. , Javazon, E.H. , Tebbets, J.C. and Flake, A.W. (2007) Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol. Blood Marrow Transplant. 13, 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zangi, L. , Margalit, R. , Reich‐Zeliger, S. , Bachar‐Lustig, E. , Beilhack, A. , Negrin, R. and Reisner, Y. (2009) Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 27, 2865–2874. [DOI] [PubMed] [Google Scholar]
- 21. Eliopoulos, N. , Stagg, J. , Lejeune, L. , Pommey, S. and Galipeau, J. (2005) Allogeneic marrow stromal cells are immune rejected by MHC class I‐ and class II‐mismatched recipient mice. Blood 106, 4057–4065. [DOI] [PubMed] [Google Scholar]
- 22. Adams, A.P. and Antczak, D.F. (2001) Ectopic transplantation of equine invasive trophoblast. Biol. Reprod. 64, 753–763. [DOI] [PubMed] [Google Scholar]
- 23. Antczak, D.F. , Bright, S.M. , Remick, L.H. and Bauman, B.E. (1982) Lymphocyte alloantigens of the horse. I. Serologic and genetic studies. Tissue Antigens 20, 172–187. [DOI] [PubMed] [Google Scholar]
- 24. Loupy, A. , Lefaucheur, C. , Vernerey, D. , Prugger, C. , Duong van Huyen, J.P. , Mooney, N. , Suberbielle, C. , Fremeaux‐Bacchi, V. , Mejean, A. , Desgrandchamps, F. , Anglicheau, D. , Nochy, D. , Charron, D. , Empana, J.P. , Delahousse, M. , Legendre, C. , Glotz, D. , Hill, G.S. , Zeevi, A. and Jouven, X. (2013) Complement‐binding anti‐HLA antibodies and kidney‐allograft survival. N. Engl. J. Med. 369, 1215–1226. [DOI] [PubMed] [Google Scholar]
- 25. Hirata, A.A. and Terasaki, P.I. (1970) Cross‐reactions between streptococcal M proteins and human transplantation antigens. Science 168, 1095–1096. [DOI] [PubMed] [Google Scholar]
- 26. Pantenburg, B. , Heinzel, F. , Das, L. , Heeger, P.S. and Valujskikh, A. (2002) T cells primed by Leishmania major infection cross‐react with alloantigens and alter the course of allograft rejection. J. Immunol. 169, 3686–3693. [DOI] [PubMed] [Google Scholar]
- 27. Amir, A.L. , D'Orsogna, L.J. , Roelen, D.L. , van Loenen, M.M. , Hagedoorn, R.S. , de Boer, R. , van der Hoorn, M.A. , Kester, M.G. , Doxiadis, I.I. , Falkenburg, J.H. , Claas, F.H. and Heemskerk, M.H. (2010) Allo‐HLA reactivity of virus‐specific memory T cells is common. Blood 115, 3146–3157. [DOI] [PubMed] [Google Scholar]
- 28. Spurlock, G.H. , Spurlock, S.L. and Parker, G.A. (1989) Ultrasonographic, gross, and histologic evaluation of a tendonitis disease model in the horse. Vet. Radiol. Ultrasound. 30, 184–188. [Google Scholar]
- 29. Haynes, M.K. , Hume, E.L. and Smith, J.B. (2002) Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin. Immunol. 105, 315–325. [DOI] [PubMed] [Google Scholar]
- 30. Gnecchi, M. , Zhang, Z. , Ni, A. and Dzau, V.J. (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 103, 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meirelles Lda, S. , Fontes, A.M. , Covas, D.T. and Caplan, A.I. (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20, 419–427. [DOI] [PubMed] [Google Scholar]


