Abstract
Objective
To describe previously unrecognized or under-recognized adverse events associated with Melody® valve implantation.
Background
In rare diseases and conditions it is typically not feasible to conduct large scale safety trials prior to drug or device approval. Therefore post-market surveillance mechanisms are necessary to detect rare but potentially serious adverse events.
Methods
We reviewed the United States The Food and Drug AdministrationManufacturer and User Facility Device Experience (MAUDE) database and conducted a structured literature review to evaluate adverse events associated with on- and off-label Melody® valve implantation. Adverse events were compared to those described in the prospective Investigational Device Exemption and Post-Market Approval Melody® transcatheter pulmonary valve trials.
Results
We identified 631 adverse events associated with “on-label” Melody® valve implants and 84 adverse events associated with “off-label” implants. The most frequent “on-label” adverse events were similar to those described in the prospective trials including stent fracture (n=210) and endocarditis (n=104). Previously unrecognized or under-recognized adverse events included stent fragment embolization (n=5), device erosion (n=4), immediate post-implant severe valvar insufficiency (n=2), and late coronary compression (n=2 cases at 5 days and 3-months post-implant). Under-recognized adverse events associated with off-label implantation included early valve failure due to insufficiency when implanted in the tricuspid position (n=7), and embolization with percutaneous implantation in the mitral position (n=5).
Conclusion
Post-market passive surveillance does not demonstrate a high frequency of previously unrecognized serious adverse events with “on-label” Melody® valve implantation. Further study is needed to evaluate safety of “off-label” uses.
Subject terms: MAUDE database, device safety, transcatheter pulmonary valve
Introduction
The Melody® transcatheter pulmonary valve (Medtronic Inc., Plymouth, MN) was the first United States The Food and Drug Administrationapproved transcatheter heart valve.1 Similar to many other invasive devices, prospective clinical trials evaluating safety and feasibility of Melody® valve implantation enrolled a relatively small number of patients; the combined U.S. Melody® trials (including the Investigational Device Exemption and post-market approval trials) and the European experience included 379 patients with 255 that were enrolled prospectively.2–5 Although adverse event rates from these initial experiences were low, the collective experiences were inadequate to detect rare but potentially serious adverse events. Since The Food and Drug Administrationapproval, the Melody® valve has gained rapid clinical acceptance and is now in widespread use.6 Moreover case reports document that the Melody® valve is increasingly being used clinically in off-label fashion.
After widespread uptake and with increased off-label use, we sought to determine whether there might be reports of previously unrecognized or under recognized adverse events associated with Melody® valve implantation. To this end we queried the United States The Food and Drug AdministrationManufacturer and User Facility Device Experience (MAUDE) database, a mandatory (for industry) and voluntary (for providers and patients) reporting mechanism designed to facilitate capture of rare device-related adverse events.7 In addition, we conducted a structured literature review to capture additional reported adverse events and to evaluate whether there might be previously unrecognized or under-recognized adverse events associated with off-label Melody® valve implantation.
Methods
MAUDE database query
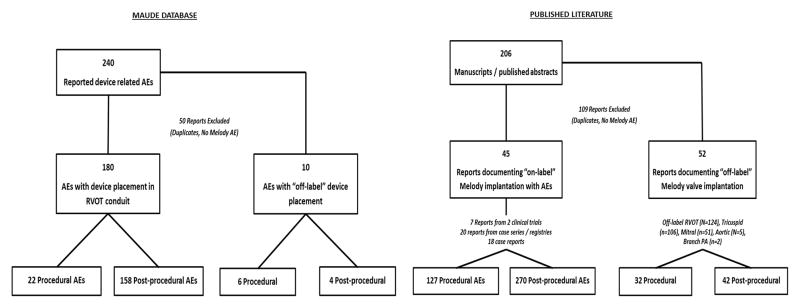
The MAUDE database is a searchable online database of medical device reports received by the Food and Drug Administration. Manufacturers and user facilities (hospitals, outpatient diagnostic or treatment facilities, nursing homes, and ambulatory surgical facilities) are required to report device-related death, serious injury, or malfunction while individual clinicians or patients can submit voluntary reports through the Food and drug administration’s “MedWatch” program. This database serves as a passive surveillance tool to monitor device performance and potentially detect adverse events associated with device use. We queried the online MAUDE database8 using keywords “MELODY” or “TRANSCATHETER PULMONARY VALVE” in the brand name field. We also performed separate searches using the keywords “MEDTRONIC”, “MEDTRONIC INC”, “MEDTRONIC HEART VALVES” or “HEART VALVES SANTA ANA” in the manufacturer field. A start date of January 1st, 2010 was specified to correspond with the Food and Drug Administration’s approval of the Melody® valve (January 25th, 2010). Device reports were collected through July 1st, 2015. All other query fields were left blank. Figure 1 summarizes results of the MAUDE database search and included studies.
Figure 1.
Melody® valve Medical Device Reports identified from the United States Food and Drug Administration database and Melody® valve adverse event reports identified from the published literature
Literature review
Embase and Medline searches were conducted with the aid of a professional librarian from Duke University Medical Center. An initial review demonstrated no Medical Subject Headings (MeSH) terms associated with the Melody® valve therefore we searched for any of the following search terms alone or in combination: “MELODY”, “MELODY VALVE”, “MELODY DEVICE”, “MELODY TPV”, “MELODY TRANSCATHETER HEART VALVE”, ‘MELODY TRANSCATHETER PULMONARY VALVE”, “TRANSCATHETER PULMONARY VALVE”. All citations were downloaded into an EndNote library and abstracts were reviewed for relevance. Articles reporting adverse events or off-label use of the Melody® valve were included; a total of 206 abstracts and/or manuscripts were identified and 97 were included in the final analysis (Figure 1, eTable 1 and 2). When adverse events were reported in multiple manuscripts describing the same study, and it was feasible to identify duplicated events, we preferentially compiled adverse events from the manuscript documenting the latest patient follow up for the particular complication.
MAUDE data collection and classification of complications
Medical device reports from the MAUDE database and from the medical literature were reviewed independently by two board certified pediatric interventional cardiologists (G.A.F and K.D.H). All device reports documenting adverse events that were considered medically significant (i.e. consistent with a grade II or greater adverse event in a clinical trial) were included. Device reports for medically insignificant adverse events (i.e. resulting in no symptoms and warranting no intervention including no need for on-going follow up) and reports that were judged by both reviewers not to represent specific Melody® valve-related adverse events were excluded. Abstract / manuscript case details were cross referenced with MAUDE device reports; Adverse events judged to represent duplicated reports based on the event description, date, or any other relevant case detail, were only included once in the analysis. Complications and relevant outcomes data were extracted and entered into a database. Adverse events were classified in two ways: 1) as procedural or post-procedural adverse events based on the event description and reported timing; and 2) as on-label or off-label complications based on the the Food and Drug Administration’s labelled indication for the Melody® valve; although the Food and Drug Administration instructions for use do not provide a specific weight limit for Melody® implantation, we considered implantations in children < 30kg to be off-label indications based upon the weight limit for the United States Investigational Device Exemption trial.
Statistical analysis
Complications were identified as discrete events and reported as absolute numbers. A primary complication categorization was assigned to each medical device report. Standard summary statistics (median, range) were used to describe time to event following implantation. All statistical analyses were conducted using SPSS 22.0 (IBM, Chicago, Il).
Results
Reports in the literature documenting adverse events associated with “on-label” Melody® valve implantation included seven reports from two prospective clinical trials (248 implants, 70 adverse events), 20 retrospective and/or registry-based case series (2,123 implants, 301 adverse events) and 18 case reports (26 implants, 26 adverse events) (eTable 1). The MAUDE database included 240 “on-label” Melody® valve medical device reports submitted between 01/01/2010 and 07/01/2015. Upon manual review, 50 of these reports were excluded as non-Melody® related adverse events or reports duplicated in the medical literature, leaving 190 MAUDE adverse event reports.
Procedural adverse events
TABLE 1 summarizes procedural adverse events reported in the literature and the MAUDE database, as well as adverse events reported in the United States Investigational Device Exemption trial and the post-approval study. Combined, these two studies, both with active surveillance protocols, reported procedural adverse events for 9.2% (23/248) of implants including conduit rupture / tear (n=8, 3.2%), access site complications (n=5, 2.0%), guidewire induced distal pulmonary artery perforation (n=3, 1.2%), coronary compression (n=2, 0.8%), ventricular tachycardia (n=1, 0.4%) and paravalvar leak (n=1, 0.4%). There were no procedural deaths reported.
Table 1.
Melody® valve procedural adverse events
| U.S. prospective studies (N=248) | Registry and case series | MAUDE medical device reports | Case Reports | |
|---|---|---|---|---|
| Conduit rupture / tear | 8 (3.2%) | 41 | 1 | - |
| Access site complications | 5 (2.0%) | 13 | - | - |
| Guidewire PA perforation | 3 (1.2%) | 4 | - | - |
| Hemodynamic change | 3 (1.2%) | - | - | 2 |
| Coronary compression | 2 (0.8%) | 5 | 5 | 1 |
| Ventricular arrhythmia | 1 (0.4%) | 1 | - | - |
| Paravalvar leak | 1 (0.4%) | - | - | - |
| Malposition / embolization | - | 5 | 5 | 1 |
| Acute device failure | - | 3 | 4 | - |
| Complete heart block | - | 2 | - | 1 |
| PA obstruction | - | 1 | 2 | - |
| Inappropriate unsheathing | - | - | 2 | - |
| Aorto-pulmonary fistula | - | - | 2 | 1 |
| Major AE NOS | - | 19 | - | - |
| Peri-procedural mortality | 0 (0%) | 4 | 1 | - |
AE: Adverse event, NOS: not otherwise specified
Review of passive surveillance mechanisms including the MAUDE device reports and non-trial literature identified additional complications including device embolization (n=11), immediate post-implant device failure requiring intervention due to insufficiency (n=2) or stenosis (n=5), complete heart block (n=3), complete branch pulmonary artery obstruction (n=3), development of an aorto-pulmonary fistula immediately after valve deployment requiring intervention (n=3) and accidental unsheathing in the right ventricle (n=2). A total of 4 procedural mortalities were reported in case series in the literature with a single procedural mortality reported in the MAUDE database. Causes of death were reported for four patients and included coronary compression (n=2), right pulmonary artery obstruction and ventricular arrhythmia.
Post-procedural device related adverse events
TABLE 2 summarizes post-procedural device related adverse events from the literature review and MAUDE database, as well as adverse events reported in the prospective Investigational Device Exemption trial (N=144 patients with median follow up 4.5 years) and the post-approval study (N=100 patients with one-year follow up). Combined, these two studies, both with active surveillance protocols, reported 74 post-procedural adverse events, all representing either stent fracture (n=57) or endocarditis (n=17). Stent fracture was less frequent in the post-approval study (n=7/100 implants, 7%) than in the earlier Investigational Device Exemption trial (n=50/144 implants, 35%), likely reflecting more frequent adoption of conduit pre-stenting in the later post-approval study (in fact, pre-stenting wasn’t permitted in the early patients enrolled in the Investigational Device Exemption trial) and a longer duration of follow-up in the Investigational Device Exemption trial.
Table 2.
Melody® valve post-procedural adverse events
| U.S. Melody® valve trials (N=244 implants) | Retrospective literature reports | Case reports | MAUDE medical device reports | |
|---|---|---|---|---|
| Stent Fracture | 57 (23.4%) | 116 | - | 94 |
| Type II (Loss of structural integrity) | 26 (10.6%) | 22 | - | 35 |
| Type III (with particle embolization) | 1 (0.4%) | - | - | 5 |
| Endocarditis | 17 (7%) | 63 | 13 | 28 |
| Valve dysfunction without stent fracture1 | - | - | - | 37 |
| Device erosion | - | - | 2 | 2 |
| Late coronary compression2 | - | - | 2 | - |
Including stenosis or insufficiency,
RCA compression identified 5 days and 3 months after implant when patients presented with myocardial infarction
Similar to the clinical trials, stent fracture and endocarditis were the most commonly reported non-procedural adverse events in the non-trial literature (median follow up 20 months, range 3–30 months for case series) and in the MAUDE database (median follow up 18 months, range 1 week to 74 months). There were 76 reports of endocarditis in the non-trial literature and 28 in the MAUDE database. Infectious organisms were documented in 65 of these cases with the most common including S. aureus (N=18, 28%), the viridans streptococci (N=17, 26%), coagulase negative staphylococcus (N=14, 22%) and the HACEK organisms (n=4,6%).. Median time to diagnosis of endocarditis was 12 months (range 1 week to 5 years) with only three cases documented within one month of implantation.
Additional adverse events in the MAUDE database / non-trial literature that were not well described in the prospective trials included five reports of complete stent fracture with stent fragment embolization, four cases of device “erosion” into the aorta or aortopulmonary fistula development 9–11 and two cases of late coronary compression that were identified at 5-days and 3-months post-implant, respectively 12, 13. Case details for these adverse events are summarized in TABLE 3.
Table 3.
Case descriptions for previously under-recognized Melody® valve post-procedural adverse events
| Time after implant (mos) | Event description and circumstances | Outcome | Source | |
|---|---|---|---|---|
| Type III stent fracture (complete fracture with stent fragment embolization) | 7 | Location of fragment not noted | Surgical valve removal | MAUDE |
| 35 | Fragment embolized to right ventricle apex | Details not provided | MAUDE | |
| 12 | Fragment embolized to right ventricle apex | Fragment left in place, 2nd trans-catheter valve implanted | MAUDE | |
| 24 | Fragment embolized to branch pulmonary artery | Fragment retrieved, 2nd trans-catheter valve implanted | MAUDE | |
| 98 | Fragment embolized to left lung | No intervention required | MAUDE | |
| Device erosion / aortopulmonary fistula | 0.75 | Valve erosion into ascending aorta in patient with history of Ross procedure, presented with heart failure | Surgical valve removal | Taggart (2013) Congenital Heart Disease |
| 0.75 | Fistula between aortic root and conduit in patient with history of interrupted aortic arch repair | Surgical valve removal | Peer (2014) Journal of Thoracic and Cardiovascular Surgery | |
| 16 | Valve erosion into ascending aorta with shunt causing “severe” heart failure | Surgical valve removal | MAUDE | |
| 36 | Valve erosion into ascending aorta in a patient with history of Ross-Konno operation, presented with heart failure | Surgical valve removal | MAUDE | |
| Late coronary obstruction | 0.2 | LAD obstruction presenting 5 days after implant; intra-procedural coronary compression resolved with nitroglycerin administration and was attributed to vasospasm | Surgical valve removal | Biermann (2012) Thoracic and Cardiovascular Surgeon |
| 3 | Late right coronary artery dissection and obstruction after exercise, felt to be related to increased cardiac output | Surgical valve removal and right coronary artery re-implantation | Deghani (2015) Catheterization and Cardiovascular Interventions |
Off-label reports
From the literature we identified 52 case reports / case series describing 342 “off-label” implantations including implants in the tricuspid (n=108), mitral (n=51), and aortic position (n=6) or “off label” uses in the right ventricular outflow tract (n=124), branch pulmonary arteries (n=2) or in children under 30kg (n=26) (eTable 2). A total of 32 procedural and 42 post-procedural adverse events were described with an additional 10 “off-label” adverse events extracted from the MAUDE database. Table 4 summarizes these adverse events by “off-label” indication. Notable events included 7 descriptions of early valve failure following implantation in the tricuspid position. In all cases, there was acute success with no significant immediate post-procedural tricuspid regurgitation but with early development (< 3 months in 6/7cases) of severe regurgitation requiring intervention. There were also adverse events reported for mitral implants including embolization in 5 reported cases implanted using a percutaneous approach. Notably mitral implants were largely performed in high-risk patients with 6/9 reports documenting an average age at implant of ≥ 65 years and one report using a surgical approach in infants (average age at implant of 7 months). There was also a single study describing procedural complications with right ventricular outflow tract conduit implantation in children < 30kg and documenting a serious intra-procedural adverse event rate of 26% (7/26 implants).14 Most of these adverse events (n=5) represented contained conduit tears during conduit balloon sizing with 2 considered major and requiring covered stent placement.
Table 4.
Adverse events associated with “off-label” Melody® valve implantation
| Native RVOT (n=124) | Tricuspid (n=108) | Mitral (n=51) | RVOT in children < 30kg (n=26) | |
|---|---|---|---|---|
| Procedural | ||||
| Vascular complication | - | 1 | 5 | 1 |
| Conduit tear | 1 | - | - | 5 |
| AP fistula | 1 | - | - | - |
| Guidewire perforation | - | - | 1 | 1 |
| Hemo / pneumothorax | 3 | |||
| Embolization | - | - | 5 | - |
| Complete AV block | - | 3 | 1 | - |
| Procedural mortality | - | - | 1 | 1* |
| Post-procedural | - | - | - | - |
| Stent fracture | 6 | 2 | - | 2 |
| Endocarditis | 3 | 1 | - | 2 |
| Early valve failure | 1 | 7 | - | - |
| Paravalvar leak | - | - | 2 | - |
RVOT: Right ventricular Outflow Tract;
Melody® valve implantation was performed in a patient on ECMO following PA laceration during a previous procedure. Mortality was not felt to be directly attributable to the Melody® implantation.
Discussion
In this analysis, we demonstrate that the most common reported adverse events associated with Melody® valve implantation in post-market surveillance mirror those reported in the prospective United States Melody® valve Investigational Device Exemption and Post Market Approval trials, and in the initial European experience.2, 4, 5, 15–18 However, we also identified several rare adverse events, including possible device erosion, device fracture with stent fragment embolization, acute onset valvar insufficiency and late coronary obstruction that were not well documented in the trial literature and are not included on the Food and Drug Administration device label.19 In addition, reports of acute valvar insufficiency with placement of the Melody® valve in the tricuspid position, embolization with mitral implantation, and risk of procedural adverse events with implantation in younger children suggest a need for systematic processes to evaluate safety when the Melody® valve is being used outside of its labelled indication and in high-risk, often high-acuity, clinical scenarios.
Post-market approval passive surveillance of medical devices is an important mechanism used to monitor for potentially harmful but under-recognized adverse events, particularly in rare diseases and conditions where large scale safety trials are typically not feasible. The United States Food and Drug Administration developed the MAUDE database for this specific purpose 20 and it was previously used in the field of interventional pediatric cardiology to highlight the risk of erosion with the Amplatzer Septal Occluder device (St. Jude Medical, Inc, Plymouth, MN).21 A limitation of the database is that it does not accurately represent event rates because most adverse events are under-reported and because the total number of device implants is not available.22 For these reasons the Food and Drug Administration recommends that the database be used to “detect a signal that might require further investigation”. This was the specific objective of our analysis.
Our findings from both the MAUDE database and our literature review are generally reassuring for use of the Melody® valve within the confines of its labeled indication. Most of the intra-procedural and post-procedural adverse events that we report, including coronary compression, conduit disruption, device embolization, stent fracture and endocarditis, have been previously well described.2–5 We did not detect any obvious “signal” suggesting a major safety concern with “on-label” Melody® valve implantation. However, several less well recognized adverse events perhaps warrant closer monitoring by the interventional community. These events included acute device failure due to insufficiency (n=2 cases), post-implant device “erosion” (n=4 cases) and late coronary compression (n=2 cases). In several of these cases there were potential extenuating circumstance (described in Table 2). Regardless these represent important device-related events and the fact that there were extenuating circumstances should not deter reporting of these events – it is well recognized that post-market adverse events are often under reported because they are judged to be due to errors in implant technique or clinical judgement.22 To facilitate passive surveillance mechanisms, implanting physicians can report device related adverse events relatively easily via the MedWatch reporting form (www.Food and Drug Administration.gov/Safety/MedWatch/default.htm).
Although both Melody® valve endocarditis and stent fracture have been previously well described, our analysis does provide some additive insight regarding these events. With respect to endocarditis, this represents the largest reported collection of Melody® endocarditis cases and confirms findings of prior reports documenting that Melody® endocarditis does not cluster around the acute implant period and that the most common bacteria (streptococci and staphylococci) represent typical endocarditis bacteria.17, 23, 24 These findings suggest that Melody® endocarditis results from de novo post-procedural blood stream infection with seeding of the Melody® apparatus rather than a pre- or peri-procedural event related to sterilization practices (e.g. use of a non-operating room environment) or the implantation protocol (e.g. valve manipulation prior to delivery). With respect to stent fracture, another previously well documented adverse event, this is the largest report of type III fracture (associated with stent fragment embolization ). A single type III fracture was identified in the United States Investigational Device Exemption trial and we identified five cases from the MAUDE database. None of these reports resulted in adverse patient outcomes. However they highlight the need for on-going surveillance after initial identification of a type I or type II fracture; in all of these cases the patients first presented with a lower grade (type I or II) fracture.
Safety of off-label use
“Off-label” use refers to use outside of the labeled indication and is very common in the field of pediatric interventional catheterization.25 We identified an increasing number of reports documenting off-label Melody® implantation. These reports may represent important breakthroughs leading to rapid advances in clinical applications, particularly in scenarios where clinical trials might be prohibitively expensive. However, safety tracking is difficult when off-label uses occur sporadically at a large number of different centers. Our limited analysis is not sufficient to appropriately evaluate safety or efficacy of these off-label indications. Large multi-center registries will be best positioned to address the safety or efficacy of infrequent off-label uses. In lieu of these data, providers should be aware of the potential complications that we identified including heart block and acute valvar insufficiency with implantation in the tricuspid position, and valve embolization with implantation in the mitral position. Although implantation in smaller children is not technically an off-label application, the original Melody® valve trials restricted enrollment to those >30kg. It is notable, although perhaps not unexpected, that the adverse event rate is somewhat higher in these smaller patients.14
There are several important limitations to the present analysis. Despite using a structured approach to our literature review, it is possible that we missed some published reports or that some of our published cases are duplicated and reported in both the literature and the MAUDE database. Moreover there are inherent biases in the published literature; positive findings are more likely to be published while negative outcomes and safety events often go unpublished. Similarly, the MAUDE database may underrepresent adverse events as it was designed for passive surveillance. The information submitted by reporters has limitations, including the possibility of inaccurate or incomplete data. In addition, most reports are not verified through objective, independent assessment mechanisms and the prevalence and incidence of adverse events cannot be determined through the MAUDE database because adverse events are underreported, may in some cases be reported in duplicate, and total number of devices implanted is not known.
Conclusion
The data presented herein are relatively reassuring that Melody® valve related adverse events have been defined through prospective clinical trials. With the notable exception of two reports of acute valvar insufficiency, intra-procedural adverse events have all either been previously reported or could be anticipated. We also did not find any evidence of previously unrecognized post-procedural adverse events occurring at a high incidence. Closer surveillance may be warranted for patients at increased risk for device erosion (e.g. after arterial switch, Ross procedure or in those with a dilated aortic root26) and after identification of an initial type I stent fracture due to risk of progression in degree of stent fracture. Finally, while off-label Melody® valve applications are increasingly being reported; our data suggest that there may be unique safety complications that warrant consideration. Specific clinical trials are unlikely for most of these off-label usages, further emphasizing the need for systematic monitoring of these implantations either via large multi-center registries or by restricting these applications to a select subset of centers to facilitate close safety surveillance.
Supplementary Material
Acknowledgments
Funding sources: This work was supported in part by a grant from The National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001115-02).
Footnotes
Disclosures: KDH receive support from The National Center for Advancing Translational Sciences of the NIH (KL2TR001115-02). PYC receives support from The National Center for Advancing Translational Sciences of the NIH (UL1TR001117).
References
- 1.United States Food and Drug Administration. [October 4th, 2015];Humanitarian Device Exemption Approval Letter. Accessed online at: http://www.accessdata.fda.gov/cdrh_docs/pdf8/H080002a.pdf.
- 2.Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–72. doi: 10.1161/CIRCULATIONAHA.107.735779. [DOI] [PubMed] [Google Scholar]
- 3.Lurz P, Gaudin R, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation. Seminars in thoracic and cardiovascular surgery Pediatric cardiac surgery annual. 2009:112–7. doi: 10.1053/j.pcsu.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 4.McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, Vincent JA. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122:507–16. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the u.s. Clinical trial. Journal of the American College of Cardiology. 2009;54:1722–9. doi: 10.1016/j.jacc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Medtronic. [October 3rd, 2015]; Press Release from February 3rd, 2015. Accessed on-line at: http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=2013134.
- 7.Gurtcheff SE. Introduction to the MAUDE database. Clinical obstetrics and gynecology. 2008;51:120–3. doi: 10.1097/GRF.0b013e318161e657. [DOI] [PubMed] [Google Scholar]
- 8. [August 1st, 2015];United States Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database. Accessed online at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Search.cfm.
- 9.Taggart NW, Connolly HM, Hagler DJ. Acute heart failure after percutaneous pulmonary valve (melody(copyright) valve) implantation. Catheterization and Cardiovascular Interventions. 2011;77:S139–S140. [Google Scholar]
- 10.Taggart NW, Hagler DJ, Connolly HM. Melody valve erosion into the ascending aorta. Congenital heart disease. 2013;8:E64. doi: 10.1111/chd.12021. [DOI] [PubMed] [Google Scholar]
- 11.Peer SM, Sinha P. Percutaneous pulmonary valve implantation after Ross-Konno aortoventriculoplasty: A cautionary word. Journal of Thoracic and Cardiovascular Surgery. 2014;147:e74–e75. doi: 10.1016/j.jtcvs.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Biermann D, Schonebeck J, Rebel M, Weil J, Reichenspurner H, Dodge-Khatami A. Coronary event after transcatheter pulmonary valve implantation. Thoracic and Cardiovascular Surgeon. 2012;94(1):e7–9. doi: 10.1016/j.athoracsur.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Dehghani P, Kraushaar G, Taylor DA. Coronary artery compression three months after transcatheter pulmonary valve implantation. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2015;85:611–4. doi: 10.1002/ccd.25628. [DOI] [PubMed] [Google Scholar]
- 14.Berman DP, McElhinney DB, Vincent JA, Hellenbrand WE, Zahn EM. Feasibility and short-term outcomes of percutaneous transcatheter pulmonary valve replacement in small (<30 kg) children with dysfunctional right ventricular outflow tract conduits. Circulation Cardiovascular interventions. 2014;7:142–8. doi: 10.1161/CIRCINTERVENTIONS.113.000881. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong AK, Balzer DT, Cabalka AK, Gray RG, Javois AJ, Moore JW, Rome JJ, Turner DR, Zellers TM, Kreutzer J. One-year follow-up of the Melody transcatheter pulmonary valve multicenter post-approval study. JACC Cardiovascular interventions. 2014;7:1254–62. doi: 10.1016/j.jcin.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Bishnoi RN, Jones TK, Kreutzer J, Ringel RE. NuMED Covered Cheatham-Platinum Stent for the treatment or prevention of right ventricular outflow tract conduit disruption during transcatheter pulmonary valve replacement. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2015;85:421–7. doi: 10.1002/ccd.25682. [DOI] [PubMed] [Google Scholar]
- 17.McElhinney DB, Benson LN, Eicken A, Kreutzer J, Padera RF, Zahn EM. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circulation Cardiovascular interventions. 2013;6:292–300. doi: 10.1161/CIRCINTERVENTIONS.112.000087. [DOI] [PubMed] [Google Scholar]
- 18.McElhinney DB, Cheatham JP, Jones TK, Lock JE, Vincent JA, Zahn EM, Hellenbrand WE. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circulation Cardiovascular interventions. 2011;4:602–14. doi: 10.1161/CIRCINTERVENTIONS.111.965616. [DOI] [PubMed] [Google Scholar]
- 19. [August 11th, 2015];Medtronic Melody Valve Sponsor Executive Summaary to the United States Food and Drug Administration Including Instructions for Use. Accessed on-line at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM172725.pdf.
- 20.Daniel Levinson, Inspector General, Department of Health and Human Services. [March 8, 2016];Adverse Event Reporting for Medical Devices. 2009 Oct; Accessed online at: http://oig.hhs.gov/oei/reports/oei-01-08-00110.pdf.
- 21.DiBardino DJ, McElhinney DB, Kaza AK, Mayer JE., Jr Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. The Journal of thoracic and cardiovascular surgery. 2009;137:1334–41. doi: 10.1016/j.jtcvs.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenzer J, Brownlee S. Why the FDA can’t protect the public. Bmj. 2010;341:c4753. doi: 10.1136/bmj.c4753. [DOI] [PubMed] [Google Scholar]
- 23.Van Dijck I, Budts W, Cools B, Eyskens B, Boshoff DE, Heying R, Frerich S, Vanagt WY, Troost E, Gewillig M. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart (British Cardiac Society) 2015;101:788–93. doi: 10.1136/heartjnl-2014-306761. [DOI] [PubMed] [Google Scholar]
- 24.Buber J, Bergersen L, Lock JE, Gauvreau K, Esch JJ, Landzberg MJ, Valente AM, Sandora TJ, Marshall AC. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation Cardiovascular interventions. 2013;6:301–10. doi: 10.1161/CIRCINTERVENTIONS.112.000348. [DOI] [PubMed] [Google Scholar]
- 25.Sutherell JS, Hirsch R, Beekman RH., 3rd Pediatric interventional cardiology in the United States is dependent on the off-label use of medical devices. Congenital heart disease. 2010;5:2–7. doi: 10.1111/j.1747-0803.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 26.Torres A, Sanders SP, Vincent JA, El-Said HG, Leahy RA, Padera RF, McElhinney DB. Iatrogenic aortopulmonary communications after transcatheter interventions on the right ventricular outflow tract or pulmonary artery: Pathophysiologic, diagnostic, and management considerations. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2015;86:438–52. doi: 10.1002/ccd.25897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.