Abstract
Objective
To assess the spatial distribution of chronic total occlusions (CTOs) within the coronary arteries and describe procedural strategies and outcomes during CTO percutaneous coronary intervention (PCI).
Background
Acute occlusions due to plaque rupture tend to cluster within the proximal third of the coronary artery.
Methods
We examined the clinical and procedural characteristics of 1,348 patients according to lesion location within the coronary tree.
Results
A total of 1,369 lesions in 1,348 patients (mean age 66 ±10 years, 85% male) were included. CTO PCI of proximal segments (n=633, 46%) was more common than of mid (n=557, 41%) and distal segments (n=179, 13%). Patients undergoing CTO PCI of proximal segments were more likely to be smokers (p<0.01), have prior coronary artery bypass graft surgery (p=0.03) and lower ejection fraction (p=0.04). CTOs occurring in proximal segments had longer length (p <0.01), proximal cap ambiguity (p<0.01), and moderate/severe calcification (p<0.01) compared to mid or distally located CTOs. Interventional collaterals were more often present in CTO PCI of proximal segments (64%, 53%, 56%, p<0.01) consistent with the higher use of retrograde approach (47%, 33%, 37%, p<0.01) relative to antegrade wire escalation (67%, 82%, 82%, p<0.01). Procedural complexity was higher in CTO PCI of proximal segments (vs. mid and distal): contrast volume= 275 ml (200-375), 260 ml (200-350), 250 ml (175-350), p=0.01; fluoroscopy time 53 minutes (32-83), 39 minutes (24-65), 40 minutes (22-72), p<0.01. However, procedural success (87%, 90%, 85%, p=0.1), technical success (89%, 91%, 88%, p=0.24), and complications rates (2.8%, 2.5%, 2.2%, p=0.88) were not different.
Conclusions
The most common target vessel location for CTO PCI is the proximal coronary segment. PCI of proximal occlusions is associated with adverse clinical and angiographic characteristics and often requires use of the retrograde approach, but can be accomplished with high procedural and technical success and low complication rates.
Keywords: chronic total occlusion, percutaneous coronary intervention, coronary artery disease
Introduction
Plaque rupture of coronary atherosclerotic lesions is widely accepted to be the most common mechanism underlying acute myocardial infarction (AMI) (1-2). Most plaque ruptures occur in predictable sites within the coronary tree usually referred to as vulnerable plaques or “hot spots” (3-4). A clustering of coronary thrombosis in the proximal third of the coronary artery has been well documented in angiographic as well as pathological studies of patients with acute ST-segment elevation acute myocardial infarction (STEMI) (5-6).
In contrast, little data exists regarding the spatial distribution of chronic total occlusions (CTOs) referred for percutaneous coronary intervention (PCI) within the coronary tree. Proximally located occlusions are associated with higher myocardium at risk and lower ejection fraction and survival after AMI (7,8). Revascularization of proximal CTOs has the potential to mitigate larger areas of myocardial ischemia and improve clinical outcomes. However, these procedures can be technically challenging given adverse angiographic characteristics such as proximal cap ambiguity, blunt stump or side-branch at the site of occlusion.
The objectives of this investigation were: 1) to assess the spatial distribution within the coronary tree of CTOs in patients referred for CTO PCI and 2) to examine clinical, angiographic and procedural characteristics of CTO PCI of proximal segments relative to mid and distal locations.
Methods
We examined the baseline clinical, angiographic characteristics, and clinical outcomes of 1,369 consecutive CTO PCIs performed in 1,348 patients between 2012 and 2015 at 12 US centers: Appleton Cardiology, Appleton Wisconsin; Columbia University, New York, New York; Henry Ford Hospital, Detroit, Michigan; Massachusetts General Hospital, Boston, Massachusetts; Medical Center of the Rockies, Loveland, Colorado; Piedmont Heart Institute, Atlanta Georgia; St. Joseph Medical Center, Bellingham Washington; St. Luke's Health System's Mid-America Heart Institute, Kansas City, Missouri; Torrance Memorial Center, Torrance, California; VA Minneapolis Healthcare System, Minneapolis, Minnesota; VA North Texas Health Care System, Dallas, Texas, and VA San Diego Healthcare System, San Diego, California. Enrollment was performed during only part of the study period in some centers due to participation in other studies. Data collection was performed prospectively and retrospectively and was recorded in a dedicated online CTO database (PROGRESS CTO: Prospective Global Registry for the Study of Chronic Total Occlusion Intervention, Clinicaltrials.gov Identifier: NCT02061436) (9-12). The study was approved by the institutional review board at each site.
Coronary CTOs were defined as coronary lesions with Thrombolysis In Myocardial Infarction (TIMI) grade 0 flow of at least 3-month duration. Estimation of the occlusion duration was based on first onset of anginal symptoms, prior history of myocardial infarction in the target vessel territory, or comparison with a prior angiogram. Calcification was assessed by angiography as mild (spots), moderate (involving ≤50% of the reference lesion diameter) and severe (involving >50% of the reference lesion diameter). Moderate proximal vessel tortuosity was defined as the presence of at least 2 bends >70 degrees or 1 bend >90 degrees and severe tortuosity as 2 bends >90 degrees or 1 bend >120 degrees in the CTO vessel. The Japanese Chronic Total Occlusion (J-CTO) score was calculated as described by Morino et al. (13). Technical success was defined as successful CTO revascularization with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. Occlusion length was visually estimated by the interventional cardiologist performing the procedure. Procedural success was defined as achievement of technical success with no in-hospital major adverse cardiac events (MACE). In-hospital MACE included any of the following adverse events prior to hospital discharge: death, Q-wave and all types of myocardial infarction, urgent repeat target vessel revascularization with either PCI or coronary artery bypass graft surgery (CABG), tamponade requiring either pericardiocentesis or surgery, and stroke.
Patients were classified in three groups based on the location of the CTO within the coronary tree. Proximal lesions were defined as those affecting the proximal segment of either the left anterior descending artery (LAD), right coronary artery (RCA), ramus intermedius (RI), left circumflex (LCX) or left main coronary artery (LMCA). Mid coronary lesions included the mid LAD, first diagonal (D1), mid CX, first obtuse marginal (OM) and mid RCA. Distal coronary lesions included the apical LAD, D2, distal CX, OM2, OM3, distal RCA, right or left PDA, and posterolateral branches (PLs). Lesion location was defined by the inteventionalist performing the CTO-PCI procedure. All procedures were performed in established CTO programs by operators that perform > 30 CTO PCIs per year. Continuous variables were presented as mean ± standard deviation or median (interquartile range) and were compared using the t-test, or Wilcoxon rank-sum test, as appropriate. Categorical data are reported as frequencies or percentages and compared using the chi square test. All statistical analyses were performed with JMP 11.0 (SAS Institute, Cary, North Carolina). Two-sided p-values of 0.05 were considered statistically significant.
Results
A total of 1,369 CTO lesions from 1,348 patients were included in the analysis. The mean age (±SD) of the population was 66 (10) years and 85% were male. Proximal lesions (n=633, 46%) were more common than mid (n=557, 41%) and distal (n=179, 13%) lesions undergoing CTO PCI. Patients with proximal CTO lesions had a higher prevalence of adverse characteristics, relative to mid and distal lesions, including smoking (proximal: 31%, mid: 22%, distal:23%, p=0.005), prior coronary artery bypass graft (CABG) surgery (proximal: 40%, mid: 28%, distal: 20%, p=0.03), heart failure (proximal: 30%, mid: 26%, distal: 20%, p=0.035) and lower ejection fraction (EF) (proximal: 49 ±14%, 51±14, 53 ±12, p=0.046) (Table 1).
Table 1.
Baseline characteristics of the study cohort according to CTO location within the coronary tree
| Overall (lesions) | Proximal | Mid | Distal | p | |
|---|---|---|---|---|---|
| N (%) | 1369 | 633 (46%) | 557 (41%) | 179 (13%) | |
| Age (years)* | 66±10 | 66±10 | 66±10 | 64±11 | 0.030 |
| Male (%) | 85 | 84 | 86 | 86 | 0.451 |
| Diabetes melitus (%) | 45 | 45 | 45 | 49 | 0.670 |
| Dyslipidemia (%) | 94 | 94 | 95 | 94 | 0.912 |
| Hypertension (%) | 89 | 91 | 86 | 89 | 0.019 |
| Prior MI (%) | 44 | 44 | 43 | 48 | 0.537 |
| Smoking (%) | 26 | 31 | 22 | 23 | 0.005 |
| Prior PCI (%) | 63 | 62 | 61 | 69 | 0.177 |
| Prior failed CTOPCI (%) | 18 | 17 | 19 | 17 | 0.673 |
| Prior CABG (%) | 35 | 40 | 28 | 37 | <0.001 |
| Heart failure (%) | 27 | 30 | 26 | 20 | 0.035 |
| History of stroke (%) | 11 | 11 | 11 | 12 | 0.873 |
| Peripheral arterial disease | 16 | 16 | 16 | 17 | 0.908 |
| Ejection fraction (%) | 50±14 | 49±14 | 51±14 | 53±12 | 0.046 |
Angiographic characteristics
A summary of angiographic characteristics is presented in Table 2. Proximal lesions were longer (43±28 mm) and had a larger vessel diameter (2.9±0.6 mm) relative to mid (CTO length: 28±18, vessel diameter: 2.8±0.5mm) and distal lesions (CTO length:26 ±16 mm, vessel diameter: 2.6±0.5 mm, both p <0.001). Proximal lesions also had more adverse angiographic features including proximal cap ambiguity, side branch at proximal cap, blunt or no stump, moderate or severe calcification and poor distal target vessel but had more interventional collaterals (Figure 1 and Table 2). The J-CTO score was higher in proximal lesions 2.7 (±1.2) relative to mid 2.4 (±1.2) and distal 2.5 (±1.2) lesions (p<0.01).
Table 2.
Angiographic characteristics of the CTO according to lesion location
| Overall | Proximal | Mid | Distal | p | |
|---|---|---|---|---|---|
| CTO length (mm) | 34±24 | 43±28 | 28±18 | 26±16 | <0.001 |
| Vessel diameter (mm) | 2.8±0.5 | 2.9±0.6 | 2.8±0.5 | 2.6±0.5 | <0.001 |
| Target vessel | <0.001 | ||||
| LAD (%) | 23 | 19 | 33 | 9 | |
| LCX (%) | 21 | 20 | 20 | 28 | |
| RCA (%) | 56 | 61 | 47 | 63 | |
| Proximal cap ambiguity (%) | 31 | 37 | 27 | 29 | 0.008 |
| Side branch at proximal cap (%) | 48 | 46 | 50 | 48 | 0.456 |
| Blunt/No stump (%) | 54 | 61 | 49 | 49 | <0.001 |
| Distal cap at bifurcation (%) | 32 | 35 | 29 | 34 | 0.173 |
| Poor distal target vessel (%) | 38 | 37 | 35 | 47 | 0.021 |
| Interventional collaterals (%) | 58 | 64 | 53 | 56 | 0.005 |
| Moderate/severe calcification (%) | 55 | 64 | 47 | 49 | <0.001 |
| Moderate/severe tortuosity (%) | 35 | 36 | 32 | 42 | 0.069 |
| In-stent restenosis (%) | 13 | 13 | 13 | 19 | 0.059 |
| J-CTO score (%) | 2.6±1.2 | 2.7±1.2 | 2.4±1.2 | 2.5±1.2 | 0.002 |
CTO: chronic total occlusion, LAD: left anterior descending, LCX: left circumflex, RCA: right coronary artery
Figure 1.
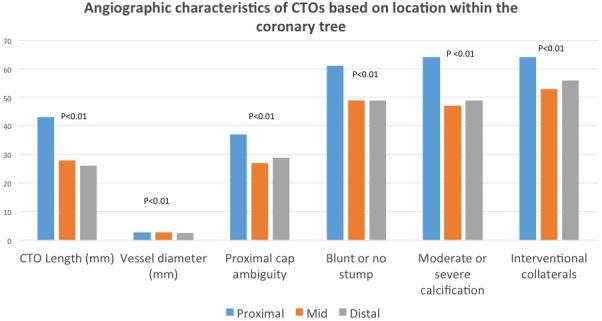
Angiographic characteristics of CTOs based on location within the coronary tree. Proximal lesions had more adverse angiographic features including proximal cap ambiguity, side branch at proximal cap, blunt or no stump, moderate or severe calcification and poor distal target vessel but had more interventional collaterals
Procedural strategies
Multiple crossing strategies were used in the overall cohort: antegrade wire escalation (75%), antegrade dissection re-entry (34%) and retrograde (40%). The use of retrograde approach was higher in proximal lesions (47%) relative to mid (33%) and distal (37%) lesions (p<0.001). Moreover, the retrograde approach was the first crossing strategy in 23% of patients with proximal lesions versus only 14% and 19% of patients with mid and distal lesions, respectively. Likewise, the retrograde approach was the successful crossing strategy in 34% of patients with proximal lesions versus 21% and 26% of patients with mid and distal lesions, respectively (Figure 2). CTO PCI of proximal lesions were more complex procedures requiring: a) higher contrast volume (275 ml, ±200-375) relative to procedures in the mid (260 ml,±200-350), and distal locations (250 ml,±175-350) (p=0.01), b) longer fluoroscopy time (proximal: 53 (32-83) minutes, mid:39 (24,65), and distal: 40 (22,72) (p<0.01) c) higher radiation exposure in air kerma dose (Gy) proximal: 4.0 (2.3-5.9), mid 3.2 (1.9-4.8), distal 3.4 (1.8-5.4) (p<0.01) and d) higher utilization of intraprocedural advanced imaging techniques (intravascular ultrasound use: proximal: 43%, mid: 36%, and distal: 28%, p=0.01).
Figure 2.
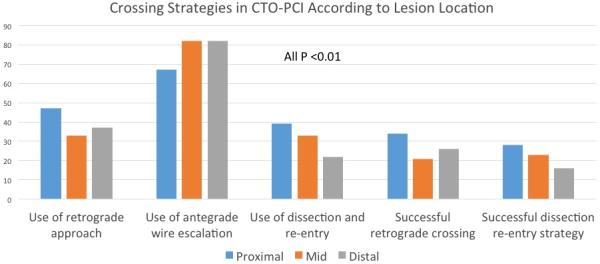
Crossing Strategies in CTO-PCI According to Lesion Location. The use of retrograde approach was higher in proximal lesions relative to mid and distal lesions
Procedural success and adverse events
Overall, procedural success was 88%. Despite higher angiographic and procedural complexity, the procedural success rate for proximal CTO-PCI was high (87%) and no different than mid (90%) or distal (85%) coronary lesions (p=0.14). Likewise, the incidence of serious major adverse events was low in the overall cohort (2.6%) with no inter-group differences according to lesion location (Figure 3).
Figure 3.
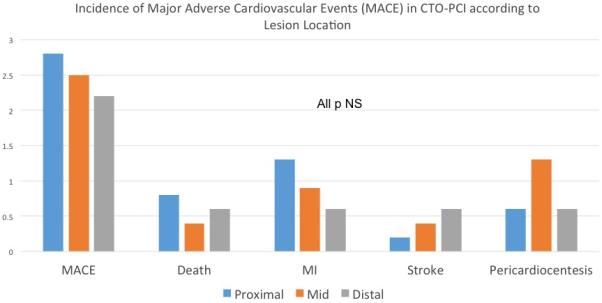
Incidence of Major Adverse Cardiovascular Events (MACE) in CTO-PCI according to Lesion Location. The incidence of serious major adverse events was low in the overall cohort with no inter-group differences according to lesion location.
Discussion
The main findings of this investigation are as follows. First, the most common target lesion location of CTO PCI within the coronary tree is the proximal third. Second, proximal lesions are associated with a higher burden of comorbidities, most notably heart failure and decreased ejection fraction. Third, proximal CTOs are associated with higher angiographic complexity, as quantified by the J-CTO score, resulting in longer and more complex procedures. Fourth, the retrograde approach was used in almost 50% of cases involving proximal CTO lesions and was the successful strategy in a third of these cases. Finally, despite increased procedural complexity associated with CTO-PCI of proximal lesions, procedural success and complications rates were similar to those of CTO-PCI of mid and distal locations.
Lesion Location and Prognosis
Most data from observational studies and randomized clinical trials suggests a relationship between myocardial ischemia and subsequent risk of death and myocardial infarction in patients with coronary artery disease (14,15). Coronary lesions located in the proximal third of the coronary artery are considered prognosticaly more important than lesions located in a more distal location due to the larger extent of myocardium at risk. This is reflected in the increased weight given to a more proximal lesion location in several angiographic scoring tools such as the Jeopardy or SYNTAX risk score (16,17). For example, the Jeopardy score (maximum number of possible points of 12) will assign 6 points to a 75% stenosis in the proximal LAD and only 2 to the same stenosis if located in the mid or distal portion of the same vessel. This has important prognostic implications; the 5-year survival of patients with single vessel disease and Jeopardy score of 2 is 97%. In contrast, the 5-year survival of patients with single vessel disease and Jeopardy score of 6 is significantly lower at 84% (16). Our analysis from the PROGRESS CTO registry show that most frequently CTO PCIs are performed to revascularize occlusions located within the proximal segment of the coronary tree, which are the ones that have the greatest potential to reduce ischemic burden, improve symptoms and clinical outcomes.
Lesion Location and Procedural Strategy
CTOs located in the proximal segment of the coronary tree had more complex angiographic features relative to distal lesions such as longer lesion length (15 mm longer on average), moderate or severe calcification, proximal cap ambiguity and a blunt stump (18). Conversely, the presence of interventional collaterals and adequate distal targets were commonly encountered in proximal stenosis all of which should be considered during pre-procedural planning. Absence of interventional collaterals is a predictor of technical failure in a recent study (19). Antegrade wire escalation and dissection re-entry have a limited role in CTO PCI of proximal coronary segments, particularly in ostial RCA occlusions where guide support and visualization can be challenging. Therefore, the presence, caliber and trajectory of interventional collaterals should be particularly considered prior to embarking in CTO PCI of proximal coronary segments. In this sub-study of PROGRESS, the retrograde approach was used in nearly half of cases involving a proximal segment and was the successful crossing strategy in one third. Experience with equipment and techniques used for retrograde CTO recanalization is essential. Despite all the procedural challenges associated with CTO PCI of proximal coronary segments, procedural success rates were high (90%) and comparable to CTO PCI in more distal locations. Procedural complications were low (2.8%) and in line with other CTO PCI procedures, highlighting the importance of adequate case selection and operator experience.
Limitations
This study has limitations. First, PROGRESS CTO is an observational registry without adjudication of clinical events by an independent committee. Second, detailed angiographic analysis was not performed by an independent core laboratory and therefore assessment of angiographic characteristics was susceptible to operator-related bias. Third, long-term follow-up of the CTO patients was not available. Finally, all procedures were performed by seasoned operators with significant CTO PCI training and extrapolation of the study results to centers with lower CTO PCI volumes should be made with caution.
Conclusions
CTO PCIs most commonly involve the proximal coronary segment and are associated with adverse clinical and angiographic characteristics. The retrograde approach is used in almost 50% of proximal CTO cases and yields high procedural and technical success rates and low complication rates.
Table 3.
Procedural Characteristic of CTO PCO according to lesion location
| Overall | Proximal | Mid | Distal | P | |
|---|---|---|---|---|---|
| Contrast volume (ml) | 260 (200, 360) | 275 (200, 375) | 260 (200, 350) | 250 (175, 350) | 0.01 |
| Fluorosccopy time (min) | 45 (27, 74) | 53 (32, 83) | 39 (24, 65) | 40 (22, 72) | <0.001 |
| Air kerma dose (Gy) | 3.5 (2.1, 5.5) | 4.0 (2.3, 5.9) | 3.2 (1.9, 4.8) | 3.4 (1.8, 5.4) | <0.001 |
| Procedure time (min) | 129 (88, 193) | 145 (98, 211) | 123 (83, 174) | 123 (72, 191) | <0.001 |
| VUS used (%) | 37 | 43 | 36 | 28 | 0.01 |
| Procedural success (%) | 88 | 87 | 90 | 85 | 0.14 |
| LVAD used (%) | 5 | 7 | 4 | 4 | 0.10 |
| Stent length (mm)* | 66±42 | 61±48 | 69±35 | 74±42 | <0.001 |
| Maximal stent diameter (mm)* | 3.1±0.5 | 3.2±0.5 | 3.0±0.5 | 3.0±0.6 | <0.001 |
IVUS: intravascular ultrasound, LVAD: left ventricular assist device
Values are mean +/− standard deviation or median (interquartile range)
Acknowledgement
Study data were collected and managed using REDCap electronic data capture tools hosted at University of Texas Southwestern Medical Center.1 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Funding: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health” under award Number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr; 42(2):377-81.
Compliance with Ethical Standards
Disclosure of potential conflicts of interest:
Dr. Garcia is a recipient of a career development award (1IK2CX000699-01) from the VA Office of Research and Development. Dr. Garcia is a consultant for Surmodics. Dr. Brilakis has received consulting and speaking honoraria from Abbott Vascular, Asahi, Boston Scientific,Elsevier, Somahlution, St. Jude Medical, and Terumo; and has received research support from InfraRedx and Boston Scientific; and his spouse is an employee of Medtronic. All other authors have nothing to disclose related to this manuscript.
Informed consent: The study was approved by the institutional review board at each site. Informed consent was waived.
References
- 1.Fuster V, Lewis A. Conner Memorial Lecture. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation. 1994;90:2126–46. doi: 10.1161/01.cir.90.4.2126. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr., Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–8. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 4.Schoenhagen P, Tuzcu EM, Ellis SG. Plaque vulnerability, plaque rupture, and acute coronary syndromes: (multi)-focal manifestation of a systemic disease process. Circulation. 2002;106:760–2. doi: 10.1161/01.cir.0000025708.36290.05. [DOI] [PubMed] [Google Scholar]
- 5.Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004;110:278–84. doi: 10.1161/01.CIR.0000135468.67850.F4. [DOI] [PubMed] [Google Scholar]
- 6.el Fawal MA, Berg GA, Wheatley DJ, Harland WA. Sudden coronary death in Glasgow: the severity and distribution of chronic coronary atherosclerotic stenoses. Br Heart J. 1987;57:420–6. doi: 10.1136/hrt.57.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RA, Zir LM, Harper RW, Leinbach RC, Hutter AM, Jr., Pohost GM, Block PC, Gold HK. Patterns of haemodynamic alteration during left ventricular ischaemia in man. Relation to angiographic extent of coronary artery disease. Br Heart J. 1979;41:441–51. doi: 10.1136/hrt.41.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dash H, Johnson RA, Dinsmore RE, Francis CK, Harthorne JW. Cardiomyopathic syndrome due to coronary arter disease. II: Increased prevalence in patients with diabetes mellitus: a matched pair analysis. Br Heart J. 1977;39:740–7. doi: 10.1136/hrt.39.7.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christopoulos G, Menon RV, Karmpaliotis D, Alaswad K, Lombardi W, Grantham JA, Michael TT, Patel VG, Rangan BV, Kotsia AP, Lembo N, Kandzari DE, Lee J, Kalynych A, Carlson H, Garcia S, Banerjee S, Thompson CA, Brilakis ES. Application of the “hybrid approach” to chronic total occlusions in patients with previous coronary artery bypass graft surgery (from a Contemporary Multicenter US registry). Am J Cardiol. 2014;113:1990–4. doi: 10.1016/j.amjcard.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Alaswad K, Menon RV, Christopoulos G, Lombardi WL, Karmpaliotis D, Grantham JA, Marso SP, Wyman MR, Pokala NR, Patel SM, Kotsia AP, Rangan BV, Lembo N, Kandzari D, Lee J, Kalynych A, Carlson H, Garcia SA, Thompson CA, Banerjee S, Brilakis ES. Transradial approach for coronary chronic total occlusion interventions: Insights from a contemporary multicenter registry. Catheter Cardiovasc Interv. 2015;85:1123–9. doi: 10.1002/ccd.25827. [DOI] [PubMed] [Google Scholar]
- 11.Christopoulos G, Wyman RM, Alaswad K, Karmpaliotis D, Lombardi W, Grantham JA, Yeh RW, Jaffer FA, Cipher DJ, Rangan BV, Christakopoulos GE, Kypreos MA, Lembo N, Kandzari D, Garcia S, Thompson CA, Banerjee S, Brilakis ES. Clinical Utility of the Japan-Chronic Total Occlusion Score in Coronary Chronic Total Occlusion Interventions: Results from a Multicenter Registry. Circ Cardiovasc Interv. 2015;8:e002171. doi: 10.1161/CIRCINTERVENTIONS.114.002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christopoulos G, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman RM, Lombardi WL, Menon RV, Grantham JA, Kandzari DE, Lembo N, Moses JW, Kirtane AJ, Parikh M, Green P, Finn M, Garcia S, Doing A, Patel M, Bahadorani J, Tarar MN, Christakopoulos GE, Thompson CA, Banerjee S, Brilakis ES. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222–8. doi: 10.1016/j.ijcard.2015.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Hinohara T, Tanaka H, Mitsudo K, Investigators JCR. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–21. doi: 10.1016/j.jcin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Stone GW, Hochman JS, Williams DO, Boden WE, Ferguson TB, Jr., Harrington RA, Maron DJ. Medical Therapy With Versus Without Revascularization in Stable Patients With Moderate and Severe Ischemia: The Case for Community Equipoise. J Am Coll Cardiol. 2016;67:81–99. doi: 10.1016/j.jacc.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, Investigators C. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 16.Califf RM, Phillips HR, 3rd, Hindman MC, Mark DB, Lee KL, Behar VS, Johnson RA, Pryor DB, Rosati RA, Wagner GS, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5:1055–63. doi: 10.1016/s0735-1097(85)80005-x. [DOI] [PubMed] [Google Scholar]
- 17.Serruys PW, Onuma Y, Garg S, Sarno G, van den Brand M, Kappetein AP, Van Dyck N, Mack M, Holmes D, Feldman T, Morice MC, Colombo A, Bass E, Leadley K, Dawkins KD, van Es GA, Morel MA, Mohr FW. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5:50–6. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
- 18.Sapontis J, Christopoulos G, Grantham JA, Wyman RM, Alaswad K, Karmpaliotis D, Lombardi WL, McCabe JM, Marso SP, Kotsia AP, Rangan BV, Christakopoulos GE, Garcia S, Thompson CA, Banerjee S, Brilakis ES. Procedural failure of chronic total occlusion percutaneous coronary intervention: Insights from a multicenter US registry. Catheter Cardiovasc Interv. 2015;85:1115–22. doi: 10.1002/ccd.25807. [DOI] [PubMed] [Google Scholar]
- 19.Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, Alaswad K, Lombardi W, Grantham JA, Moses J, Christakopoulos G, Tarar MN, Rangan BV, Lembo N, Garcia S, Cipher D, Thompson CA, Banerjee S, Brilakis ES. Development and Validation of a Novel Scoring System for Predicting Technical Success of Chronic Total Occlusion Percutaneous Coronary Interventions: The PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) Score. JACC Cardiovasc Interv. 2016;9:1–9. doi: 10.1016/j.jcin.2015.09.022. [DOI] [PubMed] [Google Scholar]


