Abstract
The incidence of type 2 diabetes mellitus (T2DM) among children and adolescents has been rising. This condition is associated with obesity, and the prevalence is higher among minority or female youth. Lifestyle modification including diet and exercise is only successful in a small portion of the patients; therefore, pharmacotherapy approaches are needed to treat T2DM among youth. Currently, in the United States, only metformin and insulin are approved for the treatment of T2DM in children. Several antihyperglycemic agents including exenatide, glimepiride, glyburide, liraglutide, pioglitazone and rosiglitazone are also used off-label in this population. Moreover, several clinical trials are ongoing that are aimed to address the safety and efficacy of newer antihyperglycemic agents in this population.
Little is known about the safety, efficacy or pharmacokinetics of antihyperglycemic agents in children or adolescents. Our ability to predict pharmacokinetics of these agents in youth is hampered first by the lack of information about the expression and activity of drug metabolizing enzymes and transporters in this population and second by the occurrence of additional conditions such as obesity and fatty liver disease. This manuscript reviews the prevalence of obesity and T2DM in children and adolescents (youth). We have then summarized published studies on safety and effectiveness of antihyperglycemic medications in youth. Drug disposition may be affected by age or puberty thus the expression and activity of different pathways for drug metabolism and xenobiotic transporters will be compared between youth and adults followed by summarizing pharmacokinetics studies of antihyperglycemic agents currently used in this population.
Keywords: diabetes mellitus, type 2, pharmacotherapy, antihyperglycemic, metformin
1. Introduction
In the past two decades, the incidence of type 2 diabetes mellitus (T2DM) in children has substantially increased. This increase is seen mostly in minority youth and is highly correlated with the presence of obesity [1]. The number of overweight children in the United States has more than doubled over the past 30 years, resulting in an increase in insulin resistance and T2DM among youth [2]. Other pediatric-specific risk factors include being overweight, family history of diabetes, and development of insulin resistance [2]. In addition, children who were not breastfed [3] or had low birth weight [4] may have an increased risk of T2DM. For example, in a Swedish study, individuals with lower birth weight had three-fold increased risk of T2DM by the age of 60 years [1]. Although, most cases of T2DM are polygenic, a genetic locus on chromosome 2, Non-Insulin-Dependent Diabetes Mellitus (Common, Type 2) 1 (NIDDM1 gene) is accounted for 30% of genetic susceptibility to this disease [1].
Due to the increased prevalence, it is evident that more research is needed on T2DM in children. An epidemiological study called SEARCH, identified the overall annual incidence rate of T2DM as 8.1 per 100,000 person-years in ages 10 to 14 and 11.8 per 100,000 person-years in ages 15 to 19 [5]. An age-specific incidence of 7.2:100,000 per year was found in Ohio, where one-third of all new cases of diabetes in ages 10 to 19 was identified as those with T2DM [1]. Notably, there is a four to six female to one male ratio suggesting that T2DM in youth is more common in the female population [1].
Similar to adults, the onset of T2DM in children is clinically manifested as polyuria, polydipsia, and polyphagia. Other manifestations include symptoms associated with insulin resistance including hypertension, lipid abnormalities, and acanthosis nigricans [2]. According to the American Diabetes Association, the primary prevention is to target modifiable risk factors such as weight loss through reduced caloric intake and increased physical activity. However, these lifestyle modifications may only be successful on a small scale [5].
Pharmacologic treatment with antihyperglycemic agents may be necessary to treat T2DM in children [1]. At this time, only metformin and insulin are approved by Food and Drug Administration of the United States for children with T2DM [6, 7]. More research is needed on the safe and effective use of other antihyperglycemic agents in this population [2].
Search strategy
The published literature was searched by the use of PubMed using search terms such as “type 2 diabetes” AND “children” with terms including “pharmacotherapy” “therapy” “antidiabetic agents” “antihyperglycemic agents” “pharmacokinetics” “pharmacodynamics”. Relevant publications were reviewed, summarized and included in this manuscript. Moreover, the website clinicaltrials.gov (https://clinicaltrials.gov/) was searched with the term “type 2 diabetes” limiting the age range “birth -17 years”. Past and on-going studies involving a pharmacotherapy approach were identified, and the drug name was included in PubMed searches.
1.1 Prevalence and pathophysiology of T2DM in children and adolescents
The World Health Organization’s definition of adolescence is between the ages of 10 and 19 years old which is associated with the beginning of the onset of physiologically normal puberty until when adult identity and behavior are established [8]. The prevalence of T2DM is higher in minority youth such as African-American, Hispanic, Asian/Pacific Islanders and American Indians in the United States. This disease often occurs in obese 12–19-year-old adolescents [9–17] and is more common in female youth than male which is opposite to the gender pattern in adults [18, 19].
Several social, behavioral, and environmental risk factors are associated with the manifestation of T2DM [20, 21]. The incidence of insulin resistance is significantly higher in youth with moderate or severe obesity, and it is significantly more prevalent in obese black than white children [22].
The peak of T2DM in children is around the age of mid-puberty due to increasing secretion of growth hormone (GH) leading to insulin resistance. Secretion of leptin, adiponectin, and tumor necrosis factor-alpha by adipose tissue can also change insulin secretion and sensitivity [23]. The major differences between children and adults at the onset of T2DM include (i) children have a higher body mass index (BMI) causing greater insulin resistance (ii) children have a lower glycated hemoglobin (HbA1c) due to a shorter latency period and less time for the development of glucose dysregulation and (iii) higher incidence of diabetes ketoacidosis (DKA) and glucose toxicity exist with children at diagnosis [24].
1.2 Clinical presentation of type 2 diabetes mellitus in youth
Obesity is the most important sign of T2DM [18] since many obese children are asymptomatic at the time of screening [25]. In the mildest form, an asymptomatic child must be diagnosed through screening or during a routine medical visit by detection of hyperglycemia and glycosuria that is usually without ketonuria. Absent or mild polyuria and polydipsia, and little or no weight loss are other features of an asymptomatic child with T2DM. Since children with autoimmune type 1 diabetes can also be obese [26] it is important to distinguish between type 1 from T2DM by measuring fasting insulin, C-peptide [26, 27] and islet autoantibodies [26, 28].
In children, with full blown T2DM, the severest form of polyuria, polydipsia, weight loss, ketonuria, and ketoacidosis are often present [18, 29, 30]. DKA can occur in approximately 25% of adolescent patients presenting with T2DM [14]. Serious complications such as hyperosmolar hyperglycemic nonketotic syndrome (HHNS) can commonly occur among youth with T2DM, which may cause increased fatality. [31] Additionally, in female patients, the polycystic ovarian syndrome may also be present [29]. Other complications such as lipid disorders and hypertension also occur more frequently in children with T2DM [30]. The presence of different co-morbidities in these children necessitates administration of other medications such as drugs to treat high blood pressure and hyperlipidemia resulting in increased incidence of drug-drug interactions and side effects.
1.3 Treatment of T2DM in youth
Management of T2DM in children and adolescents is limited to lifestyle modifications and pharmacotherapy [6]. Diet and exercise are recommended as initial interventions for T2DM in children. [4,28] However, most pediatric patients respond poorly to lifestyle intervention programs. [4,35] The addition of metformin has clinical advantages of decreasing weight, low-density lipoprotein cholesterol (LDL-C) and triglyceride levels in children without causing hypoglycemia [32]. If glycemic control is not sufficient on metformin, long-acting insulin may be recommended as an effective, adjunctive agent. Nevertheless, this regimen may not achieve therapeutic goals hence other antihyperglycemic agents may be needed [29]. Although few studies have been performed in children with T2DM [33], in this article, we have evaluated available studies on efficacy, safety, and pharmacokinetics of antihyperglycemic agents used in the pediatric population.
2. Safety and efficacy of antihyperglycemic agents in youth
In addition to metformin that is FDA-approved, several other antihyperglycemic agents are used off-label in the pediatric population. Available clinical trials on safety and efficacy will be summarized below and in Table 1:
Table 1.
Summary results of clinical trials of antihyperglycemic agents in youth with type 2 diabetes
| Medication Drug Class |
Study Dosage | Age range Number of subjects (n) | Adverse Drug Effects | Results |
|---|---|---|---|---|
| Metformin [34] Biguanide |
500 mg twice daily titrated up to 1000 mg twice daily if FPG>126mg/dl | 10–16 years n=82 | gastrointestinal irritation, including diarrhea, cramps, nausea, vomiting, and increased flatulence rarely lactic acidosis | ↓ both HbA1c and FPG Positive effect on body weight and lipid profile. |
| Metformin [35] Biguanide |
1000 mg twice daily | 6–12 years n=100 | Modest but considerable effects on body weight, body composition, and glucose homeostasis. | |
| Metformin [36] Biguanide |
Median 14 years n=1092 | ↓ HbA1c, body mass index, LDL-C, triglycerides and blood pressure. | ||
| Glimepiride [42] Sulfonylurea |
1–8 mg once-daily; mean final dose 3.8 mg/day for 24 weeks. | 8–17 years n=142 | Hyper/hypoglycemia, Abdominal pain, diarrhea, nausea, greater weight gain than metformin alone | ↔ self-monitored glucose hypoglycemia incidence in comparison with metformin. |
| Metformin/glyburide [44] Biguanide/Sulfonylurea |
Met/gly:205/1.25 mg Met: 500 mg Gly: 2.5 mg |
9–16 years n=167 | For glyburide: hypoglycemia and weight gain | Average HbA1c decreased in all three groups |
| Rosiglitazone [43] Thiazolidinedione |
2 mg twice daily titrated up to 4 mg twice daily if FPG>126mg/dl | 10–17 years n=200 | Hyper/hypoglycemia, diabetic ketoacidosis, rash, GI symptoms, edema | Mean differences in HbA1c |
| Liraglutide Glucagon-like peptide-1 receptor agonist |
1.8 mg once-daily | 10–17 years Liraglutide (n=14) placebo (n=7) |
mild GI symptoms, nausea, diarrhea, hypoglycemia | ↓ HbA1c and FPG and no effect on weight [47] |
2.1 Metformin monotherapy
In a randomized, double-blind, placebo-controlled trial, the safety and efficacy of metformin were evaluated in 82 individuals, aged 10–16 years for up to 16 weeks. The selected dose was 500 mg twice daily which was titrated up to 1000 mg twice daily if fasting plasma glucose (FPG) >126. In addition to decreasing both HbA1c and FPG, reduction in body weight and improvement of the lipid profile was observed. Adverse events were similar to those reported in adults treated with metformin. Therefore, metformin appears to be safe and effective for treatment of T2DM in pediatric patients [34]. In another study, the effect of metformin therapy on body weight was evaluated. One hundred severely obese, insulin-resistant 6–12 year olds were randomized to 1,000 mg metformin (n=53) or placebo (n=47) twice daily for 6 months. Metformin had modest but considerable effects on body weight, body composition, and glucose homeostasis [35]. In another cohort of adolescent T2DM participants (n=1092), parameters such as HbA1c, BMI, LDL-C, triglycerides and blood pressure have improved. Treatment with metformin provided short-term improvements in glycemic control and cardiometabolic risk factors, and nearly all insulin-treated youth were eventually weaned off insulin (Table 1) [36].
Although metformin studies reported clinical benefit, evidence from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study suggests metformin monotherapy is ineffective in maintaining glycemic control for about 50% of patients within one year of treatment. Pancreatic beta cell function deteriorated at a rate of 20–35% per year in the participants of the TODAY study, which is considerably higher than adult studies (7–11% decline per year) [37–39]. Over 4 years, hypertension and microalbuminuria rates increased to 33.8% from 11.6% and to 16.6% from 6.3% at baseline, respectively. Only 55.9% of the youth met the (LDL-C) level goal after 3 years, and 13.7% had retinopathy.
Bariatric surgery can be the last resort therapeutic intervention for the treatment of T2DM even in children and adolescent patients [40]. The effect of gastric bypass surgery on absorption and bioavailability of metformin was investigated in adult patients. Bioavailability of metformin in Roux-en-Y gastric bypass (RYGB) adult patients was increased by 50% [41]. In addition, the values of volume of distribution (Vd) and renal clearance [41] were higher. Glucose levels were significantly lower in bypass subjects; however, these results were likely due to baseline differences in glucose levels instead of metformin absorption. Because many of RYGB patients remain obese and may redevelop T2DM, metformin absorption was of clinical relevance.
2.2 Metformin and glimepiride
Two additional efficacy and safety trials were conducted in the adolescent population with T2DM [42, 43]. The first trial compared glimepiride (1–8 mg/day) and metformin (500–1000 mg twice daily) for 24 weeks (12-week titration and 12-week maintenance periods) [42]. Patients with T2DM on metformin therapy were randomized to receive glimepiride + metformin versus metformin monotherapy. The incidence of events were 7.7% for glimepiride-treated patients and 13.4% for metformin-treated patients [42] (Table 1). Reductions from baseline HbA1c were observed in both glimepiride (−0.54%, p = 0.001) and metformin (−0.71%, p = 0.0002) groups. Further efficacy results found 42.4% of glimepiride-treated and 48.1% of metformin-treated patients achieved HbA1c < 7% at week 24. Secondary findings observed no differences in self-monitored glucose levels, changes in lipid concentrations, or hypoglycemia incidence between the antihyperglycemic agents. However, mean change in BMI from baseline was significantly different (+0.26 vs. −0.33 kg/m2) in the glimepiride and metformin groups, respectively. Even though glimepiride patients had greater weight gain, the researchers concluded the two drugs had comparable safety and similarly reduced HbA1c [42].
2.3 Metformin and glyburide
In another study, in 9 to 16 years old children with T2DM, metformin monotherapy, and glyburide (glibenclamide), monotherapy was compared with metformin + glyburide. After 26 weeks, mean HbA1c declined in all three groups: metformin/glyburide, metformin alone, and glyburide alone, but the combination therapy failed to show superiority to the monotherapies. [44].
2.4 Metformin and rosiglitazone
In TODAY study, the efficacy of 3 treatment regimens, metformin alone or metformin + rosiglitazone 4 mg twice daily or metformin + lifestyle intervention were compared [43]. Patients aged 10 to 17 years with T2DM who were treated with metformin up to 1000 mg twice daily for at least two months, were randomized into different groups. Evaluation of glycemic control defined as a HbA1c level of <8% was the primary objective. Of the 699 study participants, 45.6% achieved glycemic control for an average of 3.86 years. Failure rates were 51.7%, 38.6% and 46.6% for metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle intervention, respectively. The proportion of patients free of glycemic failure was significantly better for metformin–rosiglitazone than metformin alone (P=0.006). There was no statistically significant difference when compared to metformin plus lifestyle intervention versus metformin alone. Subgroup analysis showed metformin + rosiglitazone was more effective in girls not in boys [43]. Further concerns of serious adverse effects reported in adults with thiazolidinediones limit the use of rosiglitazone in children and adolescents [6].
2.5 Liraglutide
A long-acting Glucagon-like peptide-1 (GLP-1) receptor agonist is used off-label in T2DM youth [45] In a publication by Klein et al., liraglutide administration was compared to placebo in T2DM adolescents, aged 10–17 years, who were treated with diet/exercise alone or with metformin[46]. No serious adverse effects were observed, including severe hypoglycemia. Mild gastrointestinal side effects were more common in the liraglutide group[47] although unlike adult, no weight loss was observed in this population [45]. A subsequent efficacy and safety trial of liraglutide compared to metformin is currently recruiting participants within the pediatric population (clinicaltrials.gov: NCT01541215).
2.6 Ongoing studies with other agents
According to Clinicaltrials.gov, a safety and efficacy study of another GLP-1 agonist, exenatide once-weekly (NCT01554618) is currently underway. Further studies are also ongoing aiming to assess the use of DPP-4 inhibitors (alogliptin, linagliptin, sitagliptin, and saxagliptin) and Sodium-Glucose co-transporter 2 (SGLT2) inhibitors (canagliflozin, dapagliflozin, empagliflozin) in the pediatric population but as of July 2016, limited published results were available.
3. Pharmacokinetic considerations of the use of antihyperglycemic agents in youth
Since T2DM mostly occurs during later stages of adulthood, little data is available on the pharmacokinetics of antihyperglycemic agents in children or adolescents. In children, several factors including developmental age of the child, severity of obesity and degree of insulin resistance can cause changes in pharmacokinetics. It will be difficult to predict the pharmacokinetics of each drug in T2DM children across the age range of 10 to 19 especially as puberty occurs during the same period. The presence of co-morbid conditions like NAFLD [48] and hyperlipidemia further complicates this relationship. In the absence of formal pharmacokinetic studies, predictive tools including physiologically based pharmacokinetics (PBPK) modeling, as implemented in Simcyp (Certara) or Gastroplus (Simulations Plus), will be highly advantageous for predicting the pharmacokinetic parameters and required drug dose in a T2DM youth.
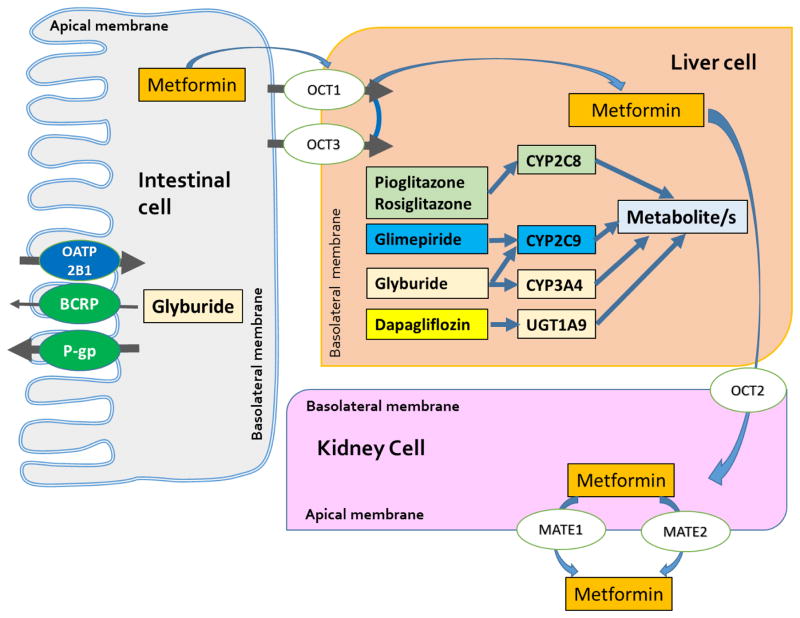
Current knowledge of the effect of disease state (i.e. obesity, diabetes, fatty liver) on pharmacokinetics is incomplete but advances in PBPK will eventually empower pharmacokinetic prediction tools when a combination of several disease states are present. Figure 1 depicts the pharmacokinetic pathways of the majority of antihyperglycemic agents that are used in children and adolescents. Fortunately, based on the elimination pathways, the drug-drug interaction potential for most antihyperglycemic agents used in children and adolescents is low to intermediate (Table 2). However, altered expression and activity of metabolizing enzymes or transporters in children cannot be dismissed nor the fact the other co-morbid conditions such as obesity, diabetes, and NAFLD can influence such pathways.
Figure 1.
Pharmacokinetics pathways of several small molecule antihyperglycemic agents used in children and adolescents with type 2 diabetes mellitus
Table 2.
Summary of drug disposition pathways relevant to disposition of antihyperglycemic agents used in children and adolescent with type 2 diabetes. Arrows indicate the available evidence on altered expression of each pathway in the youth in comparison with adult data. Data on the route of elimination and DDI risk level was extracted from University of Washington Drug Interaction Database.
| Drug Name | Routes of Elimination | Youth versus adult expression or activity | DDI Risk Level | |
|---|---|---|---|---|
| Enzymes | Transporters | |||
| Dapagliflozin | Extensive metabolism | UGT1A9 ↔ [79] | P-gp ↓ (NS) [69] Kidney OAT3 ??? |
No or low |
| Exenatide | Renal Excretion (unchanged) | No or Low | ||
| Glimepiride | Extensive Metabolism | CYP2C9 ↔ [80] | No or Low | |
| Glyburide | Extensive Metabolism | CYP2C9 ↔ [80] CYP3A4 ↔ [81] |
P-gp ↓ (NS) [69] OATP2B1↔ [69] BCRP ↑ (NS) [69] |
Intermediate |
| Liraglutide | Extensive metabolism | DPP-4s and neutral endopeptidases | No or low | |
| Metformin | Renal Excretion (unchanged) | OCT1 ↔ [69] OCT3 ??? OCT2 ??? MATE1 ↔ [69] MATE2 ? |
No or Low | |
| Pioglitazone | Extensive Metabolism | CYP2C8 ↔[67] | Intermediate | |
| Rosiglitazone | Extensive Metabolism | CYP2C8 ↔[67] | Intermediate | |
DDI: Drug-Drug Interaction; NS: not statistically different
3.1 Influence of diabetes and associated condition on drug disposition
Diabetes mellitus and associated conditions such as obesity and Non-Alcoholic Fatty Liver Disease (NAFLD) variably influence pharmacokinetics and the expression and activity of drug metabolizing enzymes and xenobiotic transporters [49–51]. Although, only anecdotal information is available on the effect of diabetes on pharmacokinetics in children and youth with diabetes. As reviewed previously [51, 52], pathological damages associated with hyperglycemia can influence all aspects of drug disposition. First, drug absorption can be delayed because of delayed gastric emptying time and changes in the gastrointestinal pH and motility. Second, plasma protein binding of drugs is altered because of increased concentration of glycated end products (i.e. increased albumin glycation) and dyslipidemia. Third, the expression and activity of several drug metabolizing enzymes is altered including significantly lower expression and activity of CYP3A4 [49] and UGT2B7 [50]. Forth, renal excretion of drugs is reduced once kidney function is compromised because of diabetic nephropathy [51]. These changes will collectively affect drug concentration and may lead to altered effect. Moreover, the effect of diabetes on pharmacokinetics cannot be viewed in isolation from other co-morbid conditions such as obesity [53] and NAFLD [54] both of which are known to influence pharmacokinetics.
3.2 ADME in children and adolescents
Generally, physiological differences in childhood and adolescence can affect the absorption, distribution, metabolism, and excretion of various drugs. [55, 56] A systematic review of 20 pharmacokinetics studies in obese children identified significantly altered pharmacokinetics in 65% of the cases. Despite this, consensus about weight-based dosing in obese children is currently lacking [57].
Absorption
Maturation changes in gastric and intestinal pH, gastrointestinal (GI) emptying time, circulation, enzyme activity, and the differences in GI flora may influence drug absorption [56, 58].
Distribution
Increased total body water–to–body fat ratio, decreased binding proteins in plasma and differences in tissue binding will affect Vd. The alteration in Vd will depend on the physiochemical characteristics of a given drug [56, 58].
Renal elimination
Renal function is 25–30% of adult values at birth, increasing to 50–75% by six months, and to adult level by age 2–3 years. Therefore, for drugs undergoing renal elimination, reduction of the drug dose must be considered although it is unlikely that T2DM develop at such young age [56, 59].
3.3 Maturation of drug metabolizing enzymes and transporters
After birth, the activities of drug metabolizing enzymes (DME) undergo change due to physical and sexual maturation. Cytochrome P450 (CYP) enzymatic activity is about 50–70% of adult levels at birth but will reach and exceed adult values afterward. Then by puberty, as hormone levels increase, CYP enzymatic activity will reach adult levels.
The expression of drug metabolizing enzymes is related to several the concentration of hormones such as growth and/or sex hormones. Puberty is marked by a coordinated and hormonally regulated physical and sexual maturation. Typically the concentration of gonadotropins, sex steroids, adrenal androgens and GH is increased during puberty which may differentially affect the expression of different DMEs between male and female. Increased GH and insulin-like growth factor-1 (IGF-1) are known to reduce the expression of CYP1A2 as characterized by caffeine breath test. CYP1A2, CYP2B6, CYP2C19 and CYP2E1 expression are mostly mediated by estrogen whereas others, such as CYP3A4, are mostly regulated by androgens and progesterone [60]. A decrease in global CYP450 activity, characterized by antipyrine pharmacokinetics, was noted in children and adolescents undergoing puberty [61].
Although CYP2C function is deficient in neonates [62–65] by puberty, CYP2C enzymatic activity will reach the adult levels. P450 activity is increased in children compared to adults, but it is not because of the amount of hepatic microsomal proteins. The activity of CYP1A2, CYP2C, and CYP3A are increased but the reason for higher clearance in children than adults is still unclear [66]. A study evaluating catalytic activity of six CYP isozymes, CYP1A2, CYP3A4/3A5, CYP2C8, CYP2C9, and CYP2E1 did not find a difference in enzyme activity in pediatric liver (younger than ten years) compared with adult livers [67]. Another study that investigated the effect of age, gender and genetic polymorphism on the expression of CYP2C8 protein did not find any significant difference between livers from 10–19 year old versus another age group [68]. A proposed explanation for the increased metabolic activity is the increased ratio of liver size to body size in children [56]. In addition to the effect of age on the maturation of CYP450 enzymes as obesity concurrently happens with T2DM in children, its effect on metabolism must be considered.
Recently Prasad and colleagues [69] have measured the expression of various transporters in the liver of neonate, children, adolescents, and adults using a targeted proteomics method. The expression of OCT1, OATP1B3, P-gp and MRP3 proteins were age-dependent. However, in the adolescent liver, only MRP3 expression was significantly lower than in adult liver [69] (Table 2).
4. Pharmacokinetic studies of antihyperglycemic agents in youth
The information about pharmacokinetic properties of antihyperglycemic agents in youth is incomplete. However, several published studies do not indicate grossly different pharmacokinetic properties between adolescents and adults.
4.1 Metformin
Metformin is eliminated in the urine and several organic cation transporters (OCTs) play an important role in metformin disposition (Figure 1). No information is available on the expression of OCTs in kidney from youth (Table 2).
Pharmacokinetic properties of metformin were investigated in a study that included 32 subjects aged 12–16 years; metformin was given to the patients 500 mg twice daily for one week. Pharmacokinetic parameters were determined by blood and urine samples analysis, which were obtained over an 11-hour period. Area under the plasma concentration–time curve (AUC), peak plasma concentration (Cmax), and elimination half-life (t1/2) values for metformin were 4.49 mg*h/mL, 0.73 mg/mL, and 3.7 hours, respectively. Systemic drug exposure (metformin AUC) in children was only 54% of that reported in adults with the same dosing [34]. Another study of single-dose metformin pharmacokinetics in patients 12 to 16 years of age showed less than a 5% difference in Cmax, AUC, and half-life when compared with healthy adult subjects [70].
In one other study, the pharmacokinetics of metformin in young, non-obese girls were investigated. The study population consisted of six 9-year-old girls who had a low birth weight and an early-normal onset of puberty. The receiving dose of metformin was 850 mg/day for eight months. Blood samples were obtained from the girls before metformin intake and for 12 hours afterward. The mean AUC was 21 mg*h/L, with a Cmax of 3 mg/L, tmax of 2.5 hours, t½ of 4 hours, Vd of 111 L and CL of 20 L/h. These values are comparable to those observed in adults [71].
4.2 Glimepiride
In the glimepiride pediatric development study, the pharmacokinetics of a 1 mg glimepiride single dose was evaluated [42]. Values of AUC, Cmax and tmax were 338.8 ng*h/mL, 102.4 ng/mL and 1 hour. Although, negative correlations were observed between drug clearance with age and weight, this association did not warrant dose adjusting. [42] Pharmacokinetics of glimepiride was studied in obese adult and children[72]. Glimepiride, a CYP2C9 substrate, is metabolized to the active M1 hydroxyl metabolite. Hepatic expression of CYP2C9 is comparable between adolescents and adults (Table 2). Clearance of parent glimepiride and metabolite M1 was not significantly different in obese versus non-obese T2DM patients. Cumulative urine excretion of M1 over 24 hours post-dose was 30% higher in obese versus non-obese subjects, while both groups received equal doses. Weight-normalized clearance values show a slight decrease in CYP2C9-mediated clearance per kilogram of total body weight [72].
4.3 Glyburide
A pharmacokinetics study was conducted for a combination therapy by glyburide/metformin and included 28 participants aged 10–16 years. Following the administration of a single 1.25 glyburide 250 mg metformin dose, blood samples were collected at selected time points over a 24-hour period. Values of AUC, Cmax, and tmax for glyburide were 167 ng*h/mL, 41.3 ng/mL, and 1 hour and for metformin were 3011 ng*h/mL, 473 ng/mL, and 2 hours. The pediatric PK parameters for the metformin/glyburide combination were only slightly different from adult pharmacokinetic parameters [73].
4.4 Pioglitazone
Pioglitazone is a CYP2C8 substrate that undergoes extensive hepatic biotransformation (Table 2). A small pharmacokinetic study assessed single- and multiple-doses of pioglitazone in 36 adolescents with T2DM [74]. Participants were aged 12 to 17 years and received once-daily doses of 15 mg, 30 mg or 45 mg pioglitazone, and the pharmacokinetics was studied as a single dose or after receiving multiple doses for 15 days. The concentration of pioglitazone and active metabolites (M-III and M-IV) were measured by LC-MS/MS. Pharmacokinetic parameters of pioglitazone were not significantly different from those observed in adult patients. Accumulation of parent pioglitazone was negligible, but M-III and M-IV concentrations at steady-state reached 3-fold higher concentration than single dose concentration. Pioglitazone was well-tolerated in the study population yet seven out of 37 subjects were experienced drug-related adverse events (AEs). These AEs were mild to moderate and when pioglitazone was discontinued.
4.5 Rosiglitazone
Rosiglitazone pharmacokinetic was evaluated following the administration of a single dose of 2 or 4 mg in patients aged 10–17 years. Values of tmax, oral clearance (CL/F), and oral volume of distribution (V/F) were 1.5 hours, 3.15 L/h, and 13.5 L. Predicted rosiglitazone AUC based on twice daily doses of 2 and 4 mg were 1520 and 3040 ng*h/mL indicating dose linearity. Pediatric pharmacokinetic parameters were found to be consistent with data from adults [24].
4.6 Exenatide
Pharmacokinetics and tolerability of a single dose subcutaneously administered exenatide was investigated in T2DM adolescent [75]. Exenatide is predominantly eliminated by glomerular filtration after undergoing proteolytic degradation [76]. The study enrolled 13 patients, aged 10–16 years, who were treated with diet/exercise or a stable dose of metformin, a sulfonylurea, or a combination of metformin and sulfonylurea for at least 3 months before screening to receive a single dose of exenatide (2.5 μg or 5 μg) or placebo on 3 separate days [75]. Exposure to exenatide was 2-fold higher in 5 μg dose group as compared with 2.5 μg. Values of AUC0–360min and Cmax obtained after 5 μg dose were comparable with those values obtained in adult studies with exenatide. Both doses significantly reduced postprandial glucose excursion as compared with placebo and resulted in lower glucagon concentration. However, the concentration of serum insulin was not different [75].
4.7 Liraglutide
Liraglutide is a long-acting GLP-1 agonist with peptide structure. It is extensively metabolized predominantly by dipeptidyl peptidase 4 (DPP-4) and neutral endopeptidases and is entirely eliminated renally as breakdown products. Liraglutide pharmacokinetics in youth were comparable to the adult and showed linear dose-concentration relationship. For a 1.8 mg dose, liraglutide tmax was 8 hour, half-life was 12 hour and apparent clearance was 1.7 L/h [47]. Population pharmacokinetics analysis was used to compare liraglutide pharmacokinetics obtained from one pediatric trial with two adult trials [77]. The geometric mean and 95% confidence interval of relative exposure (AUC) for pediatric/adult was 0.90 [0.78; 1.03] [77]. Liraglutide exposure was significantly higher in subjects with smaller body weight and was lower in male than female subjects [77].
4.8 Dapagliflozin
Pharmacokinetics of dapagliflozin was studied in children and adolescents (10–17 years old) with type 2 diabetes [78]. Dapagliflozin inhibits subtype 2 of the sodium-glucose transport proteins (SGLT2) thereby significantly reduces glucose reabsorption in the kidney. Dapagliflozin is metabolized by UGT1A9 to a major metabolite dapagliflozin 3-O-glucuronide, and this metabolite is inactive [78]. This route of metabolism is absent at birth but reaches adult level by the age of 1-year old [79]. Moreover, previous studies in liver from subjects with diabetes did not find a significant effect of diabetes on the UGT1A9 expression or activity [50]. Dapagliflozin was well-tolerated between the doses of 2.5–10 mg, and its pharmacokinetics properties did not differ significantly from adult patients.
5. Conclusions
In conclusion, there is a significant increase in the prevalence of obesity and T2DM among children and adolescents. The off-label use of antihyperglycemic agents in this population appears to be unavoidable given the limited therapeutic options in this population. Metformin monotherapy or in combination with other antihyperglycemic agents appear to be safe. Several clinical trials are underway to investigate the safety and effectiveness of other antihyperglycemic agents in the youth with T2DM. Pharmacokinetic characteristics of antihyperglycemic agents can be influenced by age and maturity or the presence of obesity, diabetes or NAFLD. Conventional pharmacokinetic studies with a smaller number of subjects maybe of limited value because such studies may not capture the presence of all sources of variability.
Key findings.
Type 2 diabetes is a major health concern among children and adolescents (youth).
Currently, only insulin and metformin are FDA approved in the youth.
Other antihyperglycemic agents are used off-label in the youth without adequate information on the safety and effectiveness of these agents.
A combination of diabetes, obesity, non-alcoholic fatty liver disease and hormonal fluctuations of puberty may variably influence the pharmacokinetics and pharmacodynamics of antihyperglycemic agents in the youth.
Acknowledgments
Funding: Support of grant # R15 GM101599 from the National Institutes of Health is gratefully acknowledged.
Use of University of Washington Drug Interaction Database is gratefully acknowledged.
List of Frequently Used Abbreviations
- AUC
Area Under the Concentration-time Curve
- BMI
Body mass index
- CL
Clearance
- Cmax
Maximum concentration
- CYP
Cytochrome P450
- GLP-1
Glucagon-like peptide-1
- HbA1c
Glycated hemoglobin
- NAFLD
Non Alcoholic Fatty Liver Disease
- PBPK
Physiologically based pharmacokinetics
- RYGB
Roux-en-Y gastric bypass
- T2DM
Type 2 diabetes mellitus
- tmax
Time to maximum concentration
- TODAY
Treatment Options for Type 2 Diabetes in Adolescents and Youth
- Vd
Volume of distribution
Footnotes
Conflict of Interest: Author FA, Author AHM, and Author AK, Author MK and Author KLM declare that they have no conflict of interest.
References
- 1.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999 Feb;22(2):345–54. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 2.Gaylor AS, Condren ME. Type 2 diabetes mellitus in the pediatric population. Pharmacotherapy. 2004 Jul;24(7):871–8. doi: 10.1592/phco.24.9.871.36099. [DOI] [PubMed] [Google Scholar]
- 3.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015 Dec;104(467):30–7. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 4.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008 Dec 24;300(24):2886–97. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 5.Mayer-Davis EJ. Type 2 diabetes in youth: epidemiology and current research toward prevention and treatment. J Am Diet Assoc. 2008 Apr;108(4 Suppl 1):S45–51. doi: 10.1016/j.jada.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Copeland KC, Silverstein J, Moore KR, Prazar GE, Raymer T, Shiffman RN, et al. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013 Feb;131(2):364–82. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 7.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015 Sep;1353:113–37. doi: 10.1111/nyas.12939. [DOI] [PubMed] [Google Scholar]
- 8.Society CP. Age limits and adolescents. Paediatr Child Health. 2003 Nov;8(9):577–8. doi: 10.1093/pch/8.9.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schober E, Holl RW, Grabert M, Thon A, Rami B, Kapellen T, et al. Diabetes mellitus type 2 in childhood and adolescence in Germany and parts of Austria. European journal of pediatrics. 2005;164(11):705–7. doi: 10.1007/s00431-005-1709-9. [DOI] [PubMed] [Google Scholar]
- 10.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129(6):1035–41. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 11.Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. Jama. 2007;297(24):2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 12.Group SfDiYS. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–8. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 13.Rotteveel J, Belksma EJ, Renders CM, Hirasing RA, Delemarre-Van de Waal HA. Type 2 diabetes in children in the Netherlands: the need for diagnostic protocols. European journal of endocrinology. 2007;157(2):175–80. doi: 10.1530/EJE-06-0754. [DOI] [PubMed] [Google Scholar]
- 14.Fagot-Campagna A, Pettitt DJ, Engelgau MM, Burrows NR, Geiss LS, Valdez R, et al. Type 2 diabetes among North adolescents: An epidemiologic health perspective. The Journal of pediatrics. 2000;136(5):664–72. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. New England Journal of Medicine. 2002;346(11):802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 16.Wabitsch M, Hauner H, Hertrampf M, Muche R, Hay B, Mayer H, et al. Type II diabetes mellitus and impaired glucose regulation in Caucasian children and adolescents with obesity living in Germany. International journal of obesity. 2004;28(2):307–13. doi: 10.1038/sj.ijo.0802555. [DOI] [PubMed] [Google Scholar]
- 17.Pinhas-Hamiel O, Lerner-Geva L, Copperman NM, Jacobson MS. Lipid and insulin levels in obese children: changes with age and puberty. Obesity. 2007;15(11):2825–31. doi: 10.1038/oby.2007.335. [DOI] [PubMed] [Google Scholar]
- 18.Reinehr T. Clinical presentation of type 2 diabetes mellitus in children and adolescents. International Journal of Obesity. 2005;29:S105–S10. doi: 10.1038/sj.ijo.0803065. [DOI] [PubMed] [Google Scholar]
- 19.Zdravkovic V, Daneman D, Hamilton J. Presentation and course of Type 2 diabetes in youth in a large multi-ethnic city. Diabetic medicine. 2004;21(10):1144–8. doi: 10.1111/j.1464-5491.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiess W, Böttner A, Raile K, Kapellen T, Müller G, Galler A, et al. Type 2 diabetes mellitus in children and adolescents: a review from a European perspective. Hormone Research in Paediatrics. 2003;59(Suppl 1):77–84. doi: 10.1159/000067829. [DOI] [PubMed] [Google Scholar]
- 21.Florez JC. The genetics of type 2 diabetes: a realistic appraisal in 2008. The Journal of Clinical Endocrinology & Metabolism. 2008;93(12):4633–42. doi: 10.1210/jc.2008-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. New England Journal of Medicine. 2004;350(23):2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 23.Roth CL, Reinehr T. Roles of gastrointestinal and adipose tissue peptides in childhood obesity and changes after weight loss due to lifestyle intervention. Archives of pediatrics & adolescent medicine. 2010;164(2):131–8. doi: 10.1001/archpediatrics.2009.265. [DOI] [PubMed] [Google Scholar]
- 24.Christensen ML, Franklin BE, Momper JD, Reed MD. Pediatric drug development programs for type 2 diabetes: A review. The Journal of Clinical Pharmacology. 2015 doi: 10.1002/jcph.497. [DOI] [PubMed] [Google Scholar]
- 25.Gungor N, Hannon T, Libman I, Bacha F, Arslanian S. Type 2 diabetes mellitus in youth: the complete picture to date. Pediatric Clinics of North America. 2005;52(6):1579–609. doi: 10.1016/j.pcl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Association AD. Type 2 diabetes in children and adolescents. Pediatrics. 2000;105(3):671–80. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 27.Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. The Journal of Clinical Endocrinology & Metabolism. 2013 doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilliam LK, Brooks-Worrell BM, Palmer JP, Greenbaum CJ, Pihoker C. Autoimmunity and clinical course in children with type 1, type 2, and type 1.5 diabetes. Journal of autoimmunity. 2005;25(3):244–50. doi: 10.1016/j.jaut.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. Type 2 diabetes mellitus in the child and adolescent. Pediatric diabetes. 2008;9(5):512–26. doi: 10.1111/j.1399-5448.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 30.Arslanian SA. Type 2 diabetes mellitus in children: pathophysiology and risk factors. Journal of Pediatric endocrinology and Metabolism. 2000;13(Supplement):1385–94. doi: 10.1515/jpem-2000-s612. [DOI] [PubMed] [Google Scholar]
- 31.Flint A, Arslanian S. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34(Supplement 2):S177–S83. doi: 10.2337/dc11-s215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IDF I. Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence. IDF; 2011. available at: https://wwwispadorg/sites/default/files/resources/files/idf-ispad_diabetes_in_childhood_and_adolescence_guidelines_2011_0pdf. [Google Scholar]
- 33.Benavides S, Striet J, Germak J, Nahata MC. Efficacy and safety of hypoglycemic drugs in children with type 2 diabetes mellitus. Pharmacotherapy. 2005 Jun;25(6):803–9. doi: 10.1592/phco.2005.25.6.803. [DOI] [PubMed] [Google Scholar]
- 34.Jones KL, Arslanian S, Peterokova VA, Park J-S, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes a randomized controlled trial. Diabetes Care. 2002;25(1):89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 35.Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, et al. Effects of Metformin on Body Weight and Body Composition in Obese Insulin-Resistant Children A Randomized Clinical Trial. Diabetes. 2011;60(2):477–85. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler PS, et al. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatric diabetes. 2015 doi: 10.1111/pedi.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, et al. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes. 2011 May;60(5):1552–60. doi: 10.2337/db10-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med. 1998 Apr;15(4):297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Kahn SE, Montgomery B, Howell W, Ligueros-Saylan M, Hsu CH, Devineni D, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001 Dec;86(12):5824–9. doi: 10.1210/jcem.86.12.8105. [DOI] [PubMed] [Google Scholar]
- 40.Reinehr T. Type 2 diabetes mellitus in children and adolescents. World journal of diabetes. 2013;4(6):270. doi: 10.4239/wjd.v4.i6.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padwal RS, Gabr RQ, Sharma AM, Langkaas LA, Birch DW, Karmali S, et al. Effect of gastric bypass surgery on the absorption and bioavailability of metformin. Diabetes Care. 2011 Jun;34(6):1295–300. doi: 10.2337/dc10-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care. 2007 Apr;30(4):790–4. doi: 10.2337/dc06-1554. [DOI] [PubMed] [Google Scholar]
- 43.Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012 Jun 14;366(24):2247–56. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.FDA Statistical Review and Evaluation CSG. 2004.
- 45.Micale SJ, Kane MP, Hogan E. Off-label use of liraglutide in the management of a pediatric patient with type 2 diabetes mellitus. Case Rep Pediatr. 2013;2013:703925. doi: 10.1155/2013/703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein DJ, Battelino T, Chatterjee D, Jacobsen LV, Hale PM, Arslanian S. Liraglutide’s Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics in Pediatric Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes technology & therapeutics. 2014;16(10):679–87. doi: 10.1089/dia.2013.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein DJ, Battelino T, Chatterjee DJ, Jacobsen LV, Hale PM, Arslanian S, et al. Liraglutide’s safety, tolerability, pharmacokinetics, and pharmacodynamics in pediatric type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Technol Ther. 2014 Oct;16(10):679–87. doi: 10.1089/dia.2013.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwimmer JB. Clinical advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2016 May;63(5):1718–25. doi: 10.1002/hep.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dostalek M, Court MH, Yan B, Akhlaghi F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br J Pharmacol. 2011 Jul;163(5):937–47. doi: 10.1111/j.1476-5381.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dostalek M, Court MH, Hazarika S, Akhlaghi F. Diabetes mellitus reduces activity of human UDP-glucuronosyltransferase 2B7 in liver and kidney leading to decreased formation of mycophenolic acid acyl-glucuronide metabolite. Drug Metab Dispos. 2011 Mar;39(3):448–55. doi: 10.1124/dmd.110.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet. 2012 Aug 1;51(8):481–99. doi: 10.2165/11631900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Gwilt PR, Nahhas RR, Tracewell WG. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin Pharmacokinet. 1991 Jun;20(6):477–90. doi: 10.2165/00003088-199120060-00004. [DOI] [PubMed] [Google Scholar]
- 53.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Clarke JD, Cherrington NJ. Nonalcoholic steatohepatitis in precision medicine: Unraveling the factors that contribute to individual variability. Pharmacol Ther. 2015 Jul;151:99–106. doi: 10.1016/j.pharmthera.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003 Sep 18;349(12):1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 56.Anderson GD. Children versus adults: Pharmacokinetic and adverse-effect differences. Epilepsia. 2002;43(s3):53–9. doi: 10.1046/j.1528-1157.43.s.3.5.x. [DOI] [PubMed] [Google Scholar]
- 57.Harskamp-van Ginkel MW, Hill KD, Becker K, Testoni D, Cohen-Wolkowiez M, Gonzalez D, et al. Drug Dosing and Pharmacokinetics in Children With Obesity: A Systematic Review. JAMA Pediatr. 2015 Jul;169(7):678–85. doi: 10.1001/jamapediatrics.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart C, Hampton E. Effect of maturation on drug disposition in pediatric patients. Clinical pharmacy. 1987;6(7):548–64. [PubMed] [Google Scholar]
- 59.Morselli PL, Franco-Morselli R, Bossi L. Clinical pharmacokinetics in newborns and infants. Clinical pharmacokinetics. 1980;5(6):485–527. doi: 10.2165/00003088-198005060-00001. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy M. Hormonal regulation of hepatic drug-metabolizing enzyme activity during adolescence. Clin Pharmacol Ther. 2008 Dec;84(6):662–73. doi: 10.1038/clpt.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linday LA, Greenblatt DJ, Warren MP, Harmatz JS, DeCresce R, Cicalese C, et al. Changes in salivary antipyrine pharmacokinetics during adolescence, correlated with age, hormonal levels and Tanner stage. Dev Pharmacol Ther. 1991;16(4):194–202. [PubMed] [Google Scholar]
- 62.Lasker JM, Wester MR, Aramsombatdee E, Raucy JL. Characterization of CYP2C19 and CYP2C9 from human liver: respective roles in microsomal tolbutamide, S-mephenytoin, and omeprazole hydroxylations. Archives of Biochemistry and Biophysics. 1998;353(1):16–28. doi: 10.1006/abbi.1998.0615. [DOI] [PubMed] [Google Scholar]
- 63.Wester MR, Lasker JM, Johnson EF, Raucy JL. CYP2C19 participates in tolbutamide hydroxylation by human liver microsomes. Drug metabolism and disposition. 2000;28(3):354–9. [PubMed] [Google Scholar]
- 64.Hadama A, Ieiri I, Morita T, Kimura M, Urae A, Irie S, et al. P-hydroxylation of phenobarbital: relationship to (S)-mephenytoin hydroxylation (CYP2C19) polymorphism. Therapeutic drug monitoring. 2001;23(2):115–8. doi: 10.1097/00007691-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi K, Kogo M, Tani M, Shimada N, Ishizaki T, Numazawa S, et al. Role of CYP2C19 in stereoselective hydroxylation of mephobarbital by human liver microsomes. Drug metabolism and disposition. 2001;29(1):36–40. [PubMed] [Google Scholar]
- 66.Tateishi T, Nakura H, Asoh M, Watanabe M, Tanaka M, Kumai T, et al. A comparison of hepatic cytochrome P450 protein expression between infancy and postinfancy. Life sciences. 1997;61(26):2567–74. doi: 10.1016/s0024-3205(97)01011-4. [DOI] [PubMed] [Google Scholar]
- 67.Blanco JG, Harrison PL, Evans WE, Relling MV. Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab Dispos. 2000 Apr;28(4):379–82. [PubMed] [Google Scholar]
- 68.Naraharisetti SB, Lin YS, Rieder MJ, Marciante KD, Psaty BM, Thummel KE, et al. Human liver expression of CYP2C8: gender, age, and genotype effects. Drug Metab Dispos. 2010 Jun;38(6):889–93. doi: 10.1124/dmd.109.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prasad B, Gaedigk A, Vrana M, Gaedigk R, Leeder JS, Salphati L, et al. Ontogeny of hepatic drug transporters as quantified by LC-MS/MS proteomics. Clin Pharmacol Ther. 2016 Jun 15; doi: 10.1002/cpt.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X, Christensen M, Burghen G, Velasquez-Mieyer P, Moore K, Donahue S, et al. Pharmacokinetics of metformin in pediatric type 2 diabetic and healthy adult subjects. Clinical Pharmacology & Therapeutics. 2003;73(2):P46-P. [Google Scholar]
- 71.Sánchez-Infantes D, Díaz M, López-Bermejo A, Marcos MV, de Zegher F, Ibáñez L. Pharmacokinetics of metformin in girls aged 9 years. Clinical pharmacokinetics. 2011;50(11):735–8. doi: 10.2165/11593970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 72.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clinical pharmacokinetics. 2012;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 73.Turner KC, Christensen M, Connor JD, Moore KT, Gao X, Donahue SR. Pharmacokinetics of a glyburide/metformin combination tablet in children and adolescents with type 2 diabetes. Clinical Pharmacology & Therapeutics. 2003;73(2):P66-P. [Google Scholar]
- 74.Christensen ML, Meibohm B, Capparelli EV, Velasquez-Mieyer P, Burghen GA, Tamborlane WV. Single- and multiple-dose pharmacokinetics of pioglitazone in adolescents with type 2 diabetes. J Clin Pharmacol. 2005 Oct;45(10):1137–44. doi: 10.1177/0091270005279578. [DOI] [PubMed] [Google Scholar]
- 75.Malloy J, Capparelli E, Gottschalk M, Guan X, Kothare P, Fineman M. Pharmacology and tolerability of a single dose of exenatide in adolescent patients with type 2 diabetes mellitus being treated with metformin: a randomized, placebo-controlled, single-blind, dose-escalation, crossover study. Clin Ther. 2009 Apr;31(4):806–15. doi: 10.1016/j.clinthera.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Fineman M, Flanagan S, Taylor K, Aisporna M, Shen LZ, Mace KF, et al. Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing. Clin Pharmacokinet. 2011 Jan;50(1):65–74. doi: 10.2165/11585880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 77.Petri KC, Jacobsen LV, Klein DJ. Comparable liraglutide pharmacokinetics in pediatric and adult populations with type 2 diabetes: a population pharmacokinetic analysis. Clin Pharmacokinet. 2015 Jun;54(6):663–70. doi: 10.1007/s40262-014-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tirucherai GS, LaCreta F, Ismat FA, Tang W, Boulton DW. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes Obes Metab. 2016 Jul;18(7):678–84. doi: 10.1111/dom.12638. [DOI] [PubMed] [Google Scholar]
- 79.Miyagi SJ, Milne AM, Coughtrie MW, Collier AC. Neonatal development of hepatic UGT1A9: implications of pediatric pharmacokinetics. Drug Metab Dispos. 2012 Jul;40(7):1321–7. doi: 10.1124/dmd.111.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008 May;118(2):250–67. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Salem F, Johnson TN, Abduljalil K, Tucker GT, Rostami-Hodjegan A. A reevaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin Pharmacokinet. 2014 Jul;53(7):625–36. doi: 10.1007/s40262-014-0140-7. [DOI] [PubMed] [Google Scholar]