Abstract
Aim
This study aimed to assess the utility and stability of intraoral stent during intensity-modulated radiation therapy (IMRT).
Background
The benefits of intraoral stents in radiotherapy are unclear.
Materials and methods
We analyzed 386 setup errors in 12 patients who received IMRT for head and neck cancers without intraoral stents (intraoral stent [−]) and 183 setup errors in 6 patients who received IMRT with intraoral stents (intraoral stent [+]). All patients were matched according to the immobilization method (masks and boards). Setup errors were measured as the distance from the initial setup based on the marking on the skin and mask to the corrected position based on bone matching on cone beam computed tomography.
Results
The mean interfractional setup errors in the right–left, craniocaudal, anterior–posterior (AP), and three-dimensional (3D) directions were −0.33, 0.08, −0.25, and 2.75 mm in the intraoral stent (−) group and −0.37, 0.24, −0.63, and 2.42 mm in the intraoral stent (+) group, respectively (P = 0.50, 0.65, 0.01, and 0.02, respectively). The systematic errors for the same directions were 0.89, 1.46, 1.15, and 0.88 mm in the intraoral stent (−) group and 0.62, 1.69, 0.68, and 0.56 mm in the intraoral stents (+) group, respectively. The random errors were 1.43, 1.43, 1.44, and 1.22 mm in the intraoral stent (−) group and 1.06, 1.11, 1.05, and 0.92 mm in the intraoral stents (+) group, respectively.
Conclusion
Setup errors can be significantly reduced in the AP and 3D-directions by using intraoral stents.
Keywords: Intensity modulated radiation therapy, Setup error, Head and neck cancer, Bite block immobilization, Radiotherapy
1. Background
Intensity-modulated radiation therapy (IMRT) has been shown to reduce xerostomia and improve the quality of life in head-and-neck cancer patients.1 Because IMRT plans are more sensitive to positioning errors compared to conventional three-dimensional (3D) treatments, geometric errors should be taken into consideration in treatment planning or planning target volume design.2 Immobilization of the head and neck is critical to maintaining the patient's position during and between treatment fractions. The stability and precision of the patient immobilization apparatus should also be considered when determining the treatment margins required for proper coverage of the target, and for adequate protection of normal critical tissues. To this end, thermoplastic masks have been used regularly to improve setup accuracy.3, 4
Image-guided radiation therapy (IGRT) involves frequent two- and three-dimensional imaging during the course of radiation treatment to determine the isocenter coordinates and ensure that it corresponds to the same position that was determined using the reference imaging dataset. An example of IGRT is the combination of the cone beam computed tomography (CBCT) dataset with the planning computed tomography (CT) dataset.4, 5 IGRT relies directly on the imaging modalities utilized during planning for the reference coordinates when positioning the patient.
Intraoral stents have reportedly been used in various clinical scenarios, especially to reduce the mucosal dose and prevent severe radiation-induced oral mucositis.6, 7, 8, 9, 10, 11, 12, 13, 14 It has been reported that using a bite block can reduce 3D variability in patient positioning during external beam radiotherapy for head and neck tumors.6, 7 Additionally, the use of intraoral stents can reduce the effects of dental alloys in radiotherapy.12, 13, 14, 15 In dental therapy, intraoral stents are used in various situations including sports, anesthesia, treatment of sleep disorders, and orthodontic treatment.16, 17, 18, 19 These intraoral devices are often convenient enough for daily use. However, they have been less utilized for modern radiotherapy purposes, including for IMRT using IGRT with CBCT, despite their ease of use. Therefore, the benefits of intraoral stents, particularly their stability in radiotherapy, ought to be clarified. The purpose of this study was to assess the utility of intraoral stents in minimizing interfraction errors in IMRT.
2. Materials and methods
Informed consent was obtained prior to treatment from all individual participants included in the study. The institutional review board of our hospital approved this retrospective study (Approval No. 2378).
2.1. Intraoral stent design
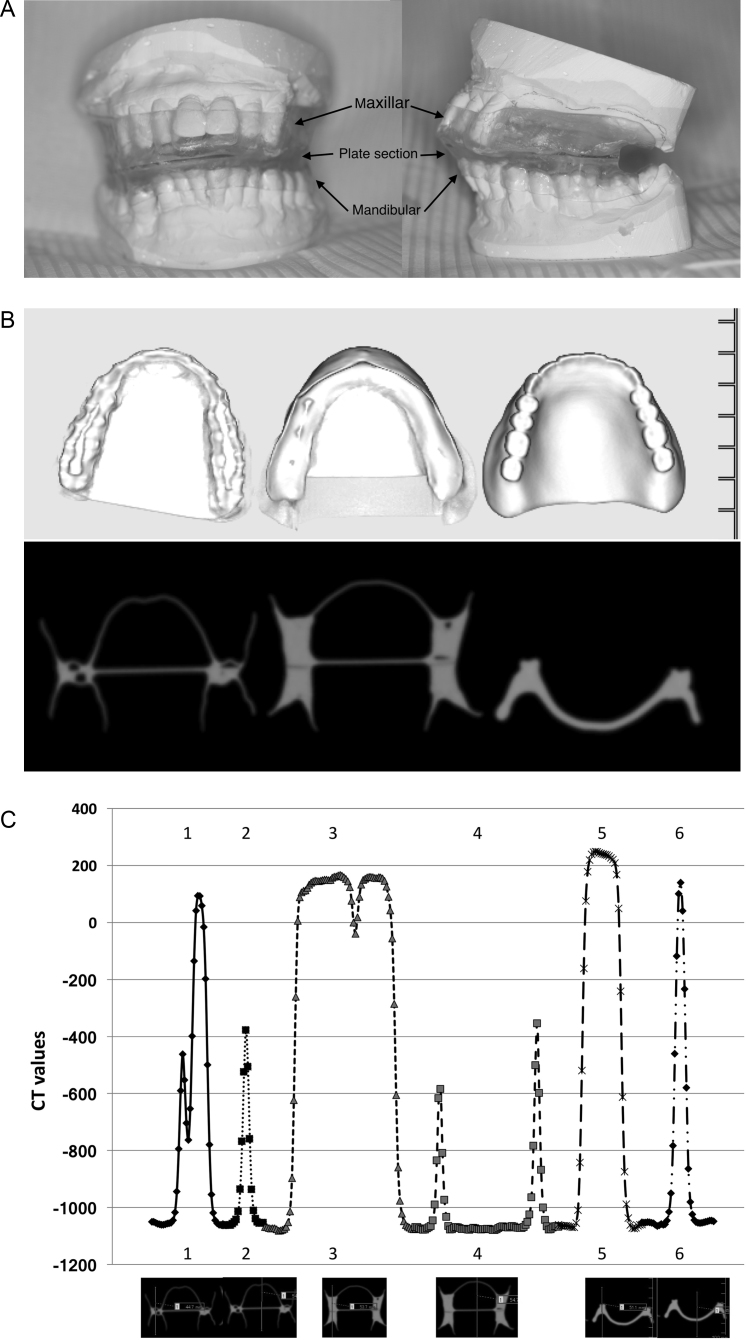
Prior to utilizing intraoral stents in radiotherapy for head and neck cancers, we assessed various stent designs to identify those that would have minimal interference with radiotherapy. Plaster models of the patients were used to construct the stents. Each stent was composed of three parts made of polyethylene terephthalate: the maxillar, mandibular, and plate sections. We fabricated a removable maxilla and mandible polyethylene terephthalate splint using a vacuum former (Erkopress®). The maxillar and mandibular sections were attached in a manner that encompassed the edge-to-edge bite, and the plate was placed between them to immobilize the tongue. The intraoral stent did not contain metal to avoid radiation scattering. The stents were adjusted by a dentist to fit the oral cavity of each patient.
2.2. Patient selection
Between November 2013 and May 2016, 18 consecutive patients who received IMRT for head and neck tumors were retrospectively analyzed (Table 1). All patients were matched according to an immobilization method; masks and boards. Twelve consecutive patients treated between November 2013 and July 2015 received IMRT without an intraoral stent (the intraoral stent [−] group) and 6 treated from August 2015 onward used intraoral stents that were individually fitted for each (the intraoral stent [+] group).
Table 1.
Patient characteristics.
| Patient | Sex | Age (y) | Primary tumor location | Clinical stage (TNM)a | Total doses of IMRT (Gy) | Total number of fractions |
|---|---|---|---|---|---|---|
| Intraoral stent (−) | ||||||
| 1 | M | 59 | MS | T4bN2cM0 | 66 | 33 |
| 2 | F | 81 | MS | T4aN0M1b | 66 | 33 |
| 3 | M | 66 | ES | T4aN0M0 | 66 | 33 |
| 4 | F | 86 | MS | T4aN0M0 | 66 | 33 |
| 5 | M | 58 | OP | T2N2cM0 | 66 | 33 |
| 6 | M | 81 | OP | T3N2cM0 | 66 | 33 |
| 7 | M | 69 | Lx | T3N2cM0c | 60 | 30 |
| 8 | M | 81 | Lx | T4aN1M0c | 60 | 30 |
| 9 | F | 77 | NC | T1N0M0 | 70 | 35 |
| 10 | F | 71 | OC | T4bN0M0 | 70 | 33 |
| 11 | F | 77 | MS | T3N0M0 | 66 | 33 |
| 12 | M | 81 | MS | T4aN1M0 | 66 | 33 |
| Intraoral stent (+) | ||||||
| 13 | M | 48 | MS | T4bN0M0 | 66 | 33 |
| 14 | M | 67 | MS | T3N0M0 | 66 | 33 |
| 15 | M | 75 | ES | T3N0M0 | 66 | 33 |
| 16 | F | 61 | NC | T3N0M0c | 40 | 20 |
| 17 | M | 72 | ES | T2N0M0 | 66 | 33 |
| 18 | M | 31 | MS | T2N0M0 | 66 | 33 |
Staging was performed based on the systematic TNM classification of the American Joint Committee on Cancer (seventh edition).
A lung nodule was later confirmed to be a metastasis after radiotherapy.
Radiotherapy was performed as an adjuvant therapy after surgery; the pathological stages are shown in the table.
Abbreviations: IMRT, intensity-modulated radiation therapy; MS, maxillary sinus; ES, ethmoid sinus; OP, oropharynx; Lx, Larynx; NC, nasal cavity; OC, oral cavity.
2.3. Radiotherapy technique
Radiotherapy was performed as previously described.20, 21 Patients were placed in the supine position and scanned using an Aquilion LB CT unit (Toshiba, Ohtawara, Japan) while wearing a thermoplastic face mask (Aquaplast RT; Q-Fix, Avondale, PA) attached to a couch overlay device (ProBoard™ Flat Carbon Fiber Headboard (Q-Fix, Avondale, PA) for immobilization. The CT images were reconstructed with a slice thickness of 2 mm. The acquired CT dataset was transferred to a XiO® treatment planning system (Elekta, Stockholm, Sweden) and the volumes of interest were outlined. IMRT was administered using volumetric modulated arc therapy, for which plans were created using the Monaco treatment planning system (Elekta, Maryland Heights, MO, USA) and were performed using a Synergy® linear accelerator (Elekta, Crawley, UK).
2.4. IGRT and image analysis
IGRT was performed as previously described.22 Daily initial setup consisted of aligning in-room lasers with mask marks and chest skin marks. A volumetric kilovoltage CBCT scan was then acquired with a linear accelerator-mounted X-ray source, XVI™ (Elekta AB, Crawley, UK). The XVI system consists of a kV X-ray source and a detector panel mounted orthogonally to the MV portal imager. A tube potential of 120 kV and a 200° gantry rotation (effectively a half-circle) with a small field of view were used. The reconstructed CBCT image was with a voxel size of 0.518 mm resolution. The planning CT imaging data was imported into the XVI system and converted to the same voxels using trilinear interpolations.
Images were assessed by radiation therapists, who were blinded to this study, by fusing the CBCT scan to the planning CT scan using commercially available software before treatment delivery in order to correct any setup errors.
Setup errors were measured as the distance from the initial set-up based on skin markings and the mask to the corrected position based on bone matching on CBCT. Setup errors for the right–left (x, RL), craniocaudal (y, CC), and vertical (anterior–posterior) (z, AP) directions were recorded in all patients. We analyzed the available 386 and 183 setup error distances in every direction in the intraoral stent (−) and (+) groups, respectively. Afterwards, 3D vector setup error displacements were calculated for each individual patient using the following formula:
where , , and are the setup errors in the x, y, and z directions, respectively.23 The individual random error was calculated as the standard deviation (SD) of all setup errors in each patient. Population random error (σ) and the distribution of systematic errors (∑) were calculated for both the intraoral stent (−) and (+) groups. σ and ∑ were calculated by using the root-mean-square of the individual patient's SDs and the SD of the individual mean errors, respectively.
Raw data from CBCT images were available in 157 and 152 setups of 5 patients in each of the intraoral stent (−) and (+) groups, respectively. The distance between the bottom of the chin and the table on the CBCT images before error correction was measured as ‘the chin distance’ in these images. The chin distance measured for the planning CT was the same as that measured in CBCT images. The differences between the chin distance in the planning CT images and those in the CBCT images were assessed. σ and ∑ were then calculated for each group.
2.5. Statistical analysis
The data are expressed as means with SDs in parentheses, unless otherwise indicated. Data were analyzed using a two-tailed Fisher's test or a Mann–Whitney test. All statistical analyses were performed using GraphPad Prism version 6.0b (GraphPad Software Inc., San Diego, CA, USA). P-values <0.05 were considered statistically significant.
3. Results
3.1. Intraoral stent
The intraoral stent we used is shown in Fig. 1. We confirmed that none of the materials contained metal. We designed the device aiming to minimize contact with the gingiva and achieve as much hollowness as possible. Initially, the part of the stent that contacts the alveolar ridge was filled with resin; however, this type of stent was found to absorb X-rays. Therefore, we chose polyethylene terephthalate as the main material and kept the device hollow in clinical practice in order to minimize X-ray scattering.
Fig. 1.
Conception and design of intraoral stents at our institute. (A) Intraoral stent in radiotherapy for head and neck cancers. We designed the device to be as hollow as possible while minimizing the area contacting the gingiva. (B) Intraoral stents of various designs were scanned with computed tomography (CT). All examined stents are shown as high densities in CT images. (C) CT values of intraoral stents are shown at each point. The six evaluated points of the three intraoral stents are shown in the X axis. Numbers 1 and 2, 3 and 4, and 5 and 6 correspond to the left, middle, and right intraoral stents in (B), respectively. The intraoral devices with greater potential for use exhibit higher CT values.
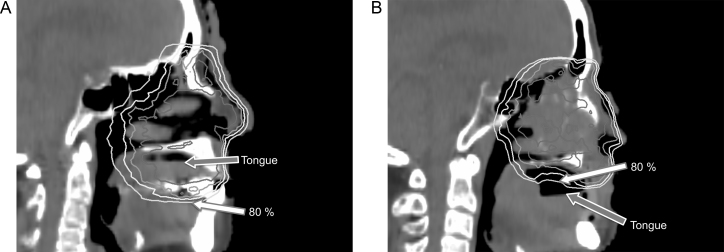
Fig. 2 illustrates the extent of dose reduction to a normal tongue achieved by typical use of an intraoral stent for locally advanced maxillary sinus cancers. Intraoral stents doubled as spacers to enable the reduction of the maximum doses, mean doses, D1cc (doses to 1 cm3) and D5cc for the normal tongue.
Fig. 2.
Dose reduction for normal tissue during radiotherapy for locally advanced maxillary sinus squamous cell carcinoma by using the intraoral stent. Isodose lines indicate 100, 95, 90, and 80% of the prescription doses in each case. The white and gray arrows indicate the 80% doses and the surface of tongue, respectively. (A) An 86-year-old woman received radiotherapy at a dose of 66 Gy in 33 fractions without an intraoral stent. The tongue surface was included in the isodose line of greater than 80% of the prescription doses. The dose volume histogram (DVH) showed that the Dmax, D1cc, and D5cc (i.e., doses covering the maximum, 1 cm3, and 5 cm3 of the tongue) were 71.0, 68.8, and 67.9 Gy, respectively. (B) A 67-year-old man received radiotherapy at a dose of 66 Gy in 33 fractions with an intraoral stent. The tongue surface was outside the 80% isodose line. The DVH showed that the Dmax, D1cc, and 5cc for the tongue were 50.3, 35.4, and 18.6 Gy, respectively.
3.2. Setup errors
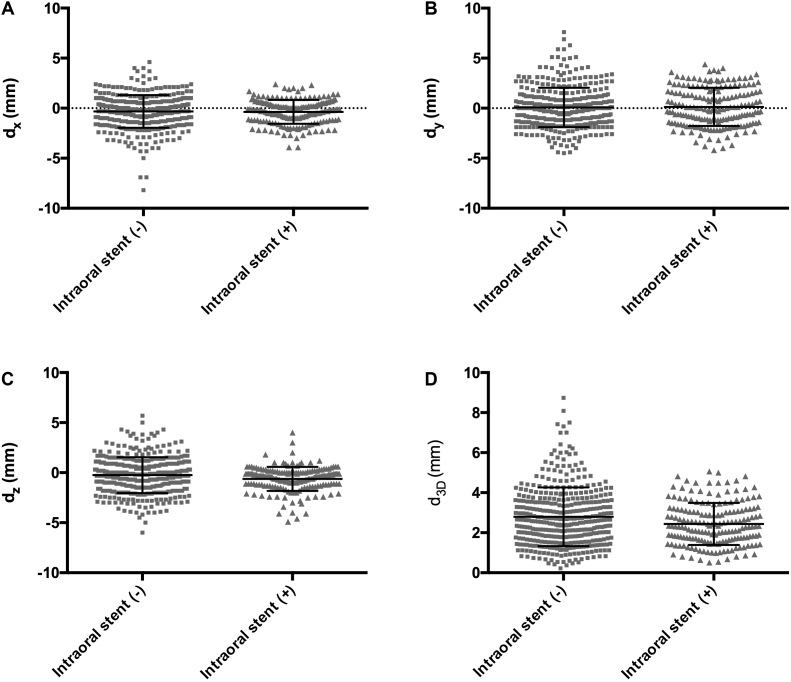
Scatter diagrams of the setup errors in the x, y, z, and 3D directions in the intraoral stent (+) and (−) groups were plotted and are shown in Fig. 3. The displacement and variability tended to be smaller in the intraoral stent (+) group than in the intraoral stent (−) group. Moreover, significant differences were observed between the two groups in the vertical (z) direction and 3D vector distance.
Fig. 3.
Distances of setup errors in all fractions. In each direction, a total of 386 and 183 setup errors in the oral stent (−) and (+) groups are plotted into scatter diagrams. (A) The interfractional setup errors of the x-direction (dx). The mean distances with standard deviations (SDs) were −0.33 ± 1.63 and −0.37 ± 1.19 mm in the oral stent (−) and (+) groups, respectively (P = 0.499). (B) The interfractional setup errors of the y-direction (dy). The mean distances with SDs were 0.075 ± 1.97 and 0.24 ± 1.92 mm in the oral stent (−) and (+) groups, respectively (P = 0.645). (C) The interfractional setup errors of the z-direction (dz). The mean distances with SDs were −0.25 ± 1.79 and −0.63 ± 1.20 mm in the oral stent (−) and (+) groups, respectively (P = 0.009). (D) The interfractional setup errors of the 3D-direction (d3D). The mean distances with SDs were 2.75 ± 1.53 and 2.42 ± 1.08 mm in the oral stent (−) and (+) groups, respectively (P = 0.022).
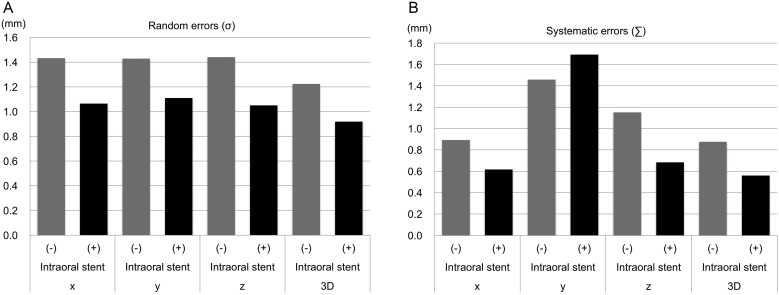
The individual random errors are shown in Fig. 4. In all directions (x, y, z, and 3D), the individual random error was smaller in the intraoral stent (+) group than in the intraoral stent (−) group. The σ and ∑ for each group are shown in Table 2 and Fig. 5. The displacement and variability were notably smaller in the intraoral stent (+) group than in the intraoral stent (−) group except for the y-direction of ∑.
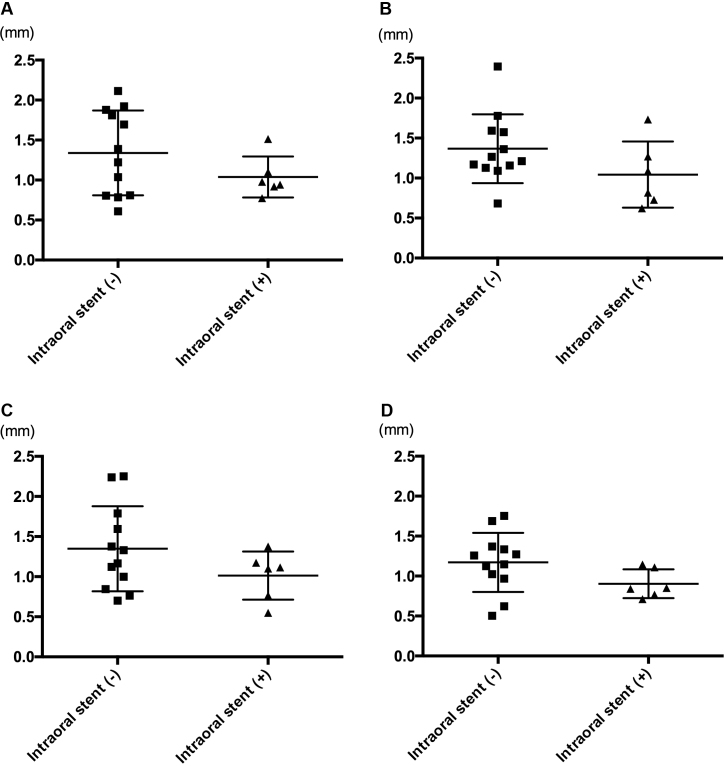
Fig. 4.
Individual random errors in the two groups. Individual random errors for patients were plotted into scatter diagrams. In each direction, 12 and 6 patients in the oral stent (−) and (+) groups are plotted. (A) In the x-direction, the individual random errors were 1.339 ± 0.530 mm and 1.038 ± 0.256 mm in the oral stent (−) and (+) groups, respectively (P = 0.319). (B) In the y-direction, the individual random errors were 1.367 ± 0.431 mm and 1.043 ± 0.414 mm in the oral stent (−) and (+) groups, respectively (P = 0.145). (C) In the z-direction, the individual random errors were 1.348 ± 0.530 mm and 1.014 ± 0.301 mm in the oral stent (−) and (+) groups, respectively (P = 0.277). (D) In the 3D-direction, the individual random errors were 1.172 ± 0.370 mm and 0.904 ± 0.181 mm in the oral stent (−) and (+) groups, respectively (P = 0.081).
Table 2.
Random and systematic errors.
| Direction | Intraoral stent (−) | Intraoral stent (+) | |
|---|---|---|---|
| σ (mm) | n = 386 | n = 183 | |
| x | 1.432 | 1.064 | |
| y | 1.428 | 1.109 | |
| z | 1.440 | 1.050 | |
| 3D | 1.224 | 0.919 |
| ∑ (mm) | n = 12 | n = 6 | |
|---|---|---|---|
| x | 0.892 | 0.616 | |
| y | 1.458 | 1.693 | |
| z | 1.152 | 0.683 | |
| 3D | 0.876 | 0.559 |
Abbreviation: 3D, three-dimensional.
Fig. 5.
Comparison of random and systematic errors between the two groups. The random (σ, A) and systematic (∑, B) errors in the intraoral stent (−) and (+) groups are showed in the bar graphs. Patients in the intraoral stent (+) group (black bars) show less random and systematic errors than those in the intraoral stent (−) group (gray bars).
Based on the formula 2.5∑ + 0.7σ, the appropriate PTV margins were 3.23, 4.65, 3.89, and 3.05 mm for the intraoral stent (−) group and 2.29, 5.01, 2.44, and 2.04 mm for the intraoral stent (+) group in the x, y, z, and 3D-directions, respectively.24, 25 As for the chin distance, the σ and ∑ were 0.191 and 0.158 mm in the intraoral stent (−) group, and 0.158 and 0.165 mm in the intraoral stent (+) group, respectively.
4. Discussion
IMRT is a well-established radical treatment for head and neck cancers, and has been shown to reduce xerostomia and result in high locoregional tumor control rates.1 Furthermore, local radiotherapy with concomitant intra-arterial infusion chemoradiotherapy performed in patients with locally advanced sinonasal carcinomas have been reported to produce favorable outcomes.20, 26 Because IMRT requires high precision during the course of treatment, a setup that uses a reliable immobilization device may help reduce the physical margins.2, 3, 4 Astreinidou et al. have shown that translational and rotational random setup errors can significantly affect the dose distribution to head-and-neck cancer patients treated with IMRT, where the goal of treatment is for 95% of the clinical target volume to receive 99% of the prescribed dose.27
The roles of intraoral stents (also known as oral appliances, mouthpieces, mouth-guards, and bite blocks) in radiotherapy have been described in numerous reports that describe these devices as physical spacers in the intraoral space. They are employed to reduce the doses to normal tissues as well as to reduce the effects of metal artifacts (dental fillings) that can make it difficult to delineate tumors and organs-at-risk on CT images. Radiation-induced mucositis is the most common toxicity, and can often interrupt the planned course of treatment and significantly reduce a patient's quality of life during and after radiotherapy. The oral mucosa is anatomically close to tumors as well as the lymph node region in patients receiving high doses of radiation. Moreover, the dose to the normal mucosa has been reported to be enhanced because of radiation scattering from dental alloys, illustrating how dental alloys can affect treatment planning and quality assurance.28, 29, 30, 31 Therefore, intraoral stents used as spacers can prevent severe mucositis.8, 9, 10 In clinical practice, intraoral stents have been manufactured from thin, hard materials and made to fit into the upper gum. However, the ideal material and optimal form factors for such stents are still unclear. We assessed various designs in this study, and found that hollow stents made of polyethylene terephthalate were ideal for reducing X-ray scattering with functional preservation of an intraoral spacer.
Several studies described the benefits of intraoral stents in terms of reducing systematic and random errors in previously used IGRT modalities.6, 7 Despite the observation that intraoral stents appear to be useful in currently used IGRT techniques, such as CBCT, the applicability of such stents is poorly described for such modalities. In the present study, the setup errors of IMRT for head and neck cancers were significantly reduced by employing stents, which is consistent with previous reports.8, 11
This study has several limitations. The utility of the intraoral stent, as well as the setup errors, were demonstrated in a limited number of patients, this can cause bias in the results of a retrospective study such as this. Moreover, ∑ was reduced by using the intraoral stent in the x- and z-directions but not in the y-direction; we posit that the stability of the aperture was less affected by errors in the y-direction than in other directions. Another limitation was the heterogeneous patient population and the variety of primary tumor sites. However, all patients were treated with IMRT and were well-matched according to the immobilization method. Moreover, we analyzed most fractions in all eligible patients to improve the reliability of the data. However, σ was obviously smaller in the oral stent (+) group in all three directions than in the oral stent (−) group; therefore, our data constitute strong evidence that using the intraoral stent can indeed contribute to error reduction for the duration of radiotherapy. In order to further clarify the roles of intraoral stents in IMRT, an additional multi-institutional clinical study ought to be conducted in a larger number of homogeneous patients that focuses on the primary tumor site, stage, and radiotherapeutic setting. A third limitation is that this study does not account for intrafractional uncertainty. However, the advantage of using the intraoral stent is clear with respect to dose as well as to treatment duration. Oita et al. described the reduction of intrafractional uncertainty by intraoral stents with three gold markers by using their real-time tumor tracking radiotherapy system.7 Because the intraoral stent can reduce doses to organs-at-risk despite being a relatively non-invasive device, there is less ethical justification for performing further prospective trials in randomized settings. Lastly, our study did not address the prognostic benefit of this device or any reduction in the severity of radiation-induced mucositis. There are only limited reports describing the clinical benefits of using intraoral stents for the treatment of head and neck cancer in terms of adverse events.8
There are also potential pitfalls when using intraoral stents; these include dislocation of the mouthpiece, calculations for neck movements not being commensurate with head movements, drawbacks due to the presence of metallic artifacts (i.e., dental alloys), and additional irradiation to the mucosal surface required at the point of the stent's contact during head and neck irradiation.
5. Conclusion
The use of intraoral stents significantly reduces setup errors in IMRT for head and neck cancers. Our data warrant conducting a prospective clinical trial in order to determine the optimal intraoral stent design in terms of utility and strength.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgements
We acknowledge Messrs. Tsukasa Wakayama and Toshiyuki Sakai of the Department of Radiological Technology, Hyogo College of Medicine College Hospital; Messrs. Hiroki Nakajima, Shogo Harui, Akira Ando, and Dr. Keiji Kamino of the Department of Radiation Oncology, Meiwa Cancer Clinic. We would also like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Nutting C.M., Morden J.P., Harrington K.J. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong T.S., Tomé W.A., Chappell R.J., Chinnaiyan P., Mehta M.P., Harari P.M. The impact of daily setup variations on head-and-neck intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;61:779–788. doi: 10.1016/j.ijrobp.2004.07.696. [DOI] [PubMed] [Google Scholar]
- 3.Bentel G.C., Marks L.B., Hendren K., Brizel D.M. Comparison of two head and neck immobilization systems. Int J Radiat Oncol Biol Phys. 1997;38:867–873. doi: 10.1016/s0360-3016(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 4.Velec M., Waldron J.N., O'Sullivan B. Cone-beam CT assessment of interfraction and intrafraction setup error of two head-and-neck cancer thermoplastic masks. Int J Radiat Oncol Biol Phys. 2010;76:949–955. doi: 10.1016/j.ijrobp.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Lu H., Lin H., Feng G. Interfractional and intrafractional errors assessed by daily cone-beam computed tomography in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy: a prospective study. J Radiat Res. 2012;53:954–960. doi: 10.1093/jrr/rrs041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willner J., Hädinger U., Neumann M., Schwab F.J., Bratengeier K., Flentje M. Three dimensional variability in patient positioning using bite block immobilization in 3D-conformal radiation treatment for ENT-tumors. Radiother Oncol. 1997;43:315–321. doi: 10.1016/s0167-8140(97)00055-8. [DOI] [PubMed] [Google Scholar]
- 7.Oita M., Ohmori K., Obinata K. Uncertainty in treatment of head-and-neck tumors by use of intraoral mouthpiece and embedded fiducials. Int J Radiat Oncol Biol Phys. 2006;64:1581–1588. doi: 10.1016/j.ijrobp.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Verrone J.R., Alves F.A., Prado J.D. Benefits of an intraoral stent in decreasing the irradiation dose to oral healthy tissue: dosimetric and clinical features. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:573–578. doi: 10.1016/j.oooo.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Kudoh T., Ikushima H., Honda E. Shielding effect of a customized intraoral mold including lead material in high-dose-rate 192-Ir brachytherapy for oral cavity cancer. J Radiat Res. 2012;53:130–137. doi: 10.1269/jrr.11102. [DOI] [PubMed] [Google Scholar]
- 10.Murakami S., Verdonschot R.G., Kakimoto N. Preventing complications from high-dose rate brachytherapy when treating mobile tongue cancer via the application of a modular lead-lined spacer. PLoS ONE. 2016;11:e0154226. doi: 10.1371/journal.pone.0154226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson B., Sales L., Winston A., Liao J., Laramore G., Parvathaneni U. Fabrication of customized tongue-displacing stents: considerations for use in patients receiving head and neck radiotherapy. J Am Dent Assoc. 2013;144:594–600. doi: 10.14219/jada.archive.2013.0170. [DOI] [PubMed] [Google Scholar]
- 12.Wang R.R., Olmsted L.W. A direct method for fabricating tongue-shielding stent. J Prosthet Dent. 1995;74:171–173. doi: 10.1016/s0022-3913(05)80182-9. [DOI] [PubMed] [Google Scholar]
- 13.Reitemeier B., Reitemeier G., Schmidt A. Evaluation of a device for attenuation of electron release from dental restorations in a therapeutic radiation field. J Prosthet Dent. 2002;87:323–327. doi: 10.1067/mpr.2002.122506. [DOI] [PubMed] [Google Scholar]
- 14.Chang K.P., Lin W.T., Shiau A.C., Chie Y.H. Dosimetric distribution of the surroundings of different dental crowns and implants during LINAC photon irradiation. Radiat Phys Chem. 2014;104:339–344. [Google Scholar]
- 15.Katsura K., Utsunomiya S., Abe E. A study on a dental device for the prevention of mucosal dose enhancement caused by backscatter radiation from dental alloy during external beam radiotherapy. J Radiat Res. 2016 doi: 10.1093/jrr/rrw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda Y., Kumamoto D., Yagi K., Ikebe K. Effectiveness and fabrication of mouthguards. Dent Traumatol. 2009;25:556–564. doi: 10.1111/j.1600-9657.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 17.Mańka-Malara K., Gawlak D., Hovhannisyan A., Klikowska M., Kostrzewa-Janicka J. Dental trauma prevention during endotracheal intubation – review of literature. Anaesthesiol Intensive Ther. 2015;47:425–429. doi: 10.5603/AIT.2015.0054. [DOI] [PubMed] [Google Scholar]
- 18.Al-Jewair T.S., Gaffar B.O., Flores-Mir C. Quality assessment of systematic reviews on the efficacy of oral appliance therapy for adult and pediatric sleep-disordered breathing. J Clin Sleep Med. 2016;12:1175–1183. doi: 10.5664/jcsm.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smithpeter J., Covell D. Relapse of anterior open bites treated with orthodontic appliances with and without orofacial myofunctional therapy. Am J Orthod Dentofac Orthop. 2010;137:605–614. doi: 10.1016/j.ajodo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Doi H., Kitajima K., Tanooka M. Radiotherapy in late elderly (aged 75 or older) patients with paranasal sinus carcinoma: a single institution experience. Eur Arch Otorhinolaryngol. 2016;273:4485–4492. doi: 10.1007/s00405-016-4151-x. [DOI] [PubMed] [Google Scholar]
- 21.Miura H., Fujiwara M., Tanooka M. Dosimetric and delivery characterizations of full-arc and half-arc volumetric-modulated arc therapy for maxillary cancer. J Radiat Res. 2012;53:785–790. doi: 10.1093/jrr/rrs031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue H., Tanooka M., Doi H. Impact of planning CT slice thickness on the accuracy of automatic target registration using the on-board cone-beam CT. Igaku Butsuri. 2011;31:2–11. [in Japanese] [PubMed] [Google Scholar]
- 23.Rotondo R.L., Sultanem K., Lavoie I., Skelly J., Raymond L. Comparison of repositioning accuracy of two commercially available immobilization systems for treatment of head-and-neck tumors using simulation computed tomography imaging. Int J Radiat Oncol Biol Phys. 2008;70:1389–1396. doi: 10.1016/j.ijrobp.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 24.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Kapanen M., Laaksomaa M., Tulijoki T., Kellokumpu-Lehtinen P.-L., Hyödynmaa S. Effects of remedies made in patient setup process on residual setup errors and margins in head and neck cancer radiotherapy based on 2D image guidance. Rep Pract Oncol Radiother. 2015;20:292–298. doi: 10.1016/j.rpor.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homma A., Sakashita T., Yoshida D. Superselective intra-arterial cisplatin infusion and concomitant radiotherapy for maxillary sinus cancer. Br J Cancer. 2013;109:2980–2986. doi: 10.1038/bjc.2013.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astreinidou E., Bel A., Raaijmakers C.P., Terhaard C.H., Lagendijk J.J. Adequate margins for random setup uncertainties in head-and-neck IMRT. Int J Radiat Oncol Biol Phys. 2005;61:938–944. doi: 10.1016/j.ijrobp.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Farman A.G., Sharma S., George D.I. Backscattering from dental restorations and splint materials during therapeutic radiation. Radiology. 1985;156:523–526. doi: 10.1148/radiology.156.2.4011918. [DOI] [PubMed] [Google Scholar]
- 29.Chin D.W., Treister N., Friedland B. Effect of dental restorations and prostheses on radiotherapy dose distribution: a Monte Carlo study. J Appl Clin Med Phys. 2009;10:2853. doi: 10.1120/jacmp.v10i1.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin M.H., Li J., Price R.A., Jr., Wang L., Lee C.C., Ma C.M. The dosimetric impact of dental implants on head-and-neck volumetric modulated arc therapy. Phys Med Biol. 2013;58:1027–1040. doi: 10.1088/0031-9155/58/4/1027. [DOI] [PubMed] [Google Scholar]
- 31.Kamomae T., Itoh Y., Okudaira K. Dosimetric impact of dental metallic crown on intensity-modulated radiotherapy and volumetric-modulated arc therapy for head and neck cancer. J Appl Clin Med Phys. 2016;17:5870. doi: 10.1120/jacmp.v17i1.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]