Abstract
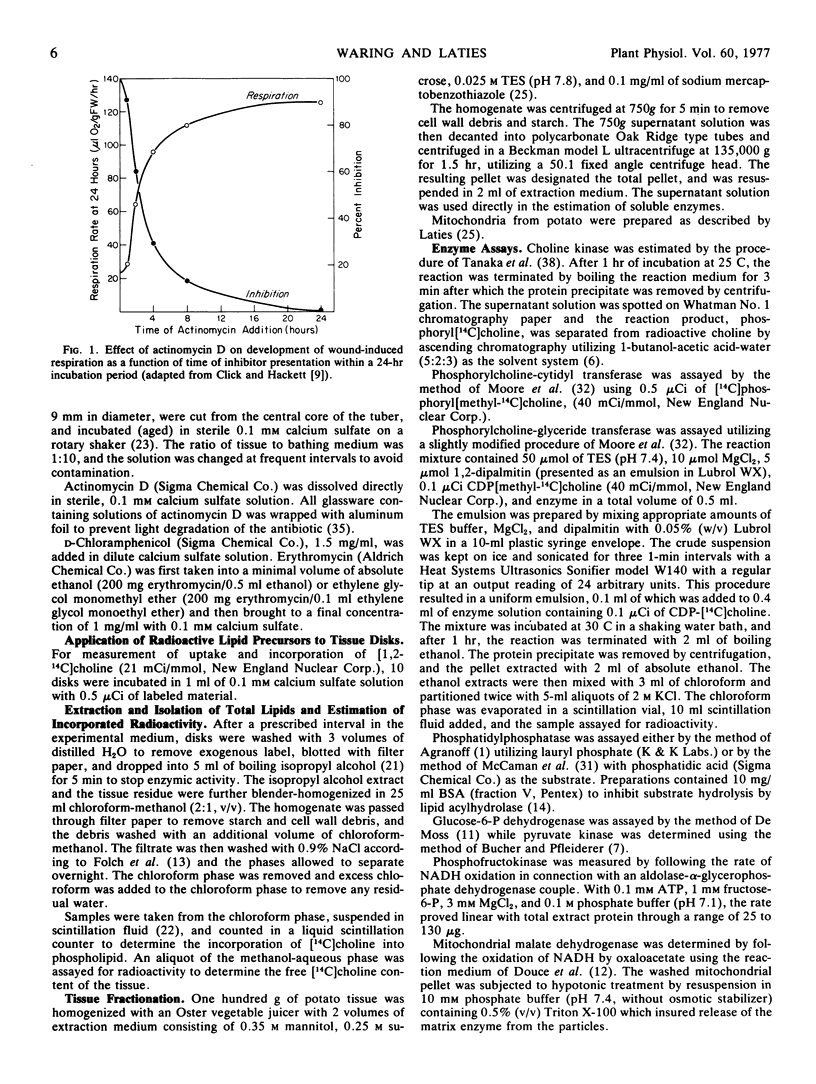
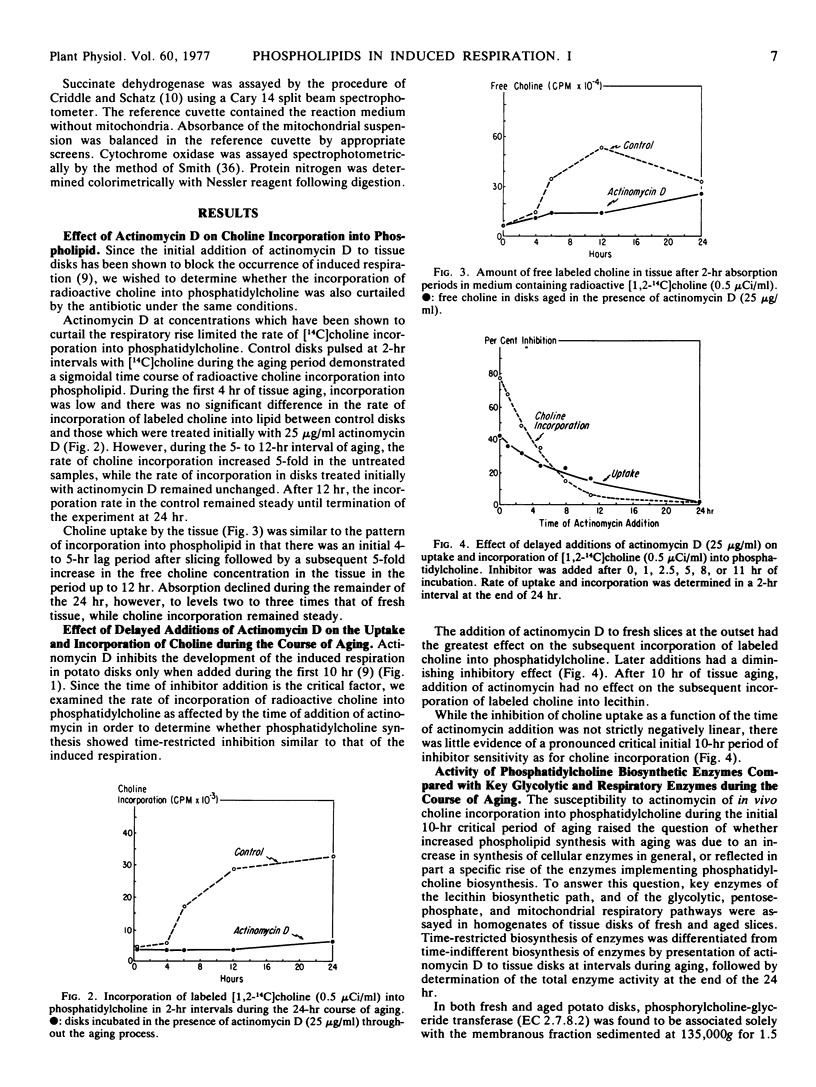
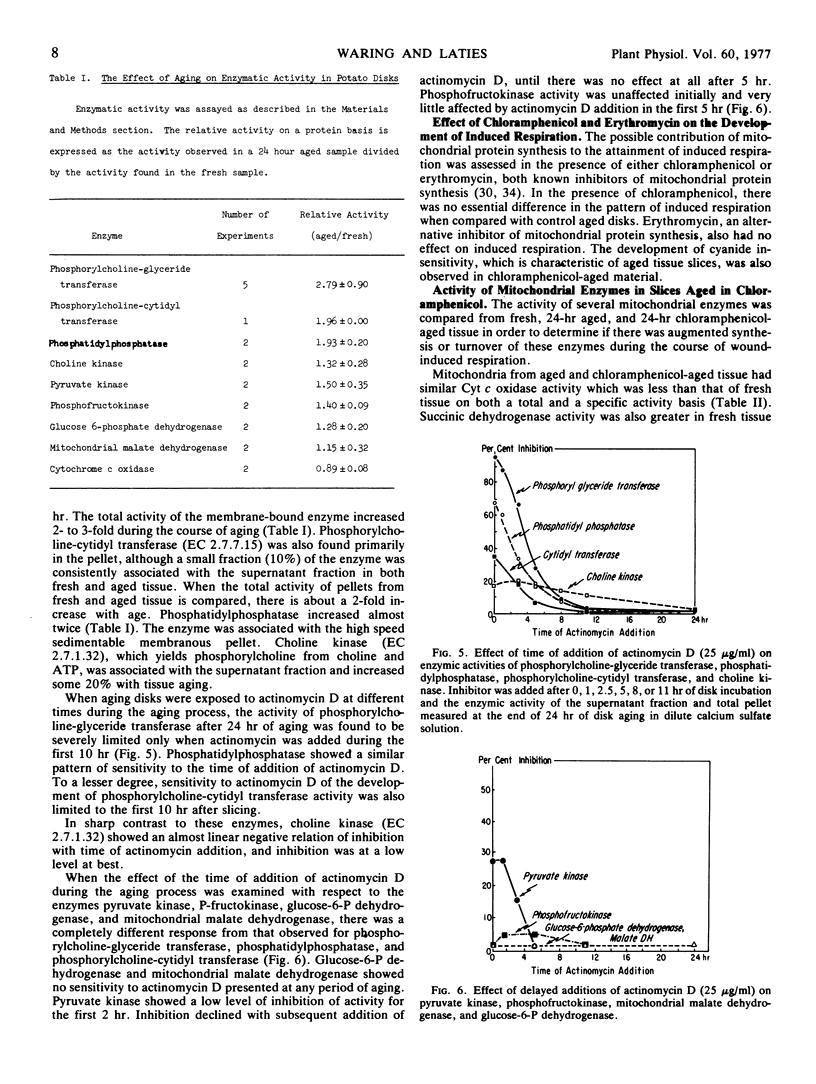
Actinomycin D prevents the full development in a 24-hour period of both wound respiration and cyanide resistance only when given in the first 10 to 12 hours following the cutting of potato tuber (Solanum tuberosum var. Russet) slices. The capacity for choline incorporation into phosphatidylcholine increases with slice aging and is inhibited by actinomycin D in the same time-restricted way. The time-restricted effectiveness of actinomycin D applies to the cutting-elicited enhanced synthesis of three critical enzymes of phosphatidylcholine synthesis, namely phosphorylcholine-glyceride transferase, phosphorylcholine-cytidyl transferase, and phosphatidylphosphatase. By contrast, actinomycim D given at any time is without effect on the measurable levels after 24 hours of a selection of glycolytic and mitochondrial respiratory enzymes. Neither succinic dehydrogenase nor cytochrome oxidase activity increases with time in aging potato slices in the presence or absence of chloramphenicol. The foregoing observations emphasize the central role of phospholipid, and ultimately membrane biosynthesis, in the development of wound-induced respiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLICK R. E., HACKETT D. P. THE ROLE OF PROTEIN AND NUCLEIC ACID SYNTHESIS IN THE DEVELOPMENT OF RESPIRATION IN POTATO TUBER SLICES. Proc Natl Acad Sci U S A. 1963 Aug;50:243–250. doi: 10.1073/pnas.50.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Tang W. J., Bolar M. L. Membrane transformations in aging potato tuber slices. Plant Physiol. 1971 Dec;48(6):795–800. doi: 10.1104/pp.48.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Galliard T. Enzymic deacylation of lipids in plants. The effects of free fatty acids on the hydrolysis of phospholipids by the lipolytic acyl hydrolase of potato tubers. Eur J Biochem. 1971 Jul 15;21(1):90–98. doi: 10.1111/j.1432-1033.1971.tb01444.x. [DOI] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W., Griffiths S. K., Niederpruem D. J. Studies on Development of Cyanide-resistant Respiration in Potato Tuber Slices. Plant Physiol. 1960 Jan;35(1):8–19. doi: 10.1104/pp.35.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Smith B. N., Epstein S., Laties G. G. The prevalence of carbon-13 in respiratory carbon dioxide as an indicator of the types of endogenous substrate. The change from lipid to carbohydrate during the respiratory rise in potato slices. J Gen Physiol. 1970 Jan;55(1):1–17. doi: 10.1085/jgp.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl G. De novo synthesis of glucose-6-phosphate- (E.C. 1.1.1.49) and 6-phosphogluconate dehydrogenase (E.C. 1.1.1.44) in plant storage tissue slices. Z Naturforsch C. 1974 Nov-Dec;29(11-12):700–704. doi: 10.1515/znc-1974-11-1209. [DOI] [PubMed] [Google Scholar]
- Laties G. G. Controlling Influence of Thickness on Development & Type of Respiratory Activity in Potato Slices. Plant Physiol. 1962 Sep;37(5):679–690. doi: 10.1104/pp.37.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G. The Onset of Tricarboxylic Acid Cycle Activity with Aging in Potato Slices. Plant Physiol. 1964 Jul;39(4):654–663. doi: 10.1104/pp.39.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G. The potentiating effect of adenosine diphosphate in the uncoupling of oxidative phosphorylation in potato mitochondria. Biochemistry. 1973 Aug 14;12(17):3350–3355. doi: 10.1021/bi00741a032. [DOI] [PubMed] [Google Scholar]
- Malhotra S. S., Spencer M. Structural Development during Germination of Different Populations of Mitochondria from Pea Cotyledons. Plant Physiol. 1973 Dec;52(6):575–579. doi: 10.1104/pp.52.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman R. E., Smith M., Cook K. Intermediary metabolism of phospholipids in brain tissue. II. Phosphatidic acid phosphatase. J Biol Chem. 1965 Sep;240(9):3513–3517. [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson M. J., Laties G. G. Ribosomal RNA synthesis in newly sliced discs of potato tuber. Plant Physiol. 1968 Jul;43(7):1011–1016. doi: 10.1104/pp.43.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. The mechanism of ethylene and cyanide action in triggering the rise in respiration in potato tubers. Plant Physiol. 1975 Jan;55(1):73–78. doi: 10.1104/pp.55.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Tolbert N. E., Gohlke A. F. Choline kinase and phosphorylcholine phosphatase in plants. Plant Physiol. 1966 Feb;41(2):307–312. doi: 10.1104/pp.41.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. J., Castelfranco P. A. Phospholipid synthesis in aging potato tuber tissue. Plant Physiol. 1968 Aug;43(8):1232–1238. doi: 10.1104/pp.43.8.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Inhibition of the Development of Induced Respiration and Cyanide-insensitive Respiration in Potato Tuber Slices by Cerulenin and Dimethylaminoethanol. Plant Physiol. 1977 Jul;60(1):11–16. doi: 10.1104/pp.60.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemot C., Stumpf P. K. Fat metabolism in higher plants. XXXIV. Development of fatty acid synthetase as a function of protein synthesis in aging potato tuber slices. Plant Physiol. 1967 Mar;42(3):391–397. doi: 10.1104/pp.42.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]


