Abstract
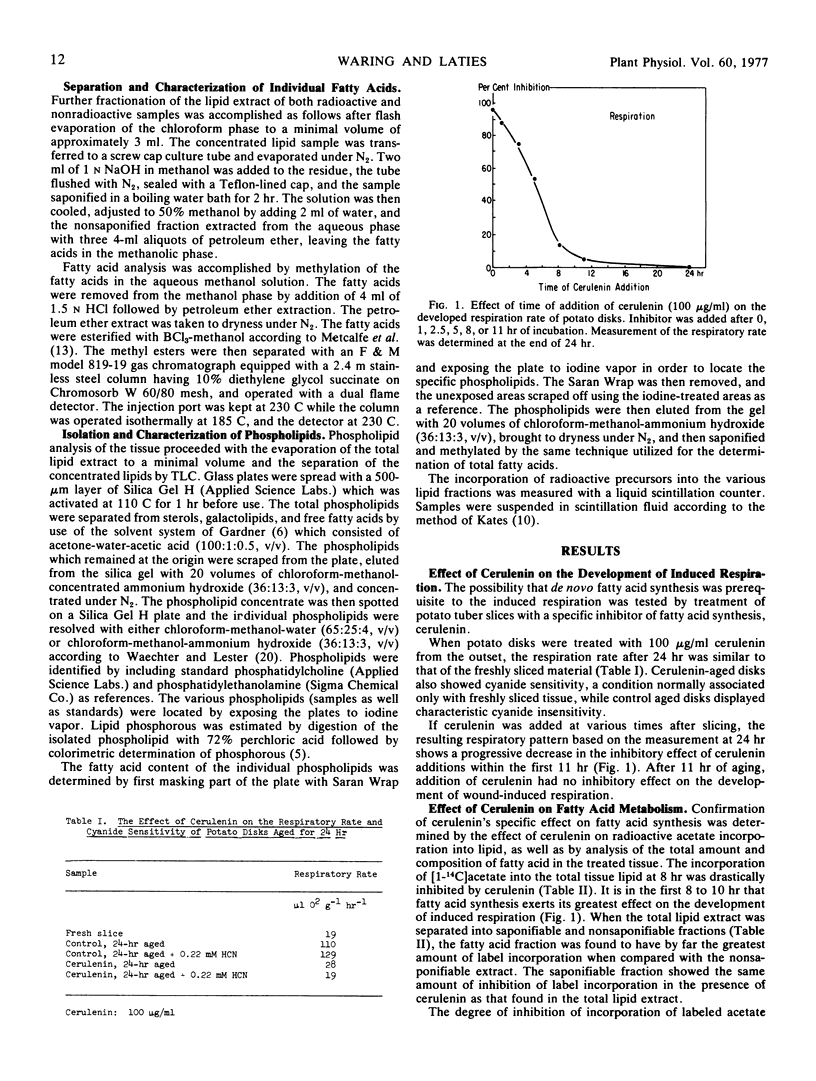
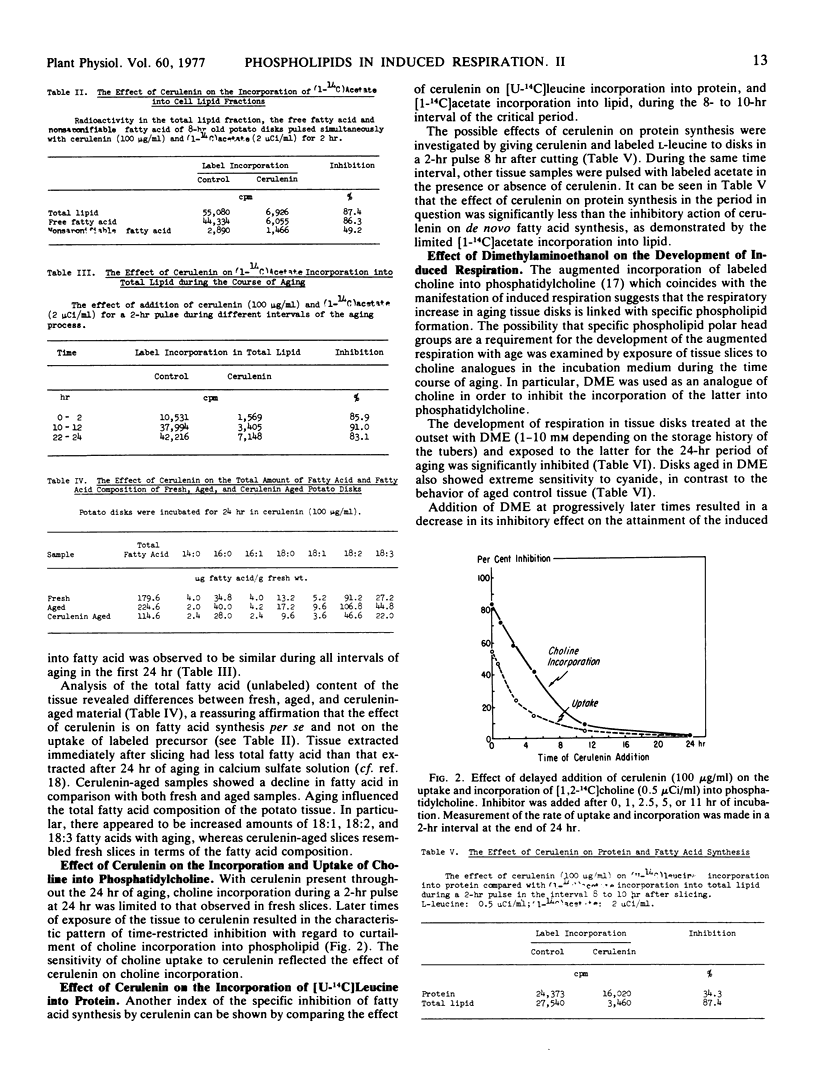
The interdependence of the development of wound-induced respiration and membrane-related phospholipid biosynthesis in potato tuber (Solanum tuberosum var. Russet) slices was established by the use of agents which selectively affect lipid and phospholipid synthesis. Cerulenin, a specific inhibitor of de novo fatty acid synthesis, inhibited the ultimate development of wound-induced respiration and of cyanide resistance only when given in the critical first 10 to 12 hours of slice aging. Similarly, when slices were exposed to the choline analogue dimethylaminoethanol within the first 10 hours, the phospholipid composition of the membrane lipids was drastically altered, the wound-induced respiration in a 24-hr period was substantially curtailed, and the development of cyanide insensitivity was sharply inhibited. These observations indicate that time-restricted membrane-related phospholipid synthesis is prerequisite to the development of wound-induced respiration and concurrent cyanide insensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arison B. H., Omura S. Revised structure of cerulenin. J Antibiot (Tokyo) 1974 Jan;27(1):28–30. doi: 10.7164/antibiotics.27.28. [DOI] [PubMed] [Google Scholar]
- CLICK R. E., HACKETT D. P. THE ROLE OF PROTEIN AND NUCLEIC ACID SYNTHESIS IN THE DEVELOPMENT OF RESPIRATION IN POTATO TUBER SLICES. Proc Natl Acad Sci U S A. 1963 Aug;50:243–250. doi: 10.1073/pnas.50.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agnolo G., Rosenfeld I. S., Awaya J., Omura S., Vagelos P. R. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973 Nov 29;326(2):155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- Douglas T. J., Paleg L. G. Inhibition of Sterol Biosynthesis by 2-Isopropyl-4-dimethylamino-5-methylphenyl-1-piperidine Carboxylate Methyl Chloride in Tobacco and Rat Liver Preparations. Plant Physiol. 1972 Mar;49(3):417–420. doi: 10.1104/pp.49.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H. W. Preparative isolation of monogalactosyl and digalactosyl diglycerides by thin-layer chromatography. J Lipid Res. 1968 Jan;9(1):139–141. [PubMed] [Google Scholar]
- Glaser M., Ferguson K. A., Vagelos P. R. Manipulation of the phospholipid composition of tissue culture cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4072–4076. doi: 10.1073/pnas.71.10.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Walker J. R., Bloch K. Inhibition of lipid synthesis in Escherichia coli cells by the antibiotic cerulenin. Antimicrob Agents Chemother. 1973 May;3(5):549–554. doi: 10.1128/aac.3.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Laties G. G. Inhibition of RNA and protein synthesis by chloral in potato slices. Plant Physiol. 1965 Nov;40(6):1237–1241. doi: 10.1104/pp.40.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Omura S., Hata T. The action mechanism of cerulenin. I. Effect of cerulenin on sterol and fatty acid biosynthesis in yeast. J Biochem. 1972 May;71(5):783–796. doi: 10.1093/oxfordjournals.jbchem.a129827. [DOI] [PubMed] [Google Scholar]
- Ono T., Kesado T., Awaya J., Omura S. Target of inhibition by the anti-lipogenic antibiotic cerulenin of sterol synthesis in yeast. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1119–1124. doi: 10.1016/0006-291x(74)90812-2. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Fiehn W. Diazacholesterol myotonia: accumulation of desmosterol and increased adenosine triphosphatase activity of sarcolemma. Science. 1973 Mar 2;179(4076):910–912. doi: 10.1126/science.179.4076.910. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Castelfranco P. A. Phospholipid synthesis in aging potato tuber tissue. Plant Physiol. 1968 Aug;43(8):1232–1238. doi: 10.1104/pp.43.8.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- WITTENBERG J., KORNBERG A. Choline phosphokinase. J Biol Chem. 1953 May;202(1):431–444. [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):401–410. doi: 10.1016/0003-9861(73)90637-1. [DOI] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Dependence of Wound-induced Respiration in Potato Slices on the Time-restricted Actinomycin-sensitive Biosynthesis of Phospholipid. Plant Physiol. 1977 Jul;60(1):5–10. doi: 10.1104/pp.60.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


