Abstract
The objective of the study was to evaluate the neuroprotective effects of bacoside A and bromelain against dichlorvos-incited toxicity. Healthy 6–8-week old, male Swiss mice were administered subacute doses of dichlorvos (40 mg/kg bw), bacoside A (5 mg/kg bw) and bromelain (70 mg/kg bw). AChE, BChE, GABA, serotonin and total protein content and their expressions were used for determination of toxic action of dichlorvos. Protective effects of bacoside A and bromelain were evaluated on the same parameters. Exposure to dichlorvos leads to significant decline in activities of AChE (p < 0.01, p < 0.001), BChE (p < 0.05) and GABA (p < 0.01) and total protein levels (p < 0.01). Antioxidant treatment significantly increased the activities of AChE (p < 0.01, p < 0.001), BChE (p < 0.05), GABA (p < 0.01) and total protein level (p < 0.05) compared to those in dichlorvos-treated mice. Overexpression of Hsp 70 protein and underexpression of phosphorylase a and b, catalase SOD and GPx were observed after dichlorvos exposure which suggests the oxidative stress. The results indicate that dichlorvos-induced neuronal damage which results in the generation of molecular expression of proteins is in agreement with the biochemical data ameliorated by bacoside A and bromelain.
Keywords: Dichlorvos, Bacoside A, Bromelain, Neurotransmitter, Cholinergic circuit
Introduction
The cholinergic system is composed of organized nerve cells which utilize the neurotransmitters (acetylcholine (AChE) and butyrylcholine (BChE)) for the transduction of action potentials to regulate cognitive functions such as memory, learning, dendrite arborization, neuronal development and differentiation (Fernandez et al. 2003; Fodale et al. 2006; Power 2004; Ravaglia et al. 2005). Dichlorvos is an organophosphate insecticide which affects the brain NT level abruptly. It leads to impairment in the cholinergic system which is responsible for disturbance of the cognitive function and neurodegeneration. AChE and BChE are the key enzymes of the cholinergic system. In the cholinergic system, AChE breaks down the Ach into choline and acetylcoenzyme A at the synaptic cleft that acts as a impulse transmitter across the synaptic gap. BChE also inactivates the neurotransmitter ACh like AChE (Bist and Bhatt 2010). Dichlorvos modulates the activity of AChE which may lead to increased ACh levels (Kobayashi et al. 1986; Sarin and Gill 1999; Sarin and Gill 2000; Yadav et al. 2012). Gamma-aminobutyric acid (GABA) is a major neurotransmitter which is distributed throughout the central nervous system (CNS). A decreased GABA level in the brain is associated with several psychiatric and neurological disorders such as anxiety, depression, insomnia and epilepsy. Serotonin, or 5-hydroxytryptamine (5-HT), has been implicated in almost every conceivable physiologic or behavioral function. Serotonin level is elevated by the exposure to dichlorvos (Maslinska et al. 1981).
In the current investigation, a combination of bacoside A and bromelain was attempted against dichlorvos-induced toxicity in the mouse brain. Bacoside A is a nerve tonic which is isolated from Bacopa monnieri (Bhattacharya et al. 2000). It renovates the damaged neurons by enhancing kinase activity, neuronal synthesis, restoration of synaptic activity and nerve impulse transmission (Singh and Dhawan 1997). Bromelain is isolated from Ananas comosus. It has the property to increase the permeability of tissues and cells for the absorption of antibiotics. Bromelain is helpful in neuroprotection and improvement of symptoms in neurodegenerative diseases (Habashi et al. 2012). Considering dichlorvos-induced disruption of brain function, involvement of key neurotransmitter enzymes is fundamental to the present investigation.
Materials and methods
Chemicals and animal monitoring
Dichlorvos, bacoside A, bromelain, acetylthiocholine iodide and butyrylthiocholine iodide were purchased from the Sigma Chemicals Company (St. Louis, MO, USA). 5,5′-Dithiobis-2-nitrobenzoic acid (DTNB) and 2-thiobarbituric acid were purchased from Himedia (Chandigarh, India). Nitroblue tetrazolium (NBT) was purchased from SRL (Mumbai, India). All other chemicals used in the present investigation were of analytical grade.
Healthy 6–8-week old, male Swiss mice (Mus musculus) were procured from C.C.S. Haryana Agriculture University, Hisar. All the studies were carried out on five groups of animals with a minimum of six animals in each group. Maintenance and treatment of animals were done in accordance with the Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA).
Experimental design
Dichlorvos, bacoside and bromelain were dissolved in water in separate vials (Table 1). Dichlorvos and bacoside were injected intraperitoneally and bromelain was given by oral mode. Duration of exposure of different doses was 21 days.
Table 1.
The investigation was carried out in five groups of animals
| Groups | Treatment |
|---|---|
| Group I | Control (normal saline as a vehicle) |
| Group II | Dichlorvos (40 mg/kg bw) |
| Group III | Bacoside (5 mg/kg bw) |
| Group IV | Bromelain (70 mg/kg bw) |
| Group V | Dichlorvos (40 mg/kg bw) + bacoside (5 mg/kg bw) + bromelain (70 mg/kg bw) |
Methods
Biochemical studies
After 21 days of duration of dosing, control and treated mice were sacrificed by cervical dislocation. Brains were immediately removed and homogenized. Selection of the homogenization medium was in accordance with the method applied. For AChE, BChE and total protein estimation, brains were homogenized in phosphate buffer (pH 7.0). For GABA and serotonin measurements, tissue homogenization media were 70% ethanol and borate buffer, respectively. Homogenates were stored at −20 °C and thawed just before the reaction.
AChE and BChE
The activities of AChE and butyrylcholinesterase (BChE) were determined by the Ellman et al. (1961) method. Thiocholine released from acetylcholine and butyrylcholine by the AChE and BChE, respectively, reacts with the DTNB, reducing it to the thiol which has absorption at 412 nm.
Serotonin
Determination of serotonin is a two-step process, i.e., serotonin extraction and colorimetric analysis of serotonin. The serotonin level for different groups was determined by Udenfriend et al. (1957).
GABA
The extraction and colorimetric analysis of GABA content were done by the Graham and Aprison (1966) method.
Total protein estimation
Quantification of total protein was done by the method of Lowry et al. (1951).
Molecular studies
For detecting any alteration in the protein expressions after various treatments, first of all, total proteins of the different regions of the brain were extracted by the method of Ericsson and Nister (2011). Extracted proteins were analyzed for any variation by SDS-PAGE.
Results
AChE
In dichlorvos-treated mice, AChE activity declined compared with that in groups I, III (p < 0.001), IV (p < 0.01) and V (p < 0.001). Activity of AChE increased in bacoside-treated mice compared with that in groups I, II (p < 0.001) and IV significantly; whereas, it diminished compared with that in group V. In bromelain-treated mice, AChE activity was found significantly elevated compared with that in groups I and II (p < 0.01); whereas, it decreased compared with that in groups III and V (p < 0.01). The maximum activity of AChE was found in group V compared with that in groups I (p < 0.001), II (p < 0.001), III and IV (p < 0.01) significantly (Table 2).
Table 2.
Change in AChE, BChE, GABA and serotonin level in the whole brain of different experimental groups
| Groups | AChE (nmole/mg protein) |
BChE (nmole/mg protein) |
GABA (nmole/mg protein) |
Serotonin (ng/g tissue weight) |
|---|---|---|---|---|
| Group I | 0.088 ± 0.0036e*** | 0.0025 ± 0.00047 | 4.35 ± 0.93b** | 13.28 ± 3.04 |
| Group II | 0.045 ± 0.0041d**,c***,e*** | 0.0007 ± 0.00021c*,e* | 1.20 ± 0.23a**,c** | 15.59 ± 3.0943 |
| Group III | 0.1287 ± 0.010b*** | 0.0039 ± 0.0012b* | 4.37 ± 0.76b** | 11.22 ± 2.29 |
| Group IV | 0.1085 ± 0.016b**,e** | 0.0025 ± 0.0007 | 2.63 ± 0.29 | 11.42 ± 4.07 |
| Group v | 0.1896 ± 0.026d**,a***,b*** | 0.0041 ± 0.0014b* | 2.78 ± 0.07 | 15.09 ± 6.15 |
Results are expressed as mean ± S.E.
*p<0.05, **p<0.01, ***p<0.001
aCompared to group I
bCompared to group II
cCompared to group III
dCompared to group IV
eCompared to group V
BChE
The level of BChE declined by the exposure to dichlorvos compared with that in groups I, III (p < 0.05), IV and V (p < 0.05). In bacoside-treated mice, BChE level increased compared with that in groups I, II (p < 0.05) and IV; whereas, it decreased compared with that in group V. BChE level elevated in bromelain-treated mice compared with that in groups I and II; whereas, it diminished compared with that in groups III and V. In group V, an elevated BChE level was found compared with that in groups I, II (p < 0.05), III and IV (Table 2).
GABA
The level of GABA in dichlorvos-treated mice decreased compared with that in groups I (p < 0.01), III (p < 0.01), IV and V. In bacoside-treated mice, the maximum level of GABA was found compared with that in groups I, II (p < 0.01), IV and V. GABA level was found increased in bromelain-treated mice compared with that in group II; whereas, it decreased compared with that in groups I, III and IV. In group V, the level of GABA was found to be increased compared with that in groups II and IV; whereas, it declined compared with that in groups I and III (Table 2).
Serotonin
In dichlorvos-treated mice, the maximum level of serotonin level was found compared with that in groups I, III, IV and V. A diminished level of serotonin was found in bacoside-treated mice compared with that in groups I, II, IV and V. The serotonin level declined in bromelain-treated mice compared with that in groups I, II and IV; whereas, it elevated compared with that in group III. In group V, the level of serotonin decreased compared with that in group II; whereas it increased compared with that in groups I, III and IV (Table 2).
Total protein
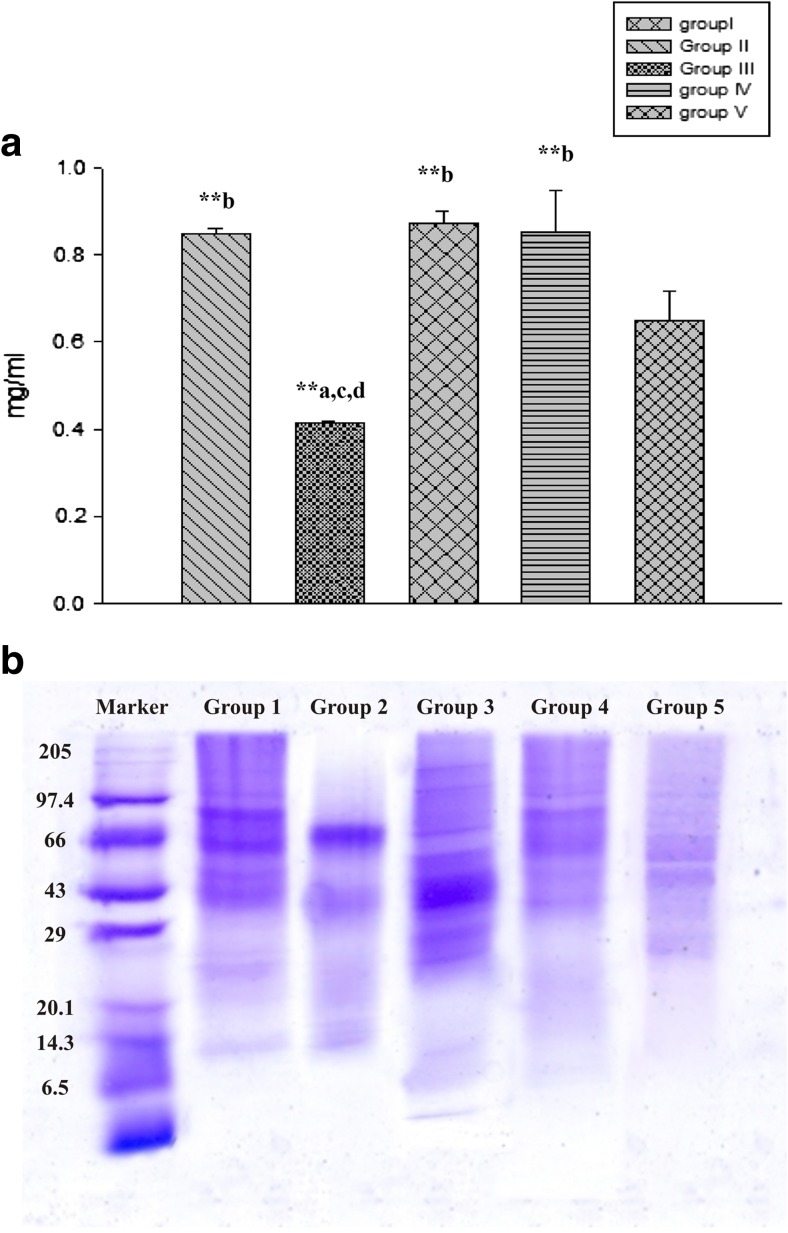
Total protein content declined in dichlorvos-treated mice compared with that in groups I, III and IV significantly (p < 0.01). In group III, total proteins increased compared with that in groups I, II (p < 0.01), IV and V. Mice which were treated with bromelain showed an elevated protein content compared with that in group II (p < 0.01). However, total protein level was recorded to be diminished after bromelain exposure compared with that in group III. In group V, a decreased level of total protein was found compared with that in groups I, III and IV; whereas, it elevated compared with that in group II (Fig. 1a).
Fig. 1.
a Total protein levels in the mouse brains of different experimental groups. Results are expressed as mean ± S.E. **p<0.01, a compared with group I, b compared with group II, c compared with group III, d compared with group IV, e compared with group V. b Gel picture showing variations in expressions of stress proteins in different experimental groups
Molecular studies
A visible change in band pattern and their intensities is recorded for the proteins of differently treated groups compared with that in the control. The differences in the intensity of staining reflect the relative abundance of individual polypeptides. Unusual patterns might indicate isoenzymes, incomplete denaturation, degradation, etc. There is no visible expression of phosphorylase a and b (92 and 94 kDa) in dichlorvos-treated mice. Expression of phosphorylase a and b is vigilant in bacoside A; whereas, it is the highest in bromelain-treated mice. However, the expression of phosphorylase a and b recovered in group V. The highest expression of stress protein Hsp70 (65–80 kDa) is vigilant in group II among all the groups. A lower expression of Hsp70 was recorded in groups III, IV and V. The protein having the molecular range of 43–66 kDa (catalase) showed no expression in the dichlorvos-treated group. The expression of catalase was recorded vigilant in groups III and V; whereas, the highest expression was found in group IV. In dichlorvos-treated mice, a lower expression of SOD (32 kDa) was observed. The highest expression of SOD is vigilant in bacoside-exposed mice; whereas, a mild expression was found in group IV. However, the expression of SOD recovered in group V. A lower expression of GPx (23–29 KDa) was observed by dichlorvos and bromelain exposure. However, the highest expression of GPx was vigilant in bacoside-treated mice; whereas, it recovered in group V (Fig. 1b).
Discussion
Cognitive dysfunction is a major health problem in neuropsychiatric and neurodegenerative disorders including schizophrenia, depression, Alzheimer’s disease, dementia, seizure disorders and Parkinsonism (Kumar and Khanum 2012). In the current study, dichlorvos inhibited the AChE and BChE activities which may lead to neurodegeneration. Dichlorvos-induced decline in AChE and BChE activities is recorded in earlier studies (Celik et al. 2009; Taylor et al. 2008; Yadav et al. 2012). It may be due to dichlorvos binding with an AChE molecule in the synaptic cleft which leads to an increase in ACh levels and inhibits AChE activities in the brain (Chaudhary et al. 2014). Treatment with bacoside A and bromelain separately increased the activity of AChE and BChE. Exposure to bacoside A and bromelain both along with dichlorvos increased the activities of AChE and BChE which implies that bacoside A and bromelain have the property to boost up the cholinergic system. Bacoside A increases AChE activity in aged rat brain cortex and the middle aged (Rastogi et al. 2012). After dichlorvos exposure, the level of GABA declined. The descent in GABA level, after dichlorvos exposure, may be due to the consequence of glutamic acid decarboxylase (GAD) inhibition following local formation of 2-keto-4 pentenoic acid (Bist and Bhatt 2009). 5-HT is effective on brain function (Pilot et al. 1983). Subacute dichlorvos exposure perturbed the mouse serotonin level. Brain is the organ having a high lipid content, becoming the main target of γ HCH, where it can easily dissolve in the membrane of pre- and postsynaptic elements thereby leading to increased levels of serotonin content in the brain (Bist and Bhatt 2009). Exposure to bacoside A and bromelain separately and both along with dichlorvos increased the GABA and decreased the serotonin activity. In a previous study, Rastogi et al. (2012) reported that bacoside A prevents the loss of serotonin and dopamine content. Bacoside A increased the GABA receptor in epileptic mice (Mathew et al. 2009). Bromelain has the property to increase the permeability of the blood-brain barrier to nutrients (Lauer et al. 2001). A previous study suggests that the interaction of bacoside A with TPH might upregulate its activity to elevate the biosynthesis of 5-HT, thereby enhancing learning and memory formation (Rajathei et al. 2014).
In the present study, dichlorvos leads to a decrease in total protein. It may be due to dichlorvos-induced oxidation of proteins which generated protein carbonyl content (PCC) (Kaur et al. 2007; Yadav et al. 2012). Treatment with bacoside and bromelain separately and both along with dichlorvos increased total protein level which may be due to their free radical-scavenging activity.
Results of molecular expression of proteins are in concordance with those of a biochemical study. It was observed that dichlorvos induced overexpression of Hsp 70 (Aridon et al. 2011) and lower expressions of GPX and SOD. Jadhav and Rajini (2009) reported the heat shock protein induction after dichlorvos exposure such as overexpression of Hsp 70 that leads to generation of free radicals and disturbance of the endogenous antioxidant system, which may involve lower expressions of GPX and SOD. There are no expressions of phosphorylase a and b and catalase in dichlorvos-treated mice; it may be due to dichlorvos leads to depletion in the glycogen content and catalase activity (Janani et al. 2010; Sarin and Gill 1999). Since metabolic maintenance of brain tissue needs glucose as major fuel, the result of the study suggests that dichlorvos exposure affects the alteration in neuronal glucose. The eradication of free radicals after bacoside A exposure, the lower expressions of Hsp 70, catalase, phosphorylase a and b and the higher expressions of SOD and GPx were observed which indicates the involvement of particular proteins responsible for cell viability and neuronal integrity. In bromelain-treated mice, a lower expression of Hsp 70 and the highest expressions of catalase and phosphorylase a and b were observed. The current finding suggests that these particular proteins are involved in the scavenging of free radicals. In group V, all the proteins intensified and recovered between the range 29–205 kDa by the treatment of bacoside A and bromelain along with dichlorvos. It may be due to bacoside A (Anbarsi et al. 2006) and bromelain have the property to modulate the expression of heat shock protein 70 (Hsp70).
Acknowledgments
We thank Prof. Aditya Shastri, Vice Chancellor, Banasthali University and Department of Science and Technology (DST), India for providing the facilities for the present investigation.
Compliance with ethical standards
Maintenance and treatment of animals were done in accordance with Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA).
Contributor Information
Bharti Chaudhary, Email: chaudhary.bharti762@gmail.com.
Renu Bist, Email: renu_bisht22@yahoo.co.in.
References
- Anbarsi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006;78(12):1378–1384. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Aridon P, Geraci F, Turturici GD, Amelio M, Savettieri G, Sconzo G. Protective role of heat shock proteins in Parkinson’s disease. Neurodegener Dis. 2011;8:155–168. doi: 10.1159/000321548. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14:174–179. doi: 10.1002/(SICI)1099-1573(200005)14:3<174::AID-PTR624>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bist R, Bhatt DK. The evaluation of effect of alpha-lipoic acid and vitamin E on the lipid peroxidation, gamma-amino butyric acid and serotonin level in the brain of mice (Mus musculus) acutely intoxicated with lindane. J Neurol Sci. 2009;276(1–2):99–102. doi: 10.1016/j.jns.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Bist R, Bhatt DK. Augmentation of cholinesterases and ATPase activities in the cerebellum and pons-medulla oblongata, by a combination of antioxidants (resveratrol, ascorbic acid, alpha-lipoic acid and vitamin E), in acutely lindane intoxicated mice. J Neurol Sci. 2010;296(1–2):83–87. doi: 10.1016/j.jns.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Celik I, Yilmaz Z, Turkoglu V. Hematotoxic and hepatotoxic effects of dichlorvos at sublethal dosages in rats. Inc Environ Toxicol. 2009;24:128–132. doi: 10.1002/tox.20390. [DOI] [PubMed] [Google Scholar]
- Chaudhary B, Agrawal S, Bist R. Obliteration in oxidative stress and Ca++ uptake in brain mitochondria leads to impairment of cholinergic system: a possible mechanism underlying neurotoxicity induced by dichlorvos. BBB. 2014;2(3):550–564. [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Ericsson C, Nister M. Protein extraction from solid tissues. Methods in Mol Bio. 2011;675:307–312. doi: 10.1007/978-1-59745-423-0_17. [DOI] [PubMed] [Google Scholar]
- Fernandez CR, Fields A, Richards T, Kaye AD. Anesthetic considerations in patients with Alzheimer’s disease. J Clin Anesth. 2003;15:5258. doi: 10.1016/S0952-8180(02)00483-X. [DOI] [PubMed] [Google Scholar]
- Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB. Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth. 2006;97:445452. doi: 10.1093/bja/ael233. [DOI] [PubMed] [Google Scholar]
- Graham LT, Jr, Aprison MH. Flurometric determination of aspartate, glutamate and gamma-aminobutyrate in nerve tissue using enzymic methods. Anal Biochem. 1966;15:487–497. doi: 10.1016/0003-2697(66)90110-2. [DOI] [PubMed] [Google Scholar]
- Habashi SA, Moghimi A, Sabouni F, Majd SA. Inhibition of NO production in LPS-stimulated primary rat microglial cells by bromelain. J of Cell and Mol Res. 2012;3(2):57–65. [Google Scholar]
- Jadhav KB, Rajini PS. Evaluation of sublethal effects of dichlorvos upon Caenorhabditis elegans based on a set of end points of toxicity. J Biochem Mol Toxicol. 2009;23(1):9–17. doi: 10.1002/jbt.20258. [DOI] [PubMed] [Google Scholar]
- Janani P, Sivakumari K, Geetha A, Ravisankar B, Parthasarathy C. Chemopreventive effect of bacoside A on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. J Cancer Res Clin Oncol. 2010;136:759–770. doi: 10.1007/s00432-009-0715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Radotra B, Minz RW, Gill KD. Impaired mitochondrial energy metabolism and neuronal apoptotic cell death after chronic dichlorvos (OP) exposure in rat brain. Neurotoxicol. 2007;28(6):1208–1219. doi: 10.1016/j.neuro.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Yuyama A, Chiba KI. Cholinergic system of brain tissue in rats poisoned with the organophosphate 0,0-demethyl 0-(2,2-dichlorovinyl) phosphate. Toxicol Appl Pharmacol. 1986;82:32–39. doi: 10.1016/0041-008X(86)90434-5. [DOI] [PubMed] [Google Scholar]
- Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6(12):81–90. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S, Reichenbach A, Birkenmeier G. Alpha 2-macroglobulin-mediated degradation of amyloid beta 1-42: a mechanism to enhance amyloid beta catabolism. Exp Neurol. 2001;167:385–392. doi: 10.1006/exnr.2000.7569. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. The original method for protein estimation. Biol Chem. 1951;193:265. [PubMed] [Google Scholar]
- Maslinska D, Lewandowska I, Prokopczyk J. Effect of prolonged acetylcholinesterase inhibition on postnatal brain development in rabbit. I. Level of serotonin in different brain regions. Acta Neuropathol, suppl Berl. 1981;7:52–55. doi: 10.1007/978-3-642-81553-9_16. [DOI] [PubMed] [Google Scholar]
- Mathew J, Paul J, Nandhu MS, Paulose CS. Bacopa monnieri and bacoside–A for ameliorating epilepsy associated behavioral deficits. Fitoterapia. 2009;81(5):315–322. doi: 10.1016/j.fitote.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Pilot MA, Thompson HH, Zara GP. Effect of hydroxytryptamine on canine intestinal motility during fasting. J Physiol. 1983;343:88–99. [Google Scholar]
- Power AE. Muscarinic cholinergic contribution tomemory consolidation: with attention to involvement of the basolateral amygdala. Curr Med Chem. 2004;11:987996. doi: 10.2174/0929867043455558. [DOI] [PubMed] [Google Scholar]
- Rajathei DM, Preethi J, Singh HK, Rajan KE. Molecular docking of bacosides with tryptophan hydroxylase: a model to understand the bacosides mechanism. Nat Prod Bioprospect. 2014;4:251–255. doi: 10.1007/s13659-014-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M, Rudra P, Ojha PPC, Devi BP, Agrawal A, Dubey GP. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontol. 2012;13:183–195. doi: 10.1007/s10522-011-9367-y. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Dalmonte E, Bianchin M, Mariani E. Incidence and etiology of dementia in a large elderly Italian population. Neurology. 2005;64:15251530. doi: 10.1212/01.WNL.0000160107.02316.BF. [DOI] [PubMed] [Google Scholar]
- Sarin S, Gill KD. Dichlorvos induced alterations in glucose homeostasis: possible implications on the state of neuronal function in rats. Mol and Cell Biochem. 1999;199:87–92. doi: 10.1023/A:1006930511459. [DOI] [PubMed] [Google Scholar]
- Sarin S, Gill KD. Biochemical characterization of dichlorvos-induced delayed neurotoxicity in rat. IUBMB Life. 2000;49:125–130. doi: 10.1080/15216540050022449. [DOI] [PubMed] [Google Scholar]
- Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi) Indian J Pharmacol. 1997;29:S359–S365. [Google Scholar]
- Taylor JT, Davis E, Dabisch P, Horsmon M, Matson K, Crouse C, Mioduszewski R. Acute toxic effects of inhaled dichlorvos vapor on respiratory mechanics and blood cholinesterase activity in guinea pigs. Inhal Toxicol. 2008;20(5):465–472. doi: 10.1080/08958370701805709. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Weissbach H, Bogdanski DF. Increase of tissue serotonin following administration of its precursor 5-hydroxytryptophan. J Biol Chem. 1957;224:803–810. [PubMed] [Google Scholar]
- Yadav P, Jadhav SE, Kumar V, Kaul KK, Pant SC, Flora SJS. Protective efficacy of 2-PAMCl, atropine and curcumin against dichlorvos induced toxicity in rats. Interdiscip Toxicol. 2012;5(1):1–8. doi: 10.2478/v10102-012-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]