Abstract
Induction of HSP72 is a natural response of stressed organisms that protects against many insults including bacterial diseases in farm (aquatic) animals. It would therefore be of great health benefit to search for natural compounds that are clinically safe yet able to induce HSP72 in animals. The phenolic compound carvacrol, an approved food component, had been shown in in vitro study to act as a co-inducer of HSP72, enhancing HSP72 production only in combination with a bona fide stress compared to the compound alone. However, in vitro model systems do not completely represent an in vivo physiology. Here, using the well-established gnotobiotic Artemia model system, we determined whether carvacrol could induce HSP72 in vivo, whether this putative effect could generate resistance in Artemia against biotic/abiotic stress and also unraveled the mechanism behind the possible HSP72-inducing effect of carvacrol. The gnotobiotic system is crucial for such studies because it avoids the interference of any extraneous factors on host-compound interaction. Here, carvacrol was shown to be a potent HSP72 inducer. Induction of HSP72 was associated with the generation of resistance in Artemia larvae against subsequent lethal heat stress or pathogenic Vibrio harveyi. Our results also provided new insight on the mode of HSP72 inducing action of carvacrol, in which the initial generation of reactive molecule H2O2 by the compound plays a key role. Overall results add new information about the bioactivity of carvacrol and advance our knowledge of this compound as potential prophylactic agent for controlling Vibrio infection in aquaculture animals.
Keywords: Gnotobiotic Artemia, Carvacrol, Heat shock protein, Vibrio harveyi, Stress
Introduction
The 70-kDa heat shock proteins (HSP70s) are a group of highly conserved proteins of which expression is constitutive or inducible under different conditions. Functionally, HSP70s are molecular chaperones that play key roles in many vital cellular functions under both normal and stressful conditions, and those include assisting in the folding of nascent proteins, translocation of these proteins between cell organelles, assembly and disassembly of multi-subunit complexes, refolding or degradation of denatured proteins due to stresses, dissolution of pathological protein aggregates, and other processes enhancing the survival of normal and diseased cells (see review Roberts et al. 2010). Additionally, the stress-inducible HSP72 is also implicated in the generation of protective immune responses against many diseases, as demonstrated in a wide variety of experimental models in vitro and in vivo (Chen and Cao 2010; Johnson and Fleshner 2006; Tsan and Gao 2009). Because of its multifaceted beneficial roles, the inducible form of HSP70 has gained considerable attention as a potential agent for the prevention and treatment of diseases in human as well as in farmed terrestrial and aquatic animals (Nagai et al. 2010; Roberts et al. 2010; Westerheide and Morimoto 2005).
So far, several strategies have been employed for enhancing the availability of HSP72 within aquaculture animals, and these include exposure of the animals to nonlethal thermal shock, preconditioning stress, and transfection of HSP72 genes (Roberts et al. 2010). However, the main constraints in the application of these techniques in aquatic farming system are the high application cost, nonuser friendly, and risk associated with increased temperature on the animals. Because of these issues, there is currently a strong impetus in identifying natural compounds that possess the property of inducing HSP70 within animals in a noninvasive manner and that are easy for application in farm animals (Roberts et al. 2010).
The phenolic compound carvacrol is a major component of essential oils obtained from the herbs oregano and thyme (Skoula et al. 1999; Fig. 1). It is recognized as a generally recognized as safe (GRAS) compound (FDA 2011) for use as feed additive and food preservative in the food industry (Shoji and Nakashims 2004). So far, carvacrol has been studied mainly for its antibacterial effects (Burt 2004), and from that perspective, it has been described to increase the expression of HSP60 but not HSP70 in prokaryotes (Burt et al. 2007). However, in an in vitro study in eukaryotic (mammalian) cells, carvacrol was shown to co-induce cellular HSP70 expression (Wieten et al. 2010). In contrast to an HSP inducer, which upregulates HSP expression by itself, a co-inducer increases HSP levels only in combination with a bona fide stress signal (Ohtsuka et al. 2005). It is important to mention that in vitro model systems (e.g., cell culture) do not completely represent in vivo physiology. The results obtained from in vitro studies therefore may not provide sufficient or realistic information to precisely understand the biological property (e.g., HSP72 induction) of the candidate compound (Astashkina et al. 2012). The fact that carvacrol is a GRAS compound, determining the HSP72-inducing activity of this compound under in vivo condition, could be of great relevance for applying this compound as anti-infective agent in farmed aquatic animals.
Fig. 1.

Chemical structure of carvacrol
A major handicap in studying the HSP-inducing activity of a compound in an in vivo system is the difficulty to eliminate (or distinguish the effect of) the microbial communities that is naturally present in the system (Baruah et al. 2014, 2015a). In fact, under germ-associated experimental conditions, the microbial communities might metabolize the compound of interest, thereby making it difficult to better comprehend the activity of the compound. Additionally, the standing microbial communities are also a good source of the endotoxin lipopolysaccharide (LPS), which has been shown to induce HSPs in different animal model systems (Chiu et al. 2007; Kojima et al. 2004). This indicates that under germ-associated conditions, the physiological responses of the host (in terms of HSP production) due to exposure to the tested compound could simply reflect the influence of the various microbial communities (LPS or other cellular component) rather than a genuine biological effect of the compound. Hence, the selection of an appropriate animal model system that allows to better understand the HSP72-inducing activity of a compound is therefore of high importance.
The brine shrimp Artemia franciscana, an aquatic invertebrate that can be reared under gnotobiotic conditions (allowing full control over the host-associated microbial communities, Marques et al. 2006), represents an exceptional experimental system for carrying out such studies as it allows to distinguish direct effects on the host [by (pre) exposing axenic Artemia larvae to the compound for a certain duration] from indirect effects. It also eliminates any possible microbial interference during the exposure period and hence facilitates the interpretation of the results in terms of a cause-effect relationship (Baruah et al. 2014). In this study, using the highly controlled gnotobiotic Artemia model system, we aimed to investigate whether carvacrol could manipulate the production of HSP72 in vivo and whether this putative effect could contribute to the induction of resistance in Artemia against pathogenic Vibrio harveyi, an opportunistic bacterium that causes significant mortalities in the farmed aquatic animals, including Artemia (Austin and Zhang 2006). Furthermore, we also unraveled the mechanism behind the possible HSP72-inducing effect of carvacrol.
Materials and methods
Bacterial strain and growth conditions
Vibrio harveyi BB120, stored in 4.3 M glycerol at −80°C, was grown at 28°C for 24 h on Marine Agar (Difco Laboratories, Detroit, MI. USA) and then to log phase in Marine Broth 2216 (Difco Laboratories, Detroit, MI. USA) by incubation at 28°C under constant agitation. Bacterial cell numbers were determined spectrophotometrically at 550 nm as previously described (Baruah et al. 2009).
Reagents
Carvacrol purchased from Sigma-Aldrich (98% Diegem, Belgium; Fig. 1) was used for the experiment. An amount of 20 mg (20.5 μl) of carvacrol was dissolved in 9.98 ml of sterile distilled water using a vortex mixer to obtain a volume of 10 ml with concentration of 2 g/l (13.3 mM). Superoxide dismutase (SOD; 2000–6000 units/mg protein) and catalase (2000–5000 units/mg protein) were obtained from Sigma. Catalase was dissolved in sterile distilled water at 0.5 g/l as a stock solution. All the stock solutions were prepared fresh for each experiment.
Hatching of germ-free Artemia larvae
All experiments were performed with high-quality hatching cysts of Artemia franciscana (EG® Type, batch 21,452, INVE Aquaculture, Dendermonde, Belgium). Approximately 1.5 g of Artemia cysts originating from the Great Salt Lake, Utah, USA (EG® Type, batch 21,452, INVE Aquaculture, Dendermonde, Belgium), were hydrated in 89 ml of distilled water for 1 h. Sterile cysts and larvae were obtained via decapsulation using 3.3 ml NaOH (8.0 M) and 50 ml NaOCl (6.7 M). During the reaction, 0.2-μm filtered aeration was provided. All manipulations were carried out under a laminar flow hood and all tools were autoclaved at 121°C for 20 min. The decapsulation was stopped after about 2 min by adding 50 ml Na2S2O3 at 10 g/l. The aeration was then terminated and the decapsulated cysts were washed with sterile artificial seawater containing 35 g/l of Instant Ocean® synthetic sea salt (Aquarium Systems, Sarrebourg, France). The cysts were suspended in 1-l glass bottles containing sterile seawater and further incubated for 28 h with constant illumination of approximately 27 μE/m2/s. After 28 h of incubation, the sterility of the hatched Artemia larvae was verified by spread plating (100 μl) as well as by adding (500 μl) hatching water on Marine Agar and Marine Broth, respectively, followed by incubating at 28°C for 5 days (Baruah et al. 2009). All these manipulations were performed under a laminar flow hood in order to maintain sterility of the cysts and larvae. Experiments started with nonsterile larvae were discarded.
In vivo pre-pretreatment of Artemia
In total, five separate tests were carried out. In the first and second tests, the putative protective effect of carvacrol was determined. To this end, a dose-response relationship of carvacrol was determined under two different challenge conditions as described previously (Baruah et al. 2012, 2015b). Briefly, hatched Artemia larvae at developmental stage II were collected, counted volumetrically by first counting a subsample of exactly 1 ml and multiplying that number by the total measured volume (ml) of hatched larvae. Thereafter, the larvae were transferred to 250-ml sterile glass bottles containing sterile seawater. The larvae were pretreated with increasing concentrations of carvacrol (16.6, 33.2, 66.4, 99.6, 132.8, 166.4 μM) for 2 h at 28°C. The larvae were rinsed repeatedly with sterile artificial seawater to wash away the compound and then allowed to recover for 2 h at 28°C. Following recovery period, groups of 20 larvae from the 250-ml glass bottles were transferred to sterile 40-ml glass tubes that contained 10 ml of sterile artificial seawater (35 g/l salinity). Subsequently, it was verified whether such pretreatment can protect the larvae against subsequent challenge with pathogenic V. harveyi at 107 cells/ml (Baruah et al. 2010) or against a lethal heat stress at 41°C for 20 min (Baruah et al. 2012). The survival of Artemia larvae was scored manually at an indicated period after challenge as described previously (Baruah et al. 2015b). In both the tests, non-pretreated larvae that were not challenged with Vibrio/lethal heat stress or challenged were maintained as controls. Five replicates were maintained for each treatment and control.
In the subsequent test, the cytotoxic effect of carvacrol was determined in the axenic Artemia larvae as previously described (Baruah et al. 2015b). The axenic larvae were pretreated with increasing concentrations of carvacrol (16.6, 33.2, 66.4, 99.6, 132.8, 166.4 μM) as described above. They were rinsed repeatedly with sterile artificial seawater to wash away the compound and then allowed to recover for 2 h at 28°C. Artemia larvae that did not receive carvacrol pretreatment served as control. Five replicates were maintained for each treatment and control. Toxicity bioassay was performed in sterile 40-ml glass tubes with 20 larvae in 10 ml of sterile artificial seawater per tube. The toxicity of the compound was determined after 48 h of recovery period by scoring the number of survivors as previously described (Baruah et al. 2015b).
In the third and fourth tests, the mode of action of carvacrol was determined. The germ-free larvae were pretreated with an optimized dose of carvacrol (dose which gave maximum protection to challenged larvae in the dose-response assay), a mixture of antioxidant enzymes catalase (10 mg/l) and SOD (75 units), or a combination of carvacrol and antioxidant enzymes mixture in a similar fashion as described above. The Artemia larvae in the control group did not undergo any pretreatment. Groups of 20 larvae were counted, distributed into sterile 40-ml glass tubes, and then challenged with V. harveyi or lethal heat stress as described in the dose-response study. Controls were maintained as described in the figure legend. Survival of the larvae was scored after 48 h of challenge.
Protein extraction and HSP72 detection
Following pretreatment, live Artemia larvae (0.1 g) from all groups of the first and third tests were collected separately on 50-μm sieves, rinsed in distilled water, immediately frozen in liquid nitrogen and stored at −80°C for Hsp70 analysis. Artemia samples were homogenized in cold buffer K (150 mM sorbitol, 70 mM potassium gluconate, 5 mM MgCl2, 5 mM NaH2PO4, 40 mM HEPES, pH 7.4) (Clegg et al. 2000), supplemented with protease inhibitor cocktail (Sigma-Aldrich®, USA) as recommended by the manufacturer. Subsequent to centrifugation at 2200×g for 1 min at 4°C, supernatant protein concentrations were determined by the Bradford method (Bradford 1976) using bovine serum albumin as standard. Supernatant samples were then combined with loading buffer, vortexed, heated at 95°C for 5 min, and electrophoresed in 10% SDS-PAGE gel, with each lane receiving equivalent amounts of protein. HeLa (heat shocked) cells (Enzo Life Sciences, USA) (6 μg) were loaded on to one well to serve as a positive control. Gels were either stained with Coomassie Biosafe (BioRad Laboratories) or transferred to polyvinylidene fluoride membrane (BioRad Immun-Blot™ PVDF) for antibody probing. Membrane was incubated with blocking buffer [50 ml of 1× phosphate-buffered saline containing 0.2% (v/v) Tween-20 and 5% (w/v) bovine serum albumin] for 60 min at room temperature and then with mouse monoclonal anti-Hsp70 antibody, clone 3A3 (Affinity BioReagents Inc., Golden, CO), which recognizes both constitutive and inducible Hsp70 (Baruah et al. 2012), at the recommended dilution of 1:5000. Horseradish peroxidase-conjugated donkey anti-mouse IgG was used as secondary antibody at the recommended dilution of 1:2500 (Affinity BioReagents Inc., Golden, CO). The membrane was then treated with enhanced chemiluminescence reagent (GE healthcare, UK), and the signals were detected by a ChemiDoc MP Imaging System (BioRad, Belgium).
Fluorescence measurement of hydrogen peroxide production in the water
The optimum dose of carvacrol and/or a mixture of antioxidant enzymes (catalase, 10 mg/l and SOD, 75 U) was added to 30 ml of sterile seawater in glass bottles and incubated at 28°C for 2 h. A control was maintained without the addition of compound/enzyme mixture in the distilled water. Water samples were taken after 2 h of incubation and immediately analyzed for hydrogen peroxide (H2O2) release/production using the Amplex® Red Hydrogen Peroxide Assay Kit (Invitrogen, Belgium) as described previously (Baruah et al. 2014).
Statistical analysis
Survival data were arcsin transformed to satisfy normality and homoscedasticity requirements as necessary. The data were then subjected to one-way analysis of variances (ANOVA) followed by Duncan’s multiple range tests using the statistical software Statistical Package for the Social Sciences version 20.0. P values ≤0.05 were considered significant.
Results
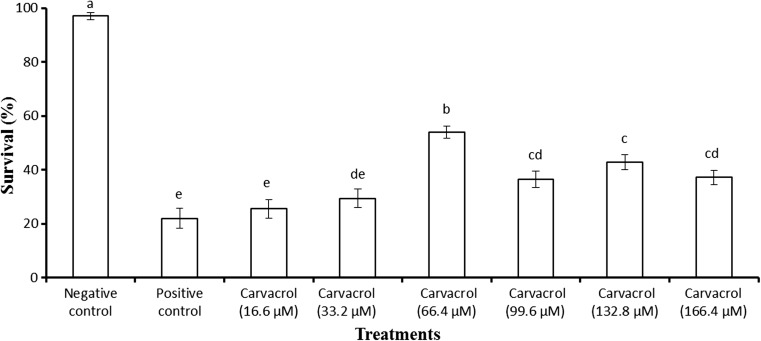
Impact of carvacrol on the survival of Artemia larvae challenged with V. harveyi
In a first experiment, we investigated whether carvacrol could confer protection prophylactically against V. harveyi to the host Artemia. To this end, germ-free Artemia larvae were pretreated with varying concentrations of carvacrol under indicated conditions, and then the larvae were challenged with V. harveyi. Artemia larvae that received carvacrol pretreatment in the range of 16.6 to 166.4 μM exhibited a significant increase in their survival compared to the control (Fig. 2). However, maximum survival (sixfold higher than the control) was observed at a concentration of 66.4 μM. Pretreatment of larvae with carvacrol at a concentration higher than 66.4 μM did not further improve the larval survival. In contrast, the survival tended to decrease with further increase in the concentration.
Fig. 2.
Effect of carvacrol pretreatment on the survival of Artemia after 48 h of challenge with V. harveyi. The larvae were pretreated with carvacrol at different doses (16.6, 33.2, 66.4, 99.6, 132.8, 166.4 μM) for 2 h, rinsed to wash away the compound, and then allowed to recover for 2 h. The larvae were subsequently challenged with V. harveyi at 107 cells/ml of rearing water. Non-pretreated larvae that were either challenged with V. harveyi (positive control) and those not challenged with V. harveyi (negative control) served as controls. Error bars represent the standard error of five replicates. Bars with same alphabet letters are not different significantly (P > 0.05, Duncan’s new multiple range test)
Impact of carvacrol on the survival of Artemia larvae challenged with lethal heat stress
Next, we tested how the Artemia larvae pretreated with different carvacrol concentrations would respond to a temperature shock that is lethal for Artemia grown at standard temperature. We found that, on exposure to a typically lethal stress (41°C for 20 min), Artemia larvae that were pretreated with carvacrol at 66.4 μM concentration had significantly higher survival than the control (Fig. 3). It is worth noting that the increase in the thermal resistance was not displayed by the Artemia larvae that were pretreated with carvacrol at concentration lower than 66.4 μM. In addition, no marked improvement in the survival was also observed in the groups that were pretreated with carvacrol at a concentration higher than 66.4 μM. These data from the thermal challenge test reported here, together with the data from the above bacterial challenge test, suggested a carvacrol concentration of 66.4 μM as the optimum dose and were therefore chosen for carrying out subsequent experiments.
Fig. 3.
Effect of carvacrol pretreatment on the survival of Artemia after 24 h of challenge with a lethal heat stress. The larvae were pretreated with carvacrol at different doses (16.6, 33.2, 66.4, 99.6, 132.8, 166.4 μM) for 2 h, rinsed to wash away the compound, and then allowed to recover for 2 h. The larvae were subsequently challenged with a lethal heat stress at 41°C for 20 min. Non-pretreated larvae that were either challenged with lethal heat stress (positive control) and those not challenged with lethal heat stress (negative control) served as controls. Error bars represent the standard error of five replicates. Bars with the same alphabet letters are not different significantly (P > 0.05, Duncan’s new multiple range test)
Toxicity of carvacrol to axenic Artemia larvae
Since the survival of Vibrio- or thermal-challenged Artemia larvae pretreated with carvacrol at concentration higher than 66.4 appeared to decrease, we next investigated whether it was due to the toxic effect of carvacrol following methodology as described in Materials and Methods. As shown in Fig. 4, Artemia larvae pretreated with carvacrol in the range of 16.6 μM to 66.4 μM did not exhibit significant difference in the survival compared with the control. However, at concentrations higher than 99.6 μM, the survival of the larvae significantly decreased. This result indicated that carvacrol appeared to exert toxic effect on the experimental animal under the present experimental conditions.
Fig. 4.
Toxicity of carvacrol to Artemia larvae. The larvae were pretreated with different doses of carvacrol as described in Fig. 2. Non-pretreated larvae served as control. Survival was scored 48 h after the recovery period. Error bars represent the standard error of five replicates. Bars with the same alphabet letters are not different significantly (P > 0.05, Duncan’s new multiple range test)
Impact of carvacrol on HSP72 production in Artemia larvae
Next, we investigated whether carvacrol could induce HSP72 in vivo. To address this, germ-free Artemia were pretreated with different doses of carvacrol and analyzed for HSP72 as described in the “Materials and Methods.” Interestingly, we found that there was a constitutive production of HSC70 in the control Artemia. However, carvacrol pretreatment at concentration of 66.4 μM significantly increased HSP72 production as compared with the control. Maximum induction of HSP72 was observed to occur in Artemia that were pretreated with a dose of 166.4 μM. However, the increase in the HSP72 induction level in the 166.4 μM pretreated group did not differ significantly from that of the groups pretreated with 99.6and 132.8 μM (Fig. 5). This result suggests that carvacrol is an effective inducer of HSP72.
Fig. 5.
Induction of HSP72 protein in Artemia larvae. Artemia larvae were pretreated with different doses of carvacrol as described in Fig. 2. Non-pretreated larvae served as control. a Protein extracted from different experimental groups was resolved on an SDS-PAGE gel and then transferred to a polyvinylidene fluoride membrane and probed with an antibody to Artemia HSP72. Five microgram of Artemia protein was loaded in each lane. Molecular mass standards (M) in kilodaltons are shown on the left. The level of HSP72 in HeLa cells was regarded as 1. Cropped blot image was shown. b Quantitative analysis of HSP72 levels in Artemia larvae is presented relative to HSP72 production in HeLa cells
Having observed this, we next sought to investigate the mechanism of action of carvacrol in inducing HSP72 within Artemia. As reactive oxygen species (e.g., hydrogen peroxide, H2O2) generated from the phenolic compounds were linked with HSP72 induction within Artemia (Baruah et al. 2014, 2015b), we hypothesized that carvacrol induces HSP72 by mechanism of generating ROS and that eliminating these reactive molecules by using ROS scavenging enzymes neutralizes the carvacrol-mediated induction of HSP72. We found that upon pretreatment with an optimized dose of carvacrol (66.4 μM), a significant increase in the production level of HSP72 was observed in the pretreated group as compared with the control (control vs. carvacrol; Fig. 6). However, co-pretreament of the Artemia larvae with carvacrol and antioxidant enzyme mixture resulted in a significant twofold decrease in the HSP72 production level as compared to the carvacrol-pretreated Artemia (carvacrol vs. carvacrol + antioxidant enzymes; Fig. 6b). Pretreatment of Artemia larvae with a mixture of antioxidant enzymes caused no significant effect on the induction of HSP72 as compared with the control.
Fig. 6.
Induction of HSP72 protein in Artemia larvae. Artemia larvae were pretreated with carvacrol, antioxidant enzymes, or in combination with both (carvacrol + antioxidant enzymes) as described in Fig. 2. Non-pretreated larvae were maintained as control. a Protein extracted from different experimental groups was resolved on an SDS-PAGE gel and then transferred to a polyvinylidene fluoride membrane and probed with an antibody to Artemia HSP72. Seven micrograms of Artemia protein was loaded in each lane. Molecular mass standards (M) in kilodaltons are shown on the left. The level of HSP72 in HeLa cells was regarded as 1. Cropped blot image was shown. b Quantitative analysis of HSP72 levels in Artemia larvae is presented relative to HSP72 production in HeLa cells. Dual bands appeared in a, in accordance with our earlier studies (Baruah et al. 2012; Norouzitallab et al. 2015). The upper and lower bands in a are predicted to represent constitutive HSP70 (HSC70 or HSP73) and the stress-inducible HSP70 (HSP70 or HSP72)
Impact of carvacrol on the generation of ROS
In the subsequent experiment, we aimed to verify whether carvacrol generates ROS in the Artemia rearing water, and for that, we measured the level of an ROS member H2O2 in the water at 2 h after addition of the compound. From Fig. 7, it is evident that addition of carvacrol into the Artemia rearing medium led to the release of a significant amount of H2O2 in the medium. However, in the presence of a mixture of the antioxidant enzymes catalase and SOD, the H2O2 level in the carvacrol added group was significantly reduced (carvacrol vs. carvacrol + antioxidant enzyme mixture). Controls without carvacrol (with or without antioxidant enzymes mixture) had no detectable levels of H2O2.
Fig. 7.
Production of H2O2 in the rearing water of Artemia larvae. a Carvacrol and/or a mixture of the antioxidant enzymes catalase and SOD at indicated doses were added to 30 ml of rearing water. After 2-h incubation, water samples were analyzed for H2O2 production. Error bars represent the standard error of three replicates. Bars with the same alphabet letters are not different significantly (P > 0.05, Duncan’s new multiple range test)
Impact of carvacrol in the presence or absence of an antioxidant enzyme mixture on the survival of Artemia larvae challenged with V. harveyi or lethal heat stress
To further confirm the contribution of carvacrol-generated H2O2 to the mediation of HSP72 induction, we conducted additional in vivo survival assays as described in the Fig. 8 legends. We assumed that scavenging of the carvacrol-generated H2O2 by the antioxidant enzymes mixture would neutralize the HSP72-inductive effect of the compound and, consequently, its protective effects toward challenge with V. harveyi or lethal heat stress. We found that Artemia larvae pretreated with carvacrol (66.4 μM) were markedly protected upon challenge with V. harveyi or lethal heat stress, with survival augmenting by fivefold and threefold in the case of V. harveyi and lethal heat stress, respectively, as compared with the controls (Fig. 8). However, co-pretreatment with a mixture of the antioxidant enzymes partially neutralized the protective effect of the compound. The survival of challenged larvae that were only pretreated with the antioxidant enzyme mixture did not show a significant difference in survival compared with the non-pretreated challenged larvae (control; Fig. 8). Furthermore, to exclude the possibility that the decreased survival in the group pretreated with a combination of antioxidant enzyme mixture and carvacrol was due to a negative effect of the antioxidant enzymes, we tested the effect of the antioxidant enzyme mixture on the survival of axenic Artemia larvae and found that there was no apparent adverse effect (data not shown, see Baruah et al. 2015b). These results indicated that H2O2 generated by carvacrol in the Artemia rearing medium appeared to be, at least in part, involved in the induction of HSP72 within Artemia larvae.
Fig. 8.
Survival (%) of Artemia larvae pretreated with carvacrol, antioxidant enzymes, or in combination with both. For the treatment groups, refer to Fig. 6 for explanations. The larvae were challenged with V. harveyi at 107 cells/ml of rearing water (a) or with a lethal heat stress at 41°C for 20 min (b). The survival was scored at 48 and 24 h of challenge with V. harveyi and lethal heat stress, respectively. Error bars represent the standard error of five replicates. Bars with the same alphabet letters are not different significantly (P > 0.05, Duncan’s new multiple range test)
Discussion
In recent years, the molecular chaperone HSP72 has become a new molecular target for development of antibacterial strategy for farmed aquatic animals, particularly given our findings that induction of HSP72 resulted in a significant reduction in the mortality of aquaculture animals mediated by bacteria (see review, Roberts et al. 2010). This motivated us to search for HSP72 inducers from natural sources, as has been done previously by a number of researchers, including ourselves (Baruah et al. 2012, 2014; Nagai et al. 2010; Sung et al. 2012; Yan et al. 2004), with the defining characteristic feature of this study being that we focused on a natural compound carvacrol, which had already received approval as a safe food component (FAO/WHO 2001). In this way, carvacrol, if it is proven to be an inducer of HSP72, could potentially be employed immediately for use as a disease control agent in aquaculture animals. In the present study, we have shown that carvacrol induces HSP72 within the host Artemia. This HSP72-inducing property of the compound was observed under controlled germ-free experimental conditions, in which there is no presence/interference of any stressing agent. Based on these observations, we can therefore conclude that carvacrol indeed acts as a potent inducer of HSP72, rather than as a coinducer as previously reported (Wieten et al. 2010). To our knowledge, this is the first direct observation in germ-free animal model of HSP72 induction in response to carvacrol exposure.
The inducible form of HSP70 seems to play an important role in defining the tolerance of organisms to several stressors. For instance, in our earlier studies, we have shown that induction of HSP72 in aquatic animals upon exposure to a classical HSP72 inducer, i.e., nonlethal heat shock or to plant-derived compounds significantly improved the resistance of the animal toward subsequent abiotic or pathogenic biotic stress (Baruah et al. 2012, 2014, 2015b; Boerrigter et al. 2014; Sung et al. 2012). Similar increased tolerance to extreme temperatures or to pathogens, following induction of HSP72, has also been demonstrated in other organisms from diverse phyla, including bacteria, coelenterates, molluscs, fish, shrimp, echinoderms, and humans (Baruah et al. 2013; Correia et al. 2013; Iwama et al. 1998; Roberts et al. 2010; Sanders 1993). This improved resistance was due to the fact that HSP72 plays a crucial function as molecular chaperone and is involved in protein biogenesis and protein homeostasis in the cells or it contributes to the generation of protective immune responses in the host (Baruah et al. 2013; Clegg et al. 2000). In this study, our results unequivocally showed that carvacrol induces protective effects against lethal heat stress and V. harveyi infection. However, it should be noted that these effects appeared to be concentration-dependent, with maximum protection at 66.4 μM carvacrol. Interestingly, the concentration required for marked induction of HSP72 and for affording maximum protection to Artemia against thermal and V. harveyi challenges was the same. These results suggested that the protective effect seen with carvacrol is mediated, at least in part, by HSP72 induction. It is also noteworthy to mention that under our experimental condition, pretreatment with carvacrol beyond an optimum concentration caused negative effect on the Artemia larvae. A possible explanation for the observed negative effect of carvacrol pretreatment at a higher dose could be attributed to exposure to higher concentration of ROS, inflecting, e.g., cellular damage, but other toxic effects may occur as well (Baruah et al. 2014; Fittipaldi et al. 2014; Fridell et al. 2005).
It is important to mention that carvacrol is lipophilic and is sparingly soluble (forms small globules) in water (Chen et al. 2014). In this study, stock solution of carvacrol was prepared by dissolving the compound in water rather than in organic solvents (such as ethanol). The rationale for following such a protocol is that organic solvents cause a significant influence on the stress responses of the host by causing HSP72 induction (Calabrese et al. 2000; Muralidharan et al. 2014) and by reducing the resistance of the host against bacterial challenge (Niu et al. 2014). As the main focus of this study was to determine the putative HSP72-inducing (and prophylactic) property of carvacrol, the compound was therefore dissolved in water to avoid the interfering effect of the organic solvents on the heat shock responses of the model organism. However, considering the limited water solubility of carvacrol, it can be argued that the concentrations of carvacrol that were shown to induce HSP72 in Artemia in reality caused the observed effect at doses relatively lower than the tested doses. To address the issue of the solubility of carvacrol and the concentrations used in this study, we conducted an additional dose-response/challenge test as described in Fig. 2. Here we used carvacrol, the stock solution of which was prepared by dissolving 20.5 µl of the compound (20 mg) first in 150 µl of ethanol (95%) and then dissolving the solution in 9.83 ml of sterile distilled water to obtain a final concentration of 2 g/l (Chen et al. 2014). Interestingly, the results showed a pattern of survival that is similar to the one described in the Fig. 2—increased protection against V. harveyi at 66.4 μM and decreasing effect at higher concentrations (data not shown). Based on the results of these two dose-response tests, we can assume that the concentrations used when dissolved in water are correct. However, these are pure speculation and needs further verification by detailed measurements of the compound in the solution following standard analytical methods.
A number of phenolic compounds with different chemical structures were shown to generate reactive oxygen species (e.g., H2O2) within the host cells and/or in the rearing/culture medium (Akagawa et al. 2003. Baruah et al. 2014, 2015b; Liang et al. 2013). In our earlier studies, we have demonstrated that ROS molecule H2O2 plays a key role in modulating the induction of HSP72 (Baruah et al. 2015b; Fittipaldi et al. 2014). For instance, it was shown that an increased production of oxygen-free radicals in cultured human neuroblastoma cells upon exposure of the cells to a classical HSP inducer, i.e., heat stress, causes an increased induction of HSP (Omar and Pappolla 1993). In another study in the Artemia experimental model, we have shown that H2O2 generated by a plant-based product/phenolic compound pyrogallol act as regulatory mediator in the induction of HSP72 within the organism, and this induction contributed to the resistance effect of the animal toward pathogenic vibrios (Baruah et al. 2014, 2015b). Since few studies have indicated that carvacrol exhibited ROS-inducing features, albeit without unknown mechanism (Huang et al. 2010; Liang et al. 2013), we therefore raised the question whether the observed Hsp70-inducing effect of carvacrol was due to the generation of ROS by the compound. As expected, our data suggested that ROS molecule H2O2 generated at the initial stage from the compound contributes to the induction of HSP72 in Artemia. This suggestion was made on the basis of our observations that the induction of HSP72 as well as the improvement in the resistance against V. harveyi or lethal heat stress in Artemia was partially nullified when the release of H2O2 by the compound was neutralized by the addition of a mixture of ROS scavenging enzymes SOD and catalase (see Figs. 6, 7 and 8). While we have not examined the contribution of other types of carvacrol-generated ROS molecules, such as superoxide anion in the induction of HSP72, we expect that these reactive molecules also account for the induction of HSP72, an assumption that needs further verification. In our study, we did not investigate the reaction mechanism underlying the ROS-inducing features of the compound. Future studies focusing on unraveling the reaction mechanism for carvacrol generating ROS molecules will provide better insight into the mode of action of carvacrol in inducing HSP72.
In conclusion, our results provide strong evidences that carvacrol is a potent inducer of HSP72 and that this compound-mediated HSP72 seems to be responsible for inducing resistance in Artemia larvae against subsequent stress mediated by lethal heat stress or pathogenic V. harveyi. This report also provides new insight on the mode of HSP72 inducing action of carvacrol, in which the initial generation of reactive molecule H2O2 by the compound plays a key role. Overall results advance our knowledge of this compound as potential prophylactic agent.
Acknowledgements
The authors acknowledge financial support from the Research Foundation Flanders, FWO-Vlaanderen, Belgium [postdoc grant to Dr. Kartik Baruah, FWO13/PDO/005), GOA project entitled ‘Host microbial interaction in aquatic production’ (project number: 01G02212), and the Belgian Science Policy Office (BELSPO) project entitled ‘AquaStress’, project number: IUAPVII/64/Sorgeloos. We highly acknowledge the Special Research Fund of Ghent University (BOF12/BAS/042) for procuring the chemiluminescence detection system.
Footnotes
Kartik Baruah and Parisa Norouzitallab have contributed equally as first authors.
Kartik Baruah and Peter Bossier have contributed equally as last authors.
References
- Akagawa M, Shigemitsu T, Suyama K (2003) Production of hydrogen peroxide by polyphenols and polyphenol-rich beverage under quasi physiological conditions. Biosci Biotechnol Biochem 67:2632–2640 [DOI] [PubMed]
- Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. 2012;134:82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Austin B, Zhang XH. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol. 2006;43:119–124. doi: 10.1111/j.1472-765X.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- Baruah K, et al. In vivo effects of single or combined N-acyl homoserine lactone quorum sensing signals on the performance of Macrobrachium rosenbergii larvae. Aquacult. 2009;288:233–238. doi: 10.1016/j.aquaculture.2008.11.034. [DOI] [Google Scholar]
- Baruah K, Ranjan JK, Sorgeloos P, Bossier P. Efficacy of homologous and heterologous heat shock protein 70s as protective agents to gnotobiotic Artemia franciscana challenged with Vibrio campbellii. Fish Shellfish Immunol. 2010;29:733–739. doi: 10.1016/j.fsi.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Baruah K, Norouzitallab P, Roberts RJ, Sorgeloos P, Bossier P. A novel heat-shock protein inducer triggers heat shock protein 70 to protect Artemia franciscana against abiotic stressors. Aquacult. 2012;334–337:152–158. doi: 10.1016/j.aquaculture.2011.12.015. [DOI] [Google Scholar]
- Baruah K, Norouzitallab P, Shihao L, Sorgeloos P, Bossier P. Feeding truncated heat shock protein 70s protects Artemia franciscana against virulent Vibrio campbellii challenge. Fish Shellfish Immunol. 2013;34:183–191. doi: 10.1016/j.fsi.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Baruah K, Norouzitallab P, Linayati L, Sorgeloos P, Bossier P. Reactive oxygen species generated by a heat shock protein (Hsp) inducing product contributes to Hsp70 production and Hsp70-mediated protective immunity in Artemia franciscana against pathogenic vibrios. Dev Comp Immunol. 2014;46:470–479. doi: 10.1016/j.dci.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Baruah K, et al. Probing the protective mechanism of poly-ß-hydroxybutyrate against vibriosis by using gnotobiotic Artemia franciscana and Vibrio campbellii as host-pathogen model. Sci Rep. 2015;5:9427. doi: 10.1038/srep09427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah K, Phong HPPD, Norouzitallab P, Defoirdt T. The gnotobiotic brine shrimp (Artemia franciscana) model system reveals that the phenolic compound pyrogallol protects against infection through its prooxidant activity. Free Radic Biol Med. 2015;89:593–601. doi: 10.1016/j.freeradbiomed.2015.10.397. [DOI] [PubMed] [Google Scholar]
- Boerrigter JG, et al. Effects of pro-Tex on zebrafish (Danio rerio) larvae, adult common carp (Cyprinus carpio) and adult yellowtail kingfish (Seriola lalandi) Fish Physiol Biochem. 2014;40:1201–1212. doi: 10.1007/s10695-014-9916-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods–a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Burt SA, et al. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl Environ Microb. 2007;73:4484–4490. doi: 10.1128/AEM.00340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Testa G, Ravagna A, Bates TE, Stella AM (2000) HSP70 induction in the brain following ethanol administration in the rat: regulation by glutathione redox state. Biochem Biophys Res Commun 269:397–400 [DOI] [PubMed]
- Chen T, Cao X. Stress for maintaining memory: HSP70 as a mobile messenger for innate and adaptive immunity. Eur J Immunol. 2010;40:1541–1544. doi: 10.1002/eji.201040616. [DOI] [PubMed] [Google Scholar]
- Chen H, Davidson PM, Zhong Q (2014) Impacts of sample preparation methods on solubility and antilisterial characteristics of essential oil components in milk. Appl Environ Microbiol 80:907–916 [DOI] [PMC free article] [PubMed]
- Chiu CH, Guu YK, Liu CH, Pan TM, Cheng W. Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol. 2007;23:364–377. doi: 10.1016/j.fsi.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA, Hoa NV, Sorgeloos P. Thermal resistance, developmental rate and heat shock proteins in Artemia franciscana, from San Francisco Bay and southern Vietnam. J Exp Mar Biol Ecol. 2000;252:85–96. doi: 10.1016/S0022-0981(00)00239-2. [DOI] [PubMed] [Google Scholar]
- Correia B, et al. Is the interplay between epigenetic markers related to the acclimation of cork oak plants to high temperatures? PLoS One. 2013;8:e53543. doi: 10.1371/journal.pone.0053543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO Committee on Food Additives . Evaluation of certain food additives and contaminants. 55th report. Geneva: WHO; 2001. [Google Scholar]
- FDA . Synthetic flavoring substances and adjuvants. 21CFR 182.60. Washington DC: U.S. Food and Drug Administration; 2011. [Google Scholar]
- Fittipaldi S, Dimauro I, Mercatelli N, Caporossi D. Role of exercise-induced reactive oxygen species in the modulation of heat shock protein response. Free Radic Res. 2014;48:52–70. doi: 10.3109/10715762.2013.835047. [DOI] [PubMed] [Google Scholar]
- Fridell YW, Sánchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein2 (hUCP2) to adult neuron sex tends life span in the fly. Cell Metab. 2005;1:145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Huang TC, Lin YT, Chuang KP. Carvacrol has the priming effects of reactive oxygen species (ROS) production in C6 glioma cells. Food Agric Immunol. 2010;21:47–55. doi: 10.1080/09540100903418842. [DOI] [Google Scholar]
- Iwama GK, Thomas PT, Forsyth RB, Vijayan MM. Heat shock protein expression in fish. Rev Fish Biol Fisheries. 1998;8:35–56. doi: 10.1023/A:1008812500650. [DOI] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Kojima K, et al. Escherichia coli LPS induces heat shock protein 25 in intestinal epithelial cells through MAP kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G645–G652. doi: 10.1152/ajpgi.00080.2003. [DOI] [PubMed] [Google Scholar]
- Liang WZ, et al. The mechanism of carvacrol-evoked [Ca2+]i rises and non-Ca2 + −triggered cell death in OC2 human oral cancer cells. Toxicol. 2013;303:152–161. doi: 10.1016/j.tox.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Marques A, Ollevier F, Verstraete W, Sorgeloos P, Bossier P. Gnotobiotically grown aquatic animals: opportunities to investigate host-microbe interactions. J Appl Microbiol. 2006;100:903–918. doi: 10.1111/j.1365-2672.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- Muralidharan S et al (2014) Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J Immunol 193:1975–1987 [DOI] [PMC free article] [PubMed]
- Nagai Y, Fujikake N, Popiel HA, Wada K. Induction of molecular chaperones as a therapeutic strategy for the polyglutamine diseases. Curr Pharm Biotechnol. 2010;11:188–197. doi: 10.2174/138920110790909650. [DOI] [PubMed] [Google Scholar]
- Niu Y, Norouzitallab P, Baruah K, Dong S, Bossier P (2014) A plant-based heat shock protein inducing compound modulates host-pathogen interactions between Artemia franciscana and Vibrio campbellii. Aquacult 430:120–127
- Norouzitallab P, Baruah K, Dechamma MM, Bossier P. Non-lethal heat shock induces HSP70 and HMGB1 proteins sequentially to protect Artemia franciscana against Vibrio campbellii. Fish Shellfish Immunol. 2015;42:395–399. doi: 10.1016/j.fsi.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Kawashima D, Gu Y, Saito K. Inducers and coinducers of molecular chaperones. Int J Hyperth. 2005;21:703–711. doi: 10.1080/02656730500384248. [DOI] [PubMed] [Google Scholar]
- Omar R, Pappolla M. Oxygen free radicals as inducers of heat shock protein synthesis in cultured human neuroblastoma cells: relevance to neurodegenerative disease. Eur Arch Psychiatry Clin Neurosci. 1993;242:262–267. doi: 10.1007/BF02190384. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis. 2010;33:789–801. doi: 10.1111/j.1365-2761.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- Sanders BM. Stress proteins in aquatic organisms: an environmental perspective. Crit Rev Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- Shoji Y, Nakashims H. Nutraceutics and delivery systems. J Drug Target. 2004;12:385–391. doi: 10.1080/10611860400003817. [DOI] [PubMed] [Google Scholar]
- Skoula M, Gotsiou P, Naxakis G, Johnson CB. A chemosystematic investigation on the mono- and sesquiterpenoids in the genus Origanum (Labiatae) Phytochemistry. 1999;52:649–657. doi: 10.1016/S0031-9422(99)00268-X. [DOI] [Google Scholar]
- Sung YY, Roberts RJ, Bossier P. Enhancement of Hsp70 synthesis protects common carp Cyprinus carpio L. against lethal ammonia toxicity. J Fish Dis. 2012;35:563–568. doi: 10.1111/j.1365-2761.2012.01397.x. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Wieten L, et al. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 2010;62:1026–1035. doi: 10.1002/art.27344. [DOI] [PubMed] [Google Scholar]
- Yan D, Saito K, Ohmi Y, Fujie N, Ohtsuka K. Paeoniflorin, a novel heat shock protein-inducing compound. Cell Stress Chaperon. 2004;9:378–389. doi: 10.1379/CSC-51R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]