Abstract
Phosphodiesterase type 5 inhibitors (PDE5Is), widely known for their beneficial effects onto male erectile dysfunction, seem to exert favorable effects onto metabolism as well. Tadalafil exposure increases oxidative metabolism of C2C12 skeletal muscle cells. A rise in fatty acid (FA) metabolism, requiring more oxygen, could induce a larger reactive oxygen species (ROS) release as a byproduct thus leading to a redox imbalance. The aim of this study was to determine how PDE5I tadalafil influences redox status in skeletal muscle cells to match the increasing oxidative metabolism. To this purpose, differentiated C2C12 skeletal muscle cells were treated with tadalafil and analyzed for total antioxidant capacity (TAC) and glutathione levels as marker of redox status; enzyme activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) engaged in antioxidant defense; and lipid peroxidation (TBARS) and protein carbonyls (PrCar) as markers of oxidative damage. Tadalafil increased total intracellular glutathione (tGSH), CAT, SOD, and GPx enzymatic activities while no changes were found in TAC. A perturbation of redox status, as showed by the decrease in the ratio between reduced/oxidized glutathione (GSH/GSSG), was observed. Nevertheless, it did not cause any change in TBARS and PrCar levels probably due to the enhancement in the antioxidant enzymatic network. Taken together, these data indicate that tadalafil, besides improving oxidative metabolism, may be beneficial to skeletal muscle cells by enhancing the enzymatic antioxidant system capacity.
Keywords: PDE5Is, Tadalafil, C2C12 skeletal muscle cells, Redox status, Oxidative metabolism
Introduction
Phosphodiesterase inhibitors (PDEIs) are a family of enzymes that act prolonging the intracellular action of cyclic nucleotide that are responsible for many physiological processes (Beavo et al. 2002). In particular, the phosphodiesterase type 5 (PDE5) inhibitors (PDE5Is) (e.g., sildenafil, tadalafil, vardenafil and so forth) act by extending the actions of cyclic guanosine monophosphate (cGMP). Recently, a role has emerged for cGMP in regulating energy metabolism (Nisoli et al. 2003) through mitochondrial biogenesis and fatty acid (FA) oxidation stimulation (Miyashita et al. 2009). It is of great interest that PDE5Is, widely used mainly to treat erectile dysfunction in males, have positive effects on metabolism (Aversa 2010) by improving insulin sensitivity (Ayala et al. 2007; Mammi C et al. 2011; Fu et al. 2015). Moreover, recent studies have shown that sildenafil promoted a brown fat cell-like phenotype in adipocyte cultures (Mitschke et al. 2013) and tadalafil reduced visceral adipose tissue (Maneschi et al. 2016).
As regards the metabolism, skeletal muscles represent the largest insulin-sensitive tissue in the body, and flexibility in fuel selection is a hallmark for metabolically healthy muscles. In fasting period, FAs are the main metabolic fuels and β-oxidation represents their degradation pathway. Noteworthy, stimulation of muscle FA oxidation has emerged as a strategy for the treatment of insulin resistance (Kiens et al. 2011).
In a recent study in vitro, we demonstrate that tadalafil improved oxidative capacity in C2C12 skeletal muscle cells as displayed by the increased reliance on fat metabolism (Sabatini et al. 2011). FAs oxidation, per ATP synthesized, requires more oxygen than glucose oxidation; the rise in mitochondrial O2 consumption, due to the oxidative metabolism, leads to the increased formation of reactive oxygen species (ROS) (Turrens 2003; Murphy 2009). Hence, the enhancement of FA oxidation could shift the cellular redox environment to a more oxidized state, therefore causing potential damage to macromolecules.
Considering the aforementioned findings, the aim of this study was to clarify whether PDE5I tadalafil, as well as to increase oxidative metabolism, may affect redox status in skeletal muscle cells. A number of studies showed the protective role of tadalafil in reducing oxidative stress in diabetic animal models (Koka et al. 2013; Koka et al. 2014; Chen et al. 2012; Mostafa et al. 2012). Moreover, the antioxidant potential of tadalafil was also observed using pathological animal models or cultured cells challenged with an oxidant stimulus (Speranza et al. 2008; Arikan et al. 2010; Adeneye et al. 2016). Based on the literature, we hypothesize that supplementation with tadalafil may improve redox status, thus allowing cells to counteract efficiently the ROS increase induced by oxidative metabolism.
To our knowledge, there is no information about the influence of tadalafil on redox balance in skeletal muscle cells. Differently from other works, our experiments were performed in cells, without the challenge of an oxidative insult. To pursue our aim, we used differentiated C2C12 skeletal muscle cells treated with tadalafil and analyzed for total antioxidant capacity (TAC), an assay that measures lipophilic and hydrophilic antioxidants; GSH homeostasis as markers of redox status; enzyme activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) engaged in antioxidant enzymatic defense; and lipid peroxidation (TBARS) and protein carbonyls (PrCar) as markers of oxidative damage.
Materials and methods
Tadalafil solution
Tadalafil was purified from Cialis© 20 mg tablets as previously described (Sabatini et al. 2011). Briefly, one tablet (corresponding to 20 mg of compound) was pulverized and dissolved in 90 mL of methanol. This solution was sonicated for 10 min and centrifuged at 14,000×g for 10 min at +4 °C. The supernatant was then transferred to tubes and tested for tadalafil purity by comparing its absorbance spectra with that published by Cheng and Chou (2005).
Cell culture
C2C12 myoblasts (2 × 103 cm2; ATCC, Manassas, VA, USA) were cultured in 25 cm2 culture flasks with Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Oud-Beijerland, Holland) supplemented with Glutamax-I (4 mM l-alanyl-l-glutamine), 4.5 g/L glucose (Invitrogen, Carlsbad, CA, USA), and 10% heat-inactivated fetal bovine serum (FBS; Hy-Clone). No antibiotics were used. The cells were incubated at 37 °C with 5% CO2 in a humidified atmosphere. Cells were split 1:6 twice weekly and fed 24 h before each experiment. Differentiation into myotubes was achieved by culturing preconfluent cells (85% confluency) in medium containing 2% FBS and monitoring them by microscopy and for myogenin expression by western blot analysis (Ceci et al. 2011). Tadalafil was dissolved in methanol at a stock concentration of 300 mM immediately before use and then diluted in the culture medium to a final concentration of 1 μM that, as calculated, correspond to the oral doses normally used for therapeutic treatment in vivo (20 mg). At this working solution, neither methanol concentration (0.1%, v/v) nor tadalafil show any toxic effect on myotubes. Cells were incubated with tadalafil-supplemented culture medium for 2, 6, and 24 h (T2-T24) according to tadalafil pharmacokinetics (Corbin et Francis 2002). Each experiment was performed in triplicate. After each treatment, the cells were trypsinized and centrifuged at 1200×g for 10 min at room temperature. The cells were then lysed in extraction buffer (50 mM Tris–acetate, 250 mM sucrose, pH 7.5) and supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail (Sigma-Aldrich). The resulting lysates were centrifuged at 14,000×g for 10 min at +4 °C, and then utilized for biochemical analysis. An aliquot of cell lysates was tested for protein content using the Bradford method (Sigma-Aldrich, St. Louis, MO, USA).
Trolox® equivalent antioxidant capacity (TAC)
Trolox® equivalent antioxidant capacity of tadalafil was evaluated spectrophotometrically, accordingly to Ghiselli et al. (2000). This assay evaluates the ability of cell lysates in preventing ABTS. + radical formation, compared to Trolox® (vitamin E analogue) standards.
Briefly, 10 μL of cell lysates or Trolox® standards (0.125–2.0 mM) were incubated in ABTS-met-Myo-PBS buffer and the absorbance at 734 nm was monitored for 2 min. The reaction was started by the addition of H2O2 (450 μM), followed for 10 min, and the variation of absorbance was then recorded. Sample ΔOD/min734 was compared to those obtained using Trolox® standards. Cell lysate TAC were expressed as micromoles/mg protein tested.
Glutathione homeostasis
Intracellular reduced (GSH) and oxidized (GSSG) glutathione contents were quantified by a DTNB–glutathione reductase recycling assay, as previously described (Colamartino et al. 2015).
Briefly, 107 cells were collected and suspended in 1:1 (v/v) μL 5% sulfosalicylic acid (SSA). Cells were lysed by freezing and thawing three times and then were centrifuged at 10,000×g for 5 min at +4 °C. The deproteinized supernatant was then analyzed for total glutathione content. Oxidized glutathione (GSSG) was selectively measured in samples where reduced GSH was masked by pretreatment with 2-vinylpyridine (2%). Ten microliters of the sample was added to the reaction buffer [700 μL NADPH (0.3 mM), 100 μL DTNB (6 mM), 190 μL H2O]. The reaction was started by adding 2.66 U/mL glutathione reductase and followed at 412 nm by the TNB stechiometrical formation. Sample ΔOD/min412 were compared to those obtained by using glutathione standards, and results were normalized for protein content.
Lipid and protein oxidation
Thiobarbituric acid reactive substances
TBARS levels were assayed by spectrophotometric analysis (Lovric et al. 2008). The methodology measures malondialdehyde (MDA) and other aldehydes produced by lipid peroxidation induced by hydroxyl free radicals. Briefly, 150-μL cell lysate was added to 25 μL 0.2% butylated hydroxytoulene (BHT) and 600 μL of 15% aqueous of trichloroacetic acid (TCA) in a 1.5-mL tube (Eppendorf, Hamburg, Germany). The mixture was centrifuged at 4000×g for 15 min at 4 °C. Three hundred microliters of the deproteinized supernatant was transferred in a Corning Cryotube 2 mL and added with 600 μL of TBA (0.375% in 0.25 M HCl). Samples were then heated at 100 °C for 15 min in boiling water. After cooling, sample absorbance was determined spectrophotometrically at 535 nm and compared to standard MDA (1,1,3,3-tetramethoxypropane) solution. The levels of TBARS were expressed in terms of nmol/mg protein.
Protein carbonyls
Protein carbonyl levels were determined by measuring the reactivity of carbonyl derivatives with 2,4-dinitrophenylhydrazine (DNPH) as described (Fagan et al. 1999) with some modifications. In brief, cell lysates (100 μL) were precipitated with 10 volumes of HCl–acetone (3:100) (v/v), then washed with 5-mL HCl–acetone to remove chromophores. The protein pellet was then washed twice and disintegrated by hard vortexing during each wash, and the supernatant was decanted after each centrifugation (800×g, for 20 min, +4 °C). Protein pellets were resuspended in 500 μL of PBS to which 500 μL of 10 mM DNPH (in 2 M HCl) was added and vortexed every 5 min for 30 min at room temperature. Protein blanks were prepared by adding 500 μL of 2 M HCl instead of DNPH. After mixing, 500 μL of 30% TCA was added to each tube, placed on ice for 10 min, and then centrifuged (800×g, for 20 min, +4 °C). The supernatant was discarded and the pellets were washed with 20% TCA followed by three ethanol–ethylacetate (1:1) (v/v) washes in order to remove any unreacted DNPH. The pellets were then solubilized in 1 mL of 6 M guanidine hydrochloride and 20 mM potassium dihydrogen phosphate (pH 2.3). The carbonyl content was calculated from the absorbance measurement at 380 nm. Millimolar extinction coefficient ε380 = 22.00. Protein carbonyl content was expressed in terms of nmol/mg protein.
Enzymatic activities
Intracellular superoxide dismutase (SOD) activity was measured using a commercial assay kit (Cayman Chemical Company, MI, USA) following the manufacturer’s instructions. This assay used xanthine oxidase and hypoxanthine to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenonal)-5-phenyltetrazolium chloride to form a formazan dye.
The SOD activity was measured by the degree of inhibition of this reaction. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. Results were expressed as units/mg of protein tested.
Catalase (CAT) activity was measured using a commercial assay kit (Cayman Chemical Company, MI, USA) following the manufacturer’s instructions. This method is based on the reaction of the enzyme with methanol in presence of H2O2. The formaldehyde produced is measured colometrically with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald) as the chromogen. Results were expressed as units/mg of protein tested.
Glutathione peroxidase (GPx) activity was measured using a commercial assay kit (Cayman Chemical Company, MI, USA) following the manufacturer’s instructions. This assay measured GPx activity indirectly by a coupled reaction with glutathione reductase. Oxidized glutathione, produced upon reduction of hydroperoxide by GPx, is recycled to its reduced state by glutathione reductase and NADPH. The oxidation of NADPH is accompanied by a decrease in absorbance at 340 nm directly proportional to the GPx activity in the sample. Results were expressed as units/mg of protein tested.
Statistical analysis
The Kolmogorov-Smirnov test was used to evaluate the variable distribution, and all data are expressed as mean values ± S.D. A one-way ANOVA for repeated measures and Bonferroni post-hoc analyses were used to determine significant variations over time and among groups for each parameter evaluated. P < 0.05 was accepted as significant.
The SPSS statistical package (Version 17.0 for Windows; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. No statistical differences were found in all parameters analyzed between untreated controls at T0 and T24 hours (data not shown).
Results
Antioxidant capacity of tadalafil
No differences were found in the total antioxidant capacity after treatment as assessed by the modified Trolox® equivalent antioxidant capacity assay (Table 1).
Table 1.
Glutathione and total antioxidant capacity analysis
| T0 | Tadalafil 1 μM | |||
|---|---|---|---|---|
| T2 | T6 | T24 | ||
| tGSHa | 130.38 ± 1.88 | 146.56 ± 3.03# | 158.78 ± 10.07# | 149.41 ± 12.13 |
| GSSGa | 14.23 ± 1.26 | 17.09 ± 0.93* | 21.18 ± 1.19# | 17.10 ± 2.85 |
| GSHa | 116.14 ± 2.08 | 129.46 ± 2.13* | 137.61 ± 10.08* | 132.31 ± 9.60* |
| TACb | 0.38 ± 0.07 | 0.39 ± 0.06 | 0.40 ± 0.02 | 0.41 ± 0.06 |
Measurement of total (tGSH), oxidized (GSSG), and reduced glutathione (GSH) and total antioxidant capacity (TAC) was performed in C2C12 myotubes after 2, 6, or 24 h (T0-T24) of tadalafil (1 μM) treatments. Data presented are the mean ± SD of three experiments
*p < 0.05 vs T0; #p < 0.01 vs T0
anmol/mg protein
bμmol Trolox/mg protein
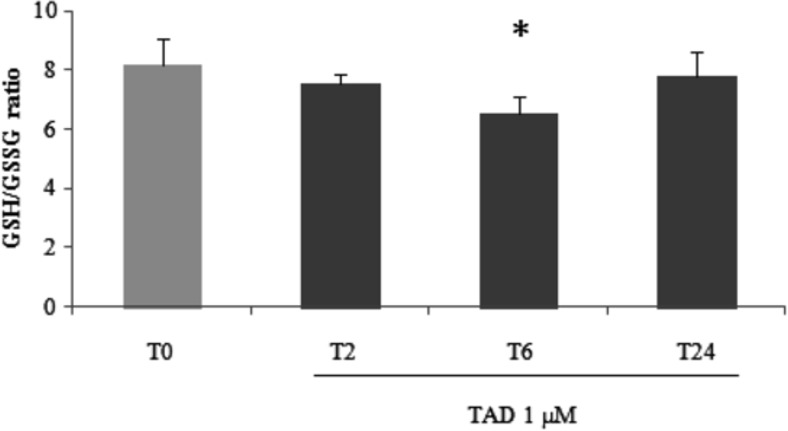
Regarding glutathione homeostasis, compared to untreated cells, tadalafil was able to increase total intracellular glutathione (tGSH) with maximal effect after 6 h of treatment (p < 0.01; Table 1). A significant increase in oxidized glutathione (p < 0.01) was also observed. The resulting GSH/GSSG ratio was found reduced in these samples if compared to untreated myotubes (6.51 ± 0.61 if compared to T0; 8.21 ± 0.84; p < 0.05; Fig. 1).
Fig. 1.
Reduced to oxidized glutathione ratio analysis. Measurement of reduced to oxidized glutathione ratio (GSH/GSSG) was performed in C2C12 myotubes after 2, 6, or 24 h (T0–T24) of tadalafil (1 μM) treatments. Data presented are the mean ± SD of three experiments. *p < 0.05 vs T0
Enzymatic activities
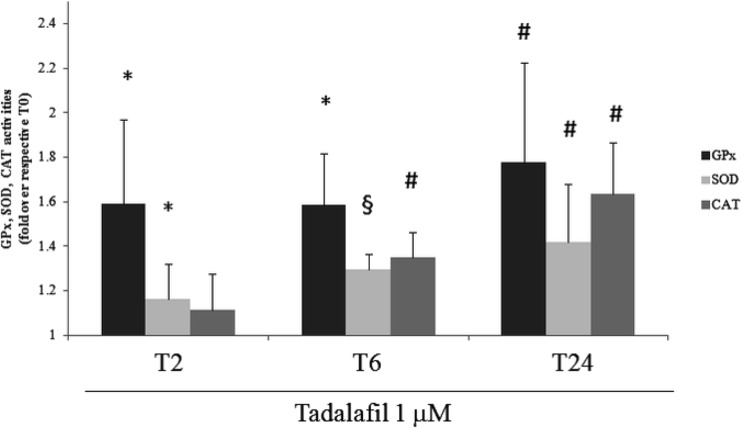
Compared to untreated cells, superoxide dismutase and glutathione peroxidase activities increased, starting from 2 h of treatment (p < 0.05). The maximal activities were found at T24 with respect to T0 (Table 2). At this experimental point, the increases in enzyme activities were 77.8 and 41.8% for GPx and SOD (P < 0.01), respectively (Fig. 2).
Table 2.
Catalase, superoxide dismutase, and glutathione peroxidase activities analysis
| T0 | Tadalafil 1 μM | |||
|---|---|---|---|---|
| T2 | T6 | T24 | ||
| CATa | 1.79 ± 0.09 | 1.98 ± 0.23 | 2.41 ± 0.16# | 2.91 ± 0.30# |
| SODa | 0.25 ± 0.01 | 0.29 ± 0.03* | 0.33 ± 0.01§ | 0.36 ± 0.06# |
| GPxa | 0.39 ± 0.06 | 0.62 ± 0.14* | 0.63 ± 0.16* | 0.69 ± 0.18# |
Measurement of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) analysis was performed in C2C12 myotubes after 2, 6, or 24 h (T0-T24) of tadalafil (1 μM) treatments. Data presented are the mean ± SD of three experiments
*p < 0.05 vs CTRL; #p < 0.01 vs CTRL; §p < 0.001 vs CTRL
aU/mg protein
Fig. 2.
Catalase, superoxide dismutase, and glutathione peroxidase activity analysis. Measurement of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) analysis was performed in C2C12 myotubes after 2, 6, or 24 h (T0–T24) of tadalafil (1 μM) treatments. Data presented as fold over respective T0 and are the mean ± SD of three experiments. *p < 0.05 vs T0; #p < 0.01 vs T0; §p < 0.001 vs T0
Tadalafil increased catalase activity starting from 6 h of treatment if compared to untreated cells. The activity of CAT was increased up to 63.5% after 24 treatments (p < 0.01; Fig. 2).
Lipid and protein oxidation
As shown in Table 3, tadalafil treatment did not induce per se any significant change in TBARS (malondialdehyde (MDA) and other aldehydes) and protein carbonyl (PrCAR) levels if compared to untreated cells.
Table 3.
Thiobarbituric acid-reactive substances (TBARS) and protein carbonyls analysis
| T0 | Tadalafil 1 μM | |||
|---|---|---|---|---|
| T2 | T6 | T24 | ||
| TBARSa | 29.31 ± 4.98 | 34.56 ± 8.08 | 36.51 ± 10.57 | 33.92 ± 9.13 |
| Pr-CARb | 3.57 ± 1.21 | 2.09 ± 0.65 | 1.61 ± 0.56 | 1.93 ± 0.86 |
Measurement of thiobarbituric acid-reactive substances (TBARS) and protein carbonyls (Pr-CAR) analysis was performed in C2C12 myotubes after 2, 6, or 24 h (T0–T24) of tadalafil (1 μM) treatments. Data presented are the mean ± SD of three experiments
anmol/g protein
bnmol/mg protein
Discussion
In the present study, we show that in C2C12 myotubes, tadalafil acts by increasing the enzymatic antioxidant system capacity. This adaptive response could potentially control the production of ROS due to the increased oxidative metabolism induced by tadalafil administration. Previously, we have demonstrated that tadalafil is able to modulate energy homeostasis in mouse skeletal muscle cells, depending on the treatment length and dose. Supplementation of C2C12 myotubes with 1 μM tadalafil influences the metabolism by improving FA oxidation as displayed by the increase of 3-OH acylCoA dehydrogenase and citrate synthase activities respectively involved in β-oxidation and Krebs cycle (Sabatini et al. 2011). Generally speaking, skeletal muscle is a tissue with an optimal redox buffer capacity; in fact, as a consequence of high metabolic demand and oxygen consumption, it is particularly exposed to ROS, to which cells respond through the action of the antioxidant network (Tsutsui et al. 2011). Glutathione is the primary antioxidant responsible for maintaining a reduced intracellular microenvironment. When ROS production is accelerated to the point that it overwhelms ROS scavenging capacity, GSH/GSSG ratio, a well-known marker of oxidative stress, decreases in significant manner (Towsend et al. 2003; Ceci et al. 2014). Indeed, a reduction in GSH/GSSG reflects a reduced antioxidant capacity and increased vulnerability to oxidative damage to macromolecules (Jones 2006). In our model, tadalafil administration produced a redox perturbation as showed by the significantly altered GSH/GSSG ratio.
We also analyzed redox status by evaluating total non-enzymatic antioxidant capacity, an assay that is indicative of the ability of cells to counteract oxidative stress-induced damage. In this assay, the combined antioxidant capacity between hydrophilic and lipophilic antioxidants is measured. It must be noted that glutathione represents the most important water-soluble antioxidants, so, considering the increase in GSSG levels, we expected a decrease in TAC values. However, our results showed that TAC was unchanged after tadalafil administration. Interestingly, the analysis of glutathione homeostasis showed a rise in reduced glutathione, probably related to an increase in its synthesis through the induction of gamma-glutamylcysteine synthetase, which is the first enzyme in the biosynthetic pathway of GSH (Lu 2013) that apparently compensates the increase in GSSG levels.
In TAC assay the role of important enzymes such as superoxide dismutase, glutathione peroxidase, and catalase (Sies 2007; Fraga et al. 2014) is not valued. Interestingly, tadalafil augmented the activity of each of these enzymes though the main increase has been observed for glutathione peroxidase activity. Furthermore, the response in enzymatic antioxidant network lasted the length of the entire treatment. Since glutathione is also a cofactor of the GSH-peroxidase family, the increase in GSH stimulated by tadalafil may be a support in glutathione peroxidase activity. We hypothesize that the significant increase in antioxidant enzymatic network, already evident at 2 h of treatment and therefore anticipating the increase in oxidative metabolism induced by tadalafil, could eventually allow cells to cope with the redox imbalance as confirmed by the fact the levels of TBARs and protein carbonyls remained unchanged.
As a limitation of this study, the underlying mechanisms by which tadalafil regulates antioxidant enzyme activity was not investigated. Considering that tadalafil has a relatively short time to onset its effects (Varma A et al., 2012), we can speculate that the increase in cGMP, directly or indirectly, may quickly lead to modulate enzyme activities. On the other hand, it is to consider also the occurrence of an increase in enzyme gene expression. It was reported that in human skeletal muscle cells, tadalafil increases the mitogen-activated protein kinase, phosphatidylinositol 3-kinase/protein kinase B, and glycogen synthase kinase 3β phosphorylation (Crescioli et al. 2013) already at 15 min of treatment. We can speculate that the activation of these intracellular pathways may lead to the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2), which has been recognized as the master regulator of the antioxidant response (Han et al. 2016). Also, the increase in GSH may be partly related to increase in its synthesis through an induction of the gamma-glutamylcysteine synthetase Nrf2-mediated (Lu 2013). Further studies are needed to have a clear insight into the molecular pathway involved in the regulation of antioxidant enzyme gene expression and/or activity by tadalafil.
It is already known that PDE5Is represent a potential tool against the evolution of diseases in which oxidative stress plays a central role. They are currently being used in the treatment of pulmonary hypertension (Igarashi et al. 2016) and Raynaud’s phenomenon (Kamata and Minota 2014). Furthermore, some studies have also reported the protective role of tadalafil in reducing oxidative stress in diabetic animal models (Koka et al. 2013; Koka et al. 2014; Chen et al. 2012; Mostafa et al. 2012). Other studies used to evaluate the antioxidant potential of tadalafil were also performed using pathological animal models or cultured cells challenged with an oxidant stimulus (Speranza et al. 2008; Arikan et al. 2010; Adeneye et al. 2016).
Differently from the abovementioned works, we investigated the adaptive response to tadalafil in absence of any oxidant stimulus showing for the first time a comprehensive framework on the ability of tadalafil, in C2C12 myotubes, to modulate an antioxidant response. Nowadays, it is known that tadalafil is used by healthy men as recreational drug owing to their perceived ability to enhance sexual performance. Indeed, in the recent years, the hypothesis that PDE5Is could influence athletic adaptation and performance (Di Luigi et al. 2003; Baldari et al. 2009; Brunelli et al. 2012) by affecting endocrine/metabolic processes (Di Luigi et al. 2008a; Di Luigi et al. 2008b; Ceci et al. 2015) led to the misuse/abuse of these drugs, such as others prohibited/not prohibited substances and supplements, by athletes (Di Luigi et al. 2005; Di Luigi et al. 2007; Di Luigi 2008; Di Luigi et al. 2012). Hence, considering the consumption of tadalafil by healthy men, it is especially interesting to understand the role of this molecule on the antioxidant network of skeletal muscle, a tissue particularly exposed to ROS, in order to evaluate any potential benefits or harmful effects.
In conclusion, this study provide evidences that tadalafil, besides improving oxidative metabolism, has beneficial effect for skeletal muscle cells on the antioxidant system, due to the improved enzymatic antioxidant system capacity. Further studies are warranted to elucidate the mechanisms involved in the antioxidant response and the potential benefits of tadalafil on the redox state for maintaining well-being in humans.
Abbreviations
- (PDE5Is)
Phosphodiesterase type 5 inhibitors
- (FAs)
Fatty acids
- Reduced
GSH
- oxidized
GSSG
- GSH/GSSG
Glutathione, reduced to oxidized glutathione ratio
- TAC
Total antioxidant capacity
- SOD
Superoxide dismutase
- CAT
Catalase
- GPx
Glutathione peroxidase
- TBARS
Thiobarbituric acid reactive substances
- PrCar
Protein carbonyls
- ROS
Reactive oxygen species
Compliance with ethical standards
Funding
This work was supported by grants from the University of Rome ‘Foro Italico’ (DIP09-13 and DIP09-14) to SS and SP. Each author of this study further declares no relationships with the companies or manufacturers who will benefit from the results of the present study.
Author contribution statement
Dr. Guglielmo Duranti, Dr. Roberta Ceci, and Dr. Paolo Sgrò were responsible for running the experiments and analyzing the data. Professor Stefania Sabatini and Professor Luigi Di Luigi were responsible for the overall direction of the project, supervising progress, and interpreting the experiments. All the authors wrote the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Guglielmo Duranti and Roberta Ceci contributed equally to the research
References
- Adeneye AA, Benebo AS. Chemopreventive effect of tadalafil in cisplatin-induced nephrotoxicity in rats. Niger J Physiol Sci. 2016;31(1):1–10. [PubMed] [Google Scholar]
- Arikan DC, Bakan V, Kurutas EB, Sayar H, Coskun A. Protective effect of tadalafil on ischemia/reperfusion injury of rat ovary. J Pediatr Surg. 2010;45(11):2203–2209. doi: 10.1016/j.jpedsurg.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Aversa A. Systemic and metabolic effects of PDE5-inhibitor drugs. World J Diabetes. 2010;1(1):3–7. doi: 10.4239/wjd.v1.i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes. 2007;56(4):1025–1033. doi: 10.2337/db06-0883. [DOI] [PubMed] [Google Scholar]
- Baldari C, Di Luigi L, Emerenziani GP, Gallotta MC, Sgrò P, Guidetti L. Is explosive performance influenced by androgen concentrations in young male soccer players? Br J Sports Med. 2009;43(3):191–194. doi: 10.1136/bjsm.2007.040386. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3(9):710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Brunelli A, Dimauro I, Sgrò P, Emerenziani GP, Magi F, Baldari C, Guidetti L, Di Luigi L, Parisi P, Caporossi D. Acute exercise modulates BDNF and pro-BDNF protein content in immune cells. Med Sci Sports Exerc. 2012;44(10):1871–1880. doi: 10.1249/MSS.0b013e31825ab69b. [DOI] [PubMed] [Google Scholar]
- Ceci R, Duranti G, Rossi A, Savini I, Sabatini S. Skeletal muscle differentiation: role of dehydroepiandrosterone sulfate. Horm Metab Res. 2011;43(10):702–707. doi: 10.1055/s-0031-1285867. [DOI] [PubMed] [Google Scholar]
- Ceci R, Beltran Valls MR, Duranti G, Dimauro I, Quaranta F, Pittaluga M, Sabatini S, Caserotti P, Parisi P, Parisi A, Caporossi D. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014;2:65–72. doi: 10.1016/j.redox.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci R, Duranti G, Sgrò P, Sansone M, Guidetti L, Baldari C, Sabatini S, Di Luigi L. Effects of tadalafil administration on plasma markers of exercise-induced muscle damage, IL6 and antioxidant status capacity. Eur J Appl Physiol. 2015;115(3):531–539. doi: 10.1007/s00421-014-3040-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li XX, Lin HC, Qiu XF, Gao J, Dai YT, Wang R. The effects of long-term administration of tadalafil on STZ-induced diabetic rats with erectile dysfunction via a local antioxidative mechanism. Asian J Androl. 2012;14(4):616–620. doi: 10.1038/aja.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Chou CH. Determination of tadalafil in small volumes of plasma by high-performance liquid chromatography with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;822:278–284. doi: 10.1016/j.jchromb.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Colamartino M, Santoro M, Duranti G, Sabatini S, Ceci R, Testa A, Padua L, Cozzi R (2015) Evaluation of levodopa and carbidopa antioxidant activity in normal human lymphocytes in vitro: implication for oxidative stress in Parkinson's disease. Neurotox Res 27(2):106–117 [DOI] [PubMed]
- Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–459. [PubMed] [Google Scholar]
- Crescioli C, Sturli N, Sottili M, Bonini P, Lenzi A. Di Luigi L insulin-like effect of the phosphodiesterase type 5 inhibitor tadalafil onto male human skeletal muscle cells. J Endocrinol Investig. 2013;36(11):1020–1026. doi: 10.3275/9034. [DOI] [PubMed] [Google Scholar]
- Di Luigi L. Supplements and the endocrine system in athletes. Clin Sports Med. 2008;27(1):131–151. doi: 10.1016/j.csm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Di Luigi L, Guidetti L, Baldari C, Romanelli F. Heredity and pituitary response to exercise-related stress in trained men. Int J Sports Med. 2003;24(8):551–558. doi: 10.1055/s-2003-43266. [DOI] [PubMed] [Google Scholar]
- Di Luigi L, Romanelli F, Lenzi A. Androgenic-anabolic steroids abuse in males. J Endocrinol Investig. 2005;28(3 Suppl):81–84. [PubMed] [Google Scholar]
- Di Luigi L, Rossi C, Sgrò P, Fierro V, Romanelli F, Baldari C, Guidetti L. Do non-steroidal anti-inflammatory drugs influence the steroid hormone milieu in male athletes? Int J Sports Med. 2007;28(10):809–814. doi: 10.1055/s-2007-964991. [DOI] [PubMed] [Google Scholar]
- Di Luigi L, Baldari C, Sgrò P, Emerenziani GP, Gallotta MC, Bianchini S, Romanelli F, Pigozzi F, Lenzi A, Guidetti L. The type 5 phosphodiesterase inhibitor tadalafil influences salivary cortisol, testosterone, and dehydroepiandrosterone sulphate responses to maximal exercise in healthy men. J Clin Endocrinol Metab. 2008;93(9):3510–3514. doi: 10.1210/jc.2008-0847. [DOI] [PubMed] [Google Scholar]
- Di Luigi L, Baldari C, Pigozzi F, Emerenziani GP, Gallotta MC, Iellamo F, Ciminelli E, Sgrò P, Romanelli F, Lenzi A, Guidetti L. The long-acting phosphodiesterase inhibitor tadalafil does not influence athletes’ VO2max, aerobic, and anaerobic thresholds in normoxia. Int J Sports Med. 2008;29(2):110–115. doi: 10.1055/s-2007-965131. [DOI] [PubMed] [Google Scholar]
- Di Luigi L, Romanelli F, Sgrò P, Lenzi A. Andrological aspects of physical exercise and sport medicine. Endocrine. 2012;42(2):278–284. doi: 10.1007/s12020-012-9655-6. [DOI] [PubMed] [Google Scholar]
- Fagan JM, Sleczka BG, Sohar I. Quantitation of oxidative damage to tissue proteins. Int J Biochem Cell B. 1999;31:751–757. doi: 10.1016/S1357-2725(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Oteiza PI, Galleano M. In vitro measurements and interpretation of total antioxidant capacity. Biochim Biophys Acta. 2014;1840:931–934. doi: 10.1016/j.bbagen.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Fu L, Li F, Bruckbauer A, Cao Q, Cui X, Wu R, Shi H, Xue B, Zemel MB. Interaction between leucine and phosphodiesterase 5 inhibition in modulating insulin sensitivity and lipid metabolism. Diabetes Metab Syndr Obes. 2015;8:227–239. doi: 10.2147/DMSO.S82338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29:1106–1114. doi: 10.1016/S0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Han KH, Hashimoto N, Fukushima M. Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J Gastroenterol. 2016;22(1):37–49. doi: 10.3748/wjg.v22.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A, Inoue S, Ishii T, Tsutani K, Watanabe H. Comparative effectiveness of oral medications for pulmonary arterial hypertension. Int Heart J. 2016;57(4):466–472. doi: 10.1536/ihj.15-459. [DOI] [PubMed] [Google Scholar]
- Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 2006;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- Kamata Y, Minota S (2014) Effects of phosphodiesterase type 5 inhibitors on Raynaud's phenomenon. Rheumatol Int 34(11):1623–1626. doi:10.1007/s00296-014-3025-z [DOI] [PubMed]
- Kiens B, Alsted TJ, Jeppesen J. Factors regulating fat oxidation in human skeletal muscle. Obes Rev. 2011;12(10):852–858. doi: 10.1111/j.1467-789X.2011.00898.x. [DOI] [PubMed] [Google Scholar]
- Koka S, Das A, Salloum FN, Kukreja RC. Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med. 2013;60:80–88. doi: 10.1016/j.freeradbiomed.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1α signaling. Am J Physiol Heart Circ Physiol. 2014;306(11):H1558–H1568. doi: 10.1152/ajpheart.00865.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovric J, Mesic M, Macan M, Koprivanac M, Kelava M, Bradamante V. Measurement of malondialdehyde (MDA) level in rat plasma after simvastatin treatment using two different analytical methods. Period Biol. 2008;110:63–67. [Google Scholar]
- Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammi C, Pastore D, Lombardo MF, Ferrelli F, Caprio M, Consoli C, Tesauro M, Gatta L, Fini M, Federici M, Sbraccia P, Donadel G, Bellia A, Rosano GM, Fabbri A, Lauro D. Sildenafil reduces insulin-resistance in human endothelial cells. PLoS One. 2011;6(1):e14542. doi: 10.1371/journal.pone.0014542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneschi E, Cellai I, Aversa A, Mello T, Filippi S, Comeglio P, Bani D, Guasti D, Sarchielli E, Salvatore G, Morelli A, Mazzanti B, Corcetto F, Corno C, Francomano D, Galli A, Vannelli GB, Lenzi A, Mannucci E, Maggi M, Vignozzi L. Tadalafil reduces visceral adipose tissue accumulation by promoting preadipocytes differentiation towards a metabolically healthy phenotype: studies in rabbits. Mol Cell Endocrinol. 2016;424:50–70. doi: 10.1016/j.mce.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, Haas B, Sassmann A, Pfeifer A, Kilic A. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 2013;27(4):1621–1630. doi: 10.1096/fj.12-221580. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa T, Rashed L, Kotb K, Taymour M. Effect of testosterone and frequent low-dose sildenafil/tadalafil on cavernous tissue oxidative stress of aged diabetic rats. Andrologia. 2012;44(6):411–415. doi: 10.1111/j.1439-0272.2012.01294.x. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299(5608):896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Sgrò P, Duranti G, Ceci R, Di Luigi L. Tadalafil alters energy metabolism in C2C12 skeletal muscle cells. Acta Biochim Pol. 2011;58(2):237–241. [PubMed] [Google Scholar]
- Sies H. Total antioxidant capacity: appraisal of a concept. J Nutr. 2007;137:1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- Speranza L, Franceschelli S, Pesce M, Vinciguerra I, De Lutiis MA, Grilli A, Felaco M, Patruno A. Phosphodiesterase type-5 inhibitor and oxidative stress. Int J Immunopathol Pharmacol. 2008;47:879–889. doi: 10.1177/039463200802100412. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/S0753-3322(03)00043-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S, Yokota T. Oxidative stress in cardiac and skeletal muscle dysfunction associated with diabetes mellitus. J Clin Biochem Nutr. 2011;48(1):68–71. doi: 10.3164/jcbn.11-012FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC. Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS One. 2012;7(9):e45243. doi: 10.1371/journal.pone.0045243. [DOI] [PMC free article] [PubMed] [Google Scholar]