Abstract
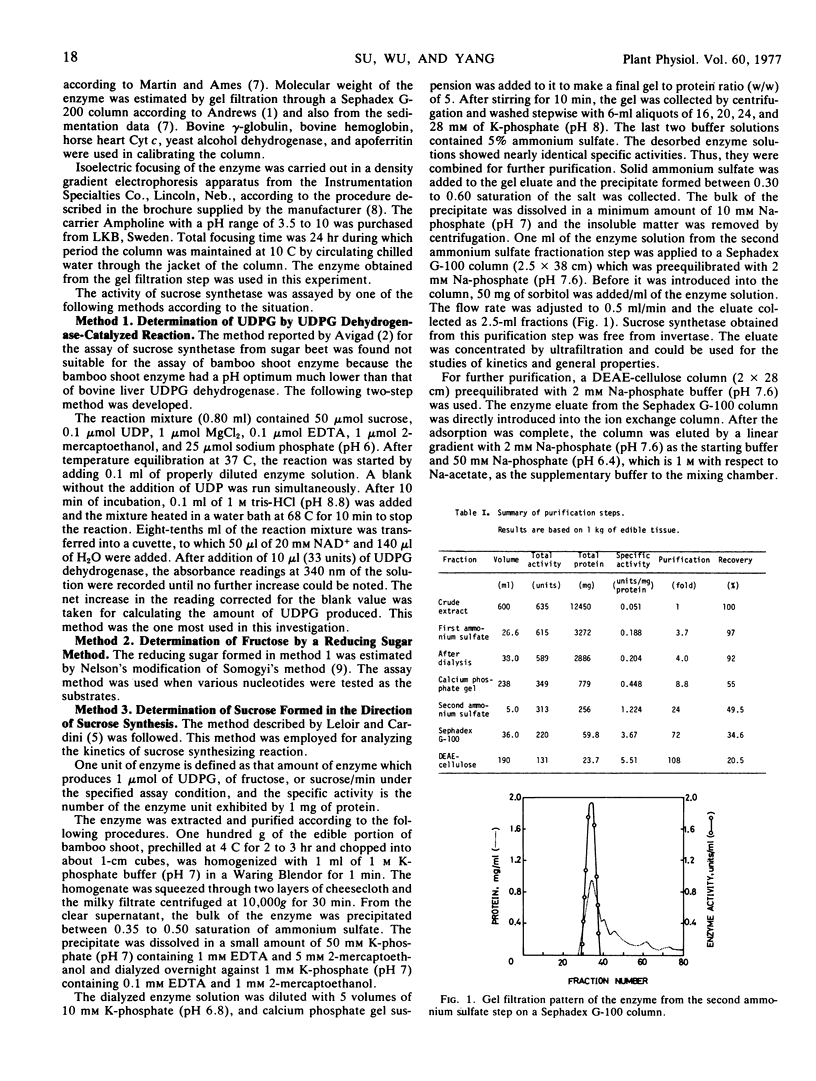
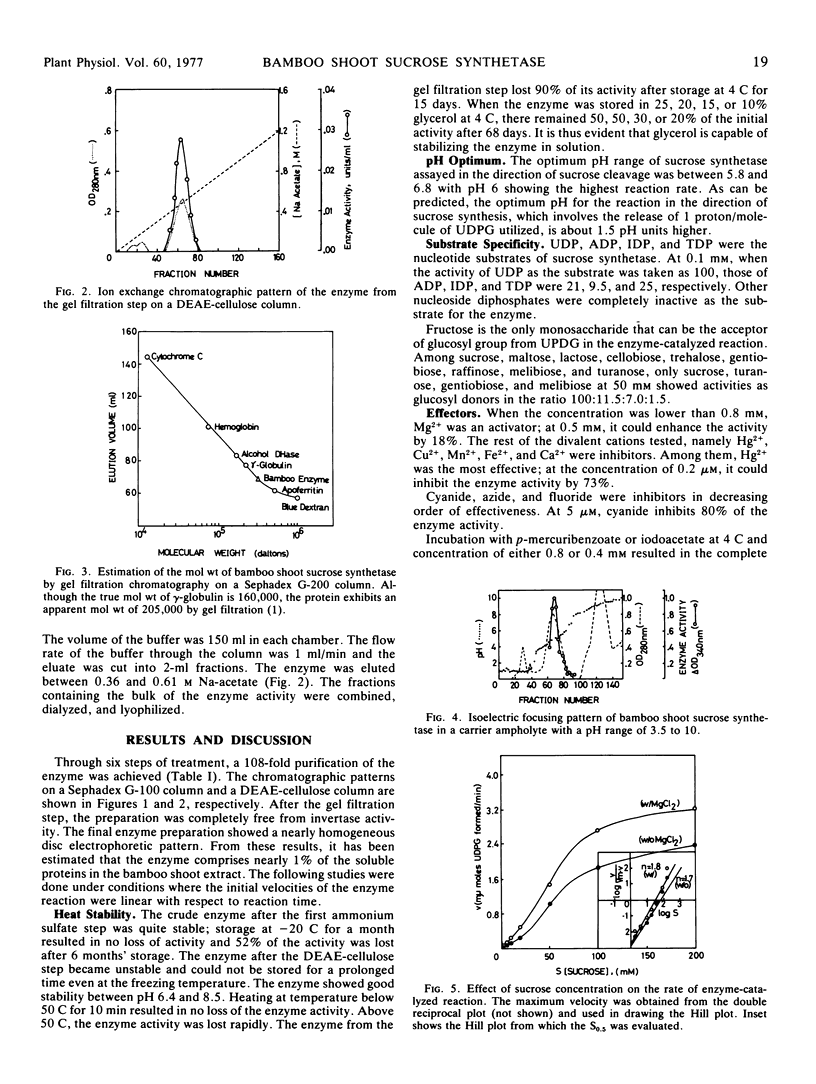
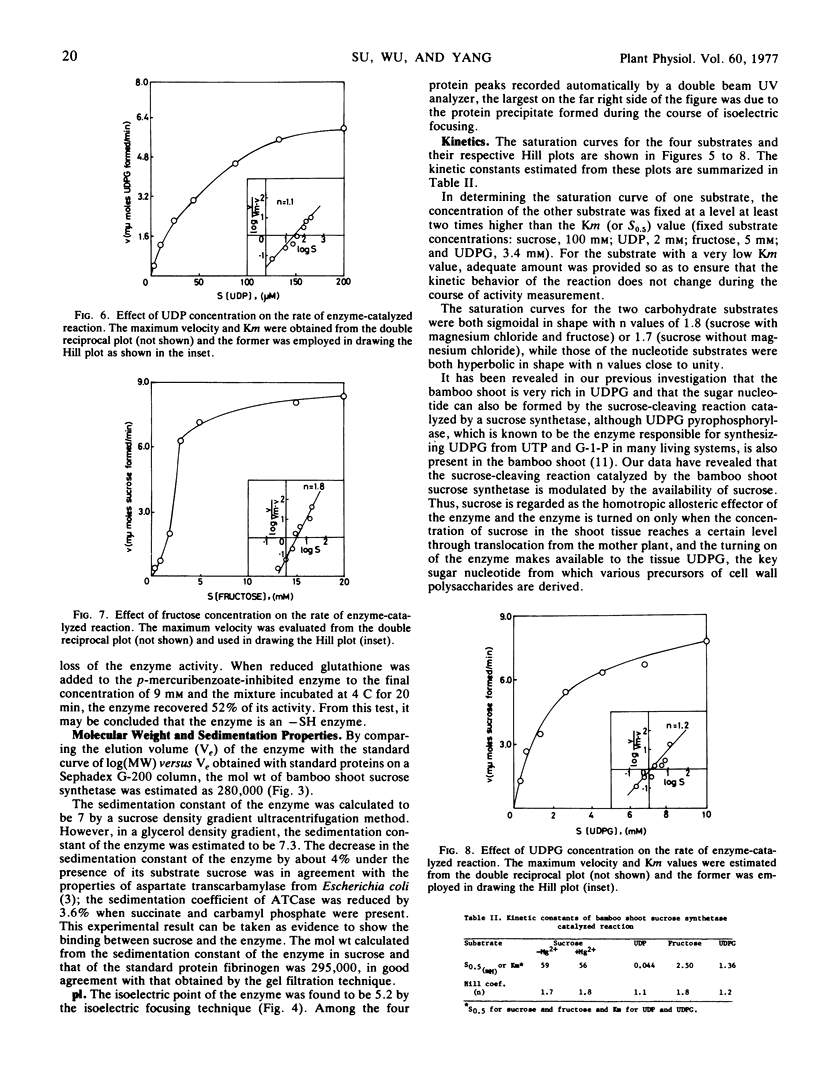
A 108-fold purification of the sucrose synthetase from the extract of the shoot of bamboo Lelaba oldhami was achieved by ammonium sulfate fractionation, calcium phosphate gel adsorption, and chromatographic separations on Sephadex G-100 and diethylaminoethyl-cellulose columns. Some properties of this enzyme, namely thermal and pH stabilities, stabilization by aqueous glycerol, pH optimum, substrate specificities, effects of metallic ions, effects of sulfhydryl reagents, molecular weight, sedimentation constants, isoelectric point, and substrate saturation kinetics had been investigated.
The substrate saturation kinetics indicated that the enzyme could be an allosteric enzyme with the saccharide substrates (sucrose and fructose) serving as the homotropic allosteric effectors in regulating the biosynthesis and degradation of sucrose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G. SUCROSE-URIDINE DIPHOSPHATE GLUCOSYLTRANSFERASE FROM JERUSALEM ARTICHOKE TUBERS. J Biol Chem. 1964 Nov;239:3613–3618. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. II. Evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry. 1968 Feb;7(2):538–552. doi: 10.1021/bi00842a600. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- PALADINI A. C., LELOIR L. F. Studies on uridine-diphosphate-glucose. Biochem J. 1952 Jun;51(3):426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]


