Abstract
Hypothermia (HT) is a well-established neuroprotective strategy against neonatal hypoxic ischemic encephalopathy (HIE). The overexpression of heat shock proteins (HSP) has been shown to provide neuroprotection in animal models of stroke. We aimed to investigate the effect of HT on HSP70 and HSP27 expression in a neonatal rat model of HIE. Seven-day-old rat pups were exposed to hypoxia for 90 min to establish the Rice-Vannucci model and were assigned to the following four groups: hypoxic injury (HI)-normothermia (NT, 36 °C), HI-HT (30 °C), sham-NT, and sham-HT. After temperature intervention for 24 h, the mRNA and protein expression of HSP70 and HSP27 were measured. The association between HSP expression and brain injury severity was also evaluated. The brain infarct size was significantly smaller in the HI-HT group than in the HI-NT group. The mRNA and protein expression of both HSPs were significantly greater in the two HI groups, compared to those in the two sham groups. Moreover, among the rat pups subjected to HI, HT significantly reduced the mRNA and protein expression of both HSPs. The mRNA expression level of the HSPs was proportional to the brain injury severity. Post-ischemic HT, i.e., a cold shock attenuated the expression of HSP70 and HSP27 in a neonatal rat model of HIE. Our study suggests that neither HSP70 nor HSP27 expression is involved in the neuroprotective mechanism through which prolonged HT protects against neonatal HIE.
Keywords: Heat shock protein, Hypoxic ischemic encephalopathy, Hypothermia, Newborn infant
Perinatal hypoxic ischemic encephalopathy (HIE) is a major cause of death and disability in newborn infants, with an incidence of 1 to 3 per 100 births (Ferriero 2004). Among the several therapeutic strategies for neonatal HIE, only hypothermia (HT) has been considered a standard of care for neonatal HIE although the efficacy is limited in cases of severe brain injury (Robertson et al. 2012). The mechanisms underlying HT involve several steps of the neurotoxic cascade after HIE, including the preservation of cerebral energy metabolism, suppression of cytotoxic amino acid accumulation and nitric oxide, suppression of free radical activity and lipid peroxidation, inhibition of inflammatory cytokine, and inhibition of apoptosis and necrosis (Shankaran 2012). The therapeutic options that augment the neuroprotective efficacy of HT are currently topics of major interest in neonatal brain research.
Heat shock proteins (HSP) are chaperones that catalyze the proper folding of nascent proteins and the refolding of denatured proteins (Lanneau et al. 2010). Although they were originally described as proteins induced by “heat shock,” HSP respond to a variety of brain injuries, including hypoxic ischemia (Dienel et al. 1986; Ferriero et al. 1990; Dwyer and Nishimura 1992; Kinouchi et al. 1993a, b; Currie et al. 2000; Sharp et al. 2000). The neuroprotective effect of HSP against ischemic stroke has been clearly demonstrated in transgenic mice models of HSP70 (Plumier et al. 1997; Lee et al. 2001; Tsuchiya et al. 2003; Lee et al. 2004; van der Weerd et al. 2005) and HSP27 (van der Weerd et al. 2010). Pharmacologic induction or intravenous administration of HSP70 also decreased the extent of infarct in rodent models of stroke (Lu et al. 2002; Doeppner et al. 2009; Zhan et al. 2010). In addition to its role as a chaperone, the underlying neuroprotective function of HSP can also largely be explained by its anti-apoptotic properties and immune modulation (Joly et al. 2010; Lanneau et al. 2010). This may reflect the key mechanisms through which HT protects against ischemic brain injury. However, reports on the impact of post-ischemic HT on HSP expression in adult rodent studies are scarce and conflicting (Chopp et al. 1992; Terao et al. 2009) and no investigators have addressed this issue in the developing brain. In the present study, we aimed to investigate the effect of HT on HSP70 and HSP27 expression in a neonatal rat model of HIE.
Materials and methods
Animal model and temperature intervention
All experiments were performed according to the National Institutes of Health guidelines for the humane handling of animals and were approved by the Committee of Animal Research of the Asan Medical Center (Seoul, Korea). Seven-day-old, male, Sprague-Dawley rats were subjected to the well-characterized Rice-Vannucci model of HIE (Rice et al. 1981; Lee et al. 2010). In brief, rats were anesthetized with isoflurane gas (induction, 4.0%; maintenance, 1.5%) in a 1:1 mixture of oxygen and nitrous oxide. Unilateral ligation of the common carotid artery was performed using 5/0 surgical silk. After a 1-h recovery period, the rat pups were moved to a chamber that was maintained at a temperature of 36.0 °C and exposed to humidified oxygen (8%) for 120 min to induce hypoxia.
Rats were assigned to the following four groups: sham-normothermia (NT), sham-hypothermia (HT), hypoxic ischemic injury (HI)-NT, and HI-HT group (n = 12 each). For temperature intervention, the rats were placed in a temperature-controlled chamber, wherein each rat was separated from the others by a lattice. With minimal adjustments throughout the intervention period, the temperature within the chamber was maintained at 35.0 and 27.0 °C for the NT and the HT groups, respectively. The rectal temperature was recorded at 1 and 4 h, and every 4 h thereafter until the end of the intervention period. Throughout the 24 h temperature intervention period, the pups were fed four times per day with 0.4 mL of formula by using feeding needles. Brains were harvested after transcardiac perfusion with 0.9% saline. The brain was cut along the coronal plane at 5 mm away from the occiput. The posterior part was fixed with 4% paraformaldehyde for pathological examination and the remaining anterior part was frozen at −80 °C for further analyses of protein and mRNA.
Brain injury severity
The posterior part of the tissue was paraffin-embedded and 5-μm-thick sections of coronal slices, containing the area equivalent of bregma −4.3 to −4.5 mm (plates 37 or 38 in the adult rat brain atlas of Paxinos), were obtained (Paxinos and Watson 1998). These sections were deparaffinized, incubated in 3% hydrogen peroxide, and then stained with a solution of Cresyl Violet (0.1%). Sections were analyzed with an image scanner (HP Scanjet G4050). Using ImageJ (v. 1.31 NIH), the infarct area, as well as the ipsilateral and contralateral hemispheric area, was manually traced and measured separately from the two consecutive coronal sections with 1-mm interval. The mean ratio of the ipsilateral infarct area to the contralateral hemispheric area was calculated. To minimize the influence of brain edema, infarct area was indirectly measured by subtracting the area of the intact cortex, hippocampus, and striatum in the ipsilateral hemisphere from that of the contralateral hemisphere (Swanson et al. 1990).
Western blotting
The brain samples were homogenized using a BioMasher (Nippi Inc.Ⓡ) and extracted in RIPA buffer (Thermo ScientificⓇ) containing Halt Protease Inhibitor Cocktail (Thermo ScientificⓇ) for 30 min on ice. Protein concentrations were measured using Pierce BCA Protein Assay Kit (Thermo scientificⓇ). Equal amounts of protein (20 μg) were resolved using SDS-PAGE and were transferred onto a nitrocellulose membrane. Blocking of the membranes were carried out with 0.1% TBST containing 3% BSA and the corresponding antibodies were added: HSP70 (1:3000, Enzo Life SciencesⓇ), HSP27 (1:3000, AbcamⓇ), and beta-actin (1:5000, SigmaⓇ). The membranes were then processed for analysis using WesternBright ECL reagent (AdvanstaⓇ). Protein bands were visualized and quantified with the C-DiGit Blot Scanner (LI-COR BiosciencesⓇ) and Image Studio software. For quantification, HSP70 and HSP27 expression levels were normalized to the beta-actin level.
Real-time PCR
Total RNA was isolated using TRIzol reagent (InvitrogenⓇ) and was reverse transcribed into cDNA using the TOPscript RT DryMIX kit (EnzynomicsⓇ). Equal amounts of cDNA were diluted and amplified via real-time PCR using a CFX Connect (Bio rad Ⓡ) in a 20 μL reaction volume containing 2× SYBR PCR master mix (EnzynomicsⓇ) and 10 μM primers. The HSP mRNA expression levels were normalized to GAPDH. The PCR conditions were as follows: initial denaturation step of 5 min at 95 °C, followed by 40 cycles of 10s at 95 °C, 20 s at 60 °C, and 30 s at 72 °C. The primers used were as follows: HSP70 forward; 5′- ACGAGGGTCTCAAGGGCAAG -3′; HSP70 reverse, 5′- CTCTTTCTCAGCCAGCGTGTTAG -3′; HSP27 forward; 5′- CACTGGCAAGCACGAAGAAA -3′; HSP27 reverse, 5′- CAGGGGACAGGGAAGAGGA -3′;GAPDH forward, 5′- TGCACCACCAACTGCTTAGC -3′; and GAPDH reverse, 5′- GGCATGGACTGTGGTCATGAG -3′.
Statistical analysis
Student’s t test was used for the quantitative analysis of the infarct volume and HSP expressions between the HI-NT and the HI-HT groups. An analysis of variance with Tukey’s B test was used to compare the parameters among the four groups. The relationship between HSP expression and brain infarct size was analyzed using Spearman’s rank correlation test. All statistical analyses were performed with SPSS 19.0 for Windows.
Results
Hypothermia and the brain infarct size
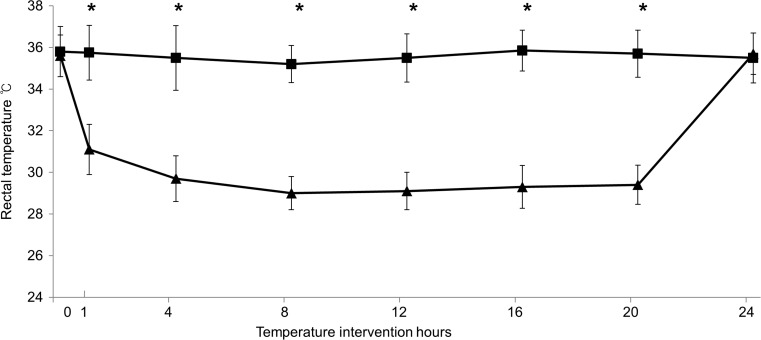
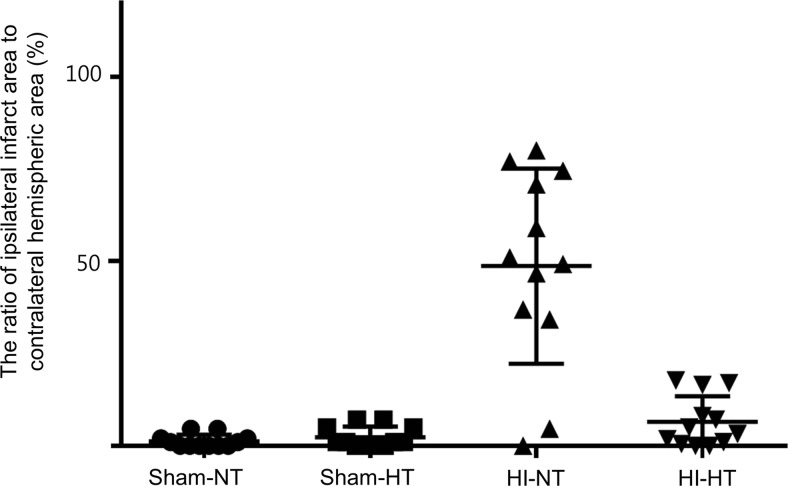
No cases of mortality were noted among the rat-pups. Prior to temperature intervention, the rectal temperature did not differ between the four groups. The temperature within each group remained stable during the intervention period, with a significant difference between the NT groups and the HT groups (Fig. 1). The size of the infarct was significantly greater in the HI-HT group (48.7 ± 26.5%) than in the HI-NT group (6.5 ± 6.9%, Fig. 2).
Fig. 1.
Trend of rectal temperature in the NT groups (rectangles) and the HT groups (triangles) (*p < 0.001 by Student’s t test)
Fig. 2.
The ratio of the ipsilateral infarct area to the contralateral hemispheric area in the four study groups. The size of the infarct was significantly greater in the HI-HT group (triangles) than in the HI-NT group (inverted triangles)
HSP70 and HSP27 expression
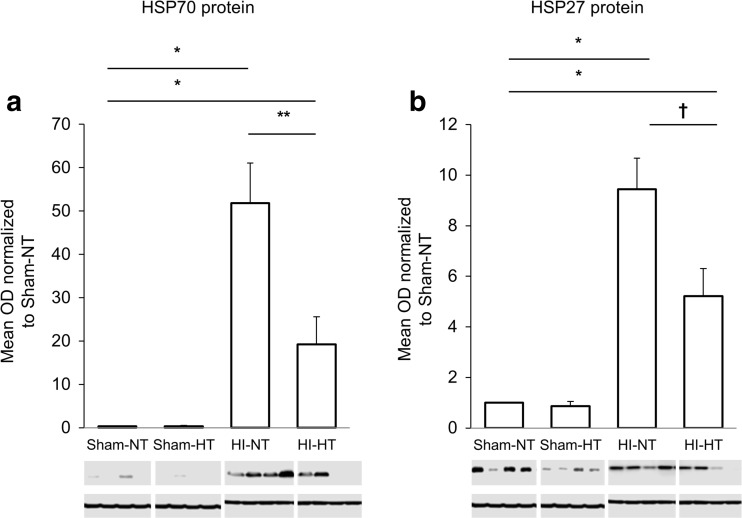
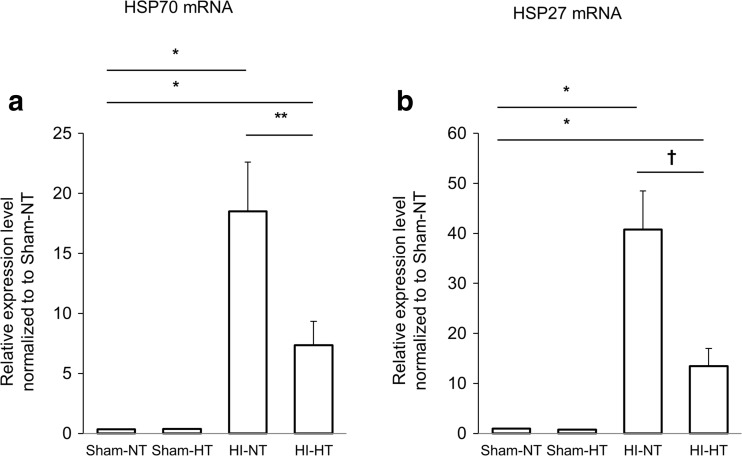
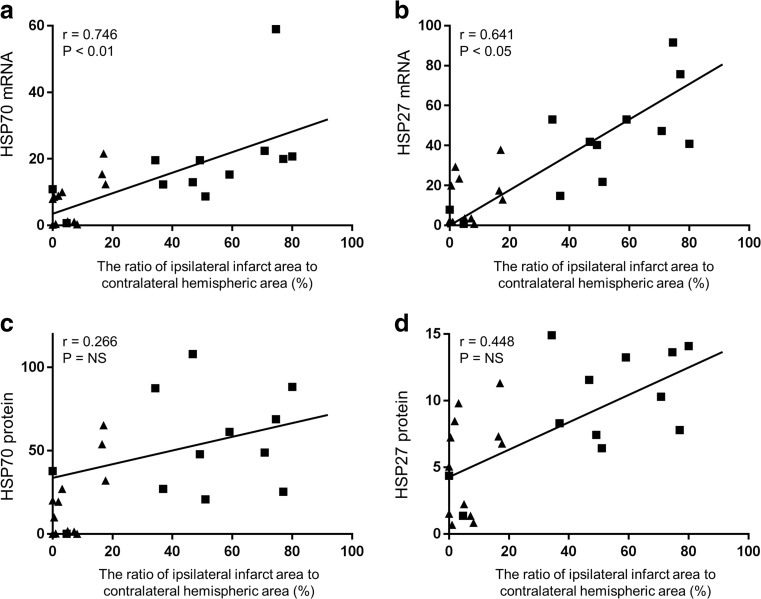
The mRNA and protein expression of the both HSPs were significantly greater in the two HI groups, compared to those in the two sham groups. Among the rat pups subjected to HI, the HT intervention significantly attenuated the protein expression of HSP70 and HSP27 (Fig. 3). Compared with the HI-NT group, the HI-HT group exhibited a significantly lower mRNA expression of HSP70 and HSP27 (Fig. 4). The protein and mRNA expression of HSP70 and HSP27 did not differ between the sham-NT and the sham-HT groups. In the HI-NT groups, the brain infarct size correlated with the mRNA expression levels of HSP70 and HSP27, but not with the protein expression levels (Fig. 5).
Fig. 3.
Protein expression of HSP70 and HSP27 in the ipsilateral hemisphere. a HSP70 expression was significantly greater in both the HI groups, as compared to the sham-NT group that demonstrated little or no HSP70 expression (*p < 0.001). Hypothermia decreased the post-ischemic HSP70 expression in the neonatal rat brain (**p < 0.01). b HSP27 expression was greater in the two HI groups than in the sham-NT groups (*P < 0.001). HSP27 expression was lower in the HI-HT group than in the HI-NT group (†p < 0.05). Constitutional expression of HSP27, which was not influenced by HT, was observed in the sham groups. OD optical density
Fig. 4.
The relative mRNA expression levels of HSP70 and HSP27 using real-time PCR of the homogenates of the ipsilateral hemisphere. After HI, the mRNA expression of HSP70 and HSP27 was significantly increased (*p < 0.001). HT significantly decreased the mRNA expression of HSP70 (A) (**p < 0.05) and HSP27 (B) († p < 0.01) at 24 h after HI. Data were normalized to GAPDH
Fig. 5.
Scatterplot of protein and mRNA expression of HSP70 and HSP27 versus the infarct size in 7-day-old rats treated with either 24 h HT (triangles) or NT (rectangles) after hypoxic ischemic injury. The size of the brain infarct was significantly correlated with the magnitude of mRNA expression of HSP70 (a) and HSP27 (b), but not with their protein expression levels (c and d). Regression lines are only drawn for the HI-NT group. The expression level of HSP was normalized to that of the Sham-NT group
Discussion
To our knowledge, this is the first study to address the effect of HT on HSP70 and HSP27 expression in a neonatal rodent model. A 24-h HT significantly reduced the post-ischemic expression of HSP70 and HSP27 in the brain of neonatal rats. Moreover, HT suppressed HSP expression at the transcriptional level. The post-ischemic induction of HSP70 expression is consistent with previous findings (Li et al. 2016; Chopp et al. 1991; Kinouchi et al. 1993a, b Sharp et al. 1993; Currie et al. 2000) including neonatal rodent model (Ferriero et al. 1990; Matsumori et al. 2005). We reported on the increased HSP27 expression in neonatal rats with HIE, which is a novel finding.
In the present study, despite the more robust protein and mRNA expressions of both HSPs in the HI-NT group versus the HI-HT group, the brain infarct size was significantly decreased in the HI-HT group. This is consistent with another adult rodent study, wherein 3 h of HT reduced the HSP70 immunoreactivity at 48 h after middle cerebral artery occlusion (MCAO) (Chopp et al. 1991). Considering the other previous studies that showed the remarkable neuroprotection conferred by HSP70 pre-induction (Rajdev et al. 2000; Lu et al. 2002; Tsuchiya et al. 2003; Franklin et al. 2005; Matsumori et al. 2005), we speculate that HSP expression can exert its neuroprotective effects only when it precedes hypoxic ischemic injury. Moreover, we could not completely exclude the possibility that the post-ischemic induction of HSP70 and/or HSP27 is rather detrimental to the developing brain at a stage where compensatory energy metabolism remains incompletely developed (Lee et al. 2001). In addition, prolonged HT did not influence the expression of HSP70 and HSP27 in the brains of sham-treated neonatal rats. This is not in agreement with several in vitro studies which consistently demonstrated increased HSP70 expression after rewarming from HT rather than during the period of severe (Holland et al. 1993; Liu et al. 1994) or mild (Fujita 1999) HT per se. Our finding suggests that moderate degree of HT per se has a minimal impact on the expression of HSPs in the brain provided that there is no ischemic injury, as previously demonstrated in an adult rat exposed to HT for 48 h (Kaneko and Kibayashi 2012). Interestingly, an in vitro study with similar setting of HT (32 °C for 24 h) to our study, demonstrated decreased mRNA expression of HSP70 in inflammatory cell culture (Sonna et al. 2006). The influence of moderate degree of HT on HSP induction in developing brain could be addressed in a follow-up study that also targets the cold shock proteins including cold-induced RNA-binding protein (CIRBP) and RNA-binding motif 3(RBM3) (Sonna et al. 2002).
A low but clear expression of HSP27 in the sham-treated group suggests a constitutional expression of HSP27 in the neonatal brain. In adult rats, there is minimal or no constitutive expression of HSP70 and HSP27 in the cerebrum (Franklin et al. 2005), although HSP27 has been detected in the neurons of the retina, brain stem, and spinal cord (Chen and Brown 2007). Following stress due to ischemic injury, HSP27 induction is predominantly observed in the glial cells rather than in the neurons (Currie et al. 2000). The role of neuroprotection exerted by persistent HSP27 induction, mainly in astrocytes, remains unclear, even though some evidences of its anti-apoptotic action at different stages of cell death has been reported (Sharp et al. 2000, Stetler et al. 2009). Another plausible explanation for the HSP27 expression in the sham groups is the lower induction threshold of HSP27, compared to that of HSP70, in response to surgical stress. Unlike HSP70, HSP27 is an ATP-independent chaperone. However, only minimal information is available on whether the ATP-dependency of HSP27 is associated with the threshold for protein expression in response to minor stress (Lanneau et al. 2010). Interestingly, significant HSP27 expression was observed in adult rats following only 10 min of MCAO, which is usually not considered sufficient to cause neuronal cell death (Currie et al. 2000). HSP27 immunostaining in the hemisphere contralateral to that with ischemic injury also supports the hypothesis of a lower threshold for HSP27 expression in response to minor stress (Kato et al. 1995).
A linear relationship between the mRNA expression levels of HSP70 and HSP27 and the severity of ischemic brain injury is also observed in the present study. Although few reports have described the correlation between the HSP70 expression level and the severity of injury in organs such as liver (Aoe et al. 1997) and spinal cord (Cizkova et al. 2004), such a relationship in the neonatal brain has not been described yet. Following pharmacologically induced-status epilepticus, a strong correlation was observed between the number of HSP70-positive neurons and the duration of type IV electroencephalogram activity (Lowenstein et al. 1990). Meanwhile, the relationship was not clear in the setting of stroke, particularly in the ischemic core region (Chopp et al. 1991; Kinouchi et al. 1993a, b). Following focal ischemia, only minimal HSP70 expression was detected in severely damaged or necrotic hippocampal neurons, while marked HSP70 expression was noted in the morphologically intact neurons. These discrepancies may be due to the differences in the developmental stage of the brain or the severity of the injury.
Our study had certain limitations, of note. First, the outcome measure was recorded only one time point; in fact, HT might induce HSP expression earlier than 24-h post-injury. In a previous study, cells immunoreactive to HSP70 were observed as early as 1 h after the completion of hypoxia in neonatal rats (Ferriero et al. 1990). However, even if HSP70 was induced early, particularly within 6 h after HIE, the critical time window for hypothermic neuroprotection, the HSP expression seems to be insufficient to exert neuroprotection against HI injury in neonatal rats. Further study with multiple time points for the outcome measure could address this issue. Second, the impact of HT on HSP70 and HSP27 induction should be evaluated in the setting of less severe HI injury. As demonstrated, a >50-fold increase in HSP70 expression was observed in rat pups subjected to HI versus sham-treated rat-pups, which is remarkably robust considering approximately 2-fold increase in HSP70 expressions in another neonatal study (Matsumori et al. 2005). Third, our study did not describe the regional distribution of HSP expression. Since the distribution of HSP70 induction differs according to the type of cells and the degree of ischemia (Gonzalez et al. 1991; Sharp et al. 1991), the non-significant correlation between HSP protein expression and the severity of brain injury, represented by the infarct area, could be reevaluated in the region-specific analysis.
In conclusion, post-ischemic HT, i.e., a cold shock, reduced the expression of HSP70 and HSP27 in a neonatal rat model of HIE. Our study suggests that the neuroprotection conferred by prolonged HT is not associated with HSP overexpression in the neonatal brain. Thus, we believe that neither HSP70 nor HSP27 expression is involved in the neuroprotective mechanism through which prolonged HT protects against HIE in neonatal rats.
Acknowledgements
This research was supported by a Grant (10-465) from the Asan Institute for Life Sciences, Seoul, Korea.
References
- Aoe T, Inaba H, Kon S, Imai M, Aono M, Mizuguchi T, Saito T, Nishino T. Heat shock protein 70 messenger RNA reflects the severity of ischemia/hypoxia-reperfusion injury in the perfused rat liver. Crit Care Med. 1997;25(2):324–329. doi: 10.1097/00003246-199702000-00022. [DOI] [PubMed] [Google Scholar]
- Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12(1):51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Li Y, Dereski MO, Levine SR, Yoshida Y, Garcia JH. Neuronal injury and expression of 72-kDa heat-shock protein after forebrain ischemia in the rat. Acta Neuropathol. 1991;83(1):66–71. doi: 10.1007/BF00294432. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, Dereski MO, Levine SR, Yoshida Y, Garcia JH. Hypothermia reduces 72-kDa heat-shock protein induction in rat brain after transient forebrain ischemia. Stroke. 1992;23(1):104–107. doi: 10.1161/01.STR.23.1.104. [DOI] [PubMed] [Google Scholar]
- Cizkova D, Carmel JB, Yamamoto K, Kakinohana O, Sun D, Hart RP, Marsala M. Characterization of spinal HSP72 induction and development of ischemic tolerance after spinal ischemia in rats. Exp Neurol. 2004;185(1):97–108. doi: 10.1016/j.expneurol.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Currie RW, Ellison JA, White RF, Feuerstein GZ, Wang X, Barone FC. Benign focal ischemic preconditioning induces neuronal Hsp70 and prolonged astrogliosis with expression of Hsp27. Brain Res. 2000;863(1–2):169–181. doi: 10.1016/S0006-8993(00)02133-8. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Kiessling M, Jacewicz M, Pulsinelli WA. Synthesis of heat shock proteins in rat brain cortex after transient ischemia. J Cereb Blood Flow Metab. 1986;6(4):505–510. doi: 10.1038/jcbfm.1986.86. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, Nagel F, Dietz GP, Weise J, Tonges L, Schwarting S, Bahr M. TAT-Hsp70-mediated neuroprotection and increased survival of neuronal precursor cells after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2009;29(6):1187–1196. doi: 10.1038/jcbfm.2009.44. [DOI] [PubMed] [Google Scholar]
- Dwyer BE, Nishimura RN. Heat shock proteins in hypoxic-ischemic brain injury: a perspective. Brain Pathol. 1992;2(3):245–251. doi: 10.1111/j.1750-3639.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, Soberano HQ, Simon RP, Sharp FR. Hypoxia-ischemia induces heat shock protein-like (HSP72) immunoreactivity in neonatal rat brain. Dev Brain Res. 1990;53(1):145–150. doi: 10.1016/0165-3806(90)90136-M. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Krueger-Naug AM, Clarke DB, Arrigo AP, Currie RW. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperth. 2005;21(5):379–392. doi: 10.1080/02656730500069955. [DOI] [PubMed] [Google Scholar]
- Fujita J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999;1(2):243–255. [PubMed] [Google Scholar]
- Gonzalez MF, Lowenstein D, Fernyak S, Hisanaga K, Simon R, Sharp FR. Induction of heat shock protein 72-like immunoreactivity in the hippocampal formation following transient global ischemia. Brain Res Bull. 1991;26(2):241–250. doi: 10.1016/0361-9230(91)90234-B. [DOI] [PubMed] [Google Scholar]
- Holland DB, Roberts SG, Wood EJ, Cunliffe WJ. Cold shock induces the synthesis of stress proteins in human keratinocytes. J Invest Dermatol. 1993;101(2):196–199. doi: 10.1111/1523-1747.ep12363791. [DOI] [PubMed] [Google Scholar]
- Joly A-L, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. Journal of innate immunity. 2010;2(3):238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Kibayashi K. Mild hypothermia facilitates the expression of cold-inducible RNA-binding protein and heat shock protein 70.1 in mouse brain. Brain Res. 2012;1466:128–136. doi: 10.1016/j.brainres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Kato H, Kogure K, Liu XH, Araki T, Kato K, Itoyama Y. Immunohistochemical localization of the low molecular weight stress protein HSP27 following focal cerebral ischemia in the rat. Brain Res. 1995;679(1):1–7. doi: 10.1016/0006-8993(95)00198-Y. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Sharp FR, Hill MP, Koistinaho J, Sagar SM, Chan PH. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993;13(1):105–115. doi: 10.1038/jcbfm.1993.13. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Sharp FR, Koistinaho J, Hicks K, Kamii H, Chan PH. Induction of heat shock hsp70 mRNA and HSP70 kDa protein in neurons in the ‘penumbra’ following focal cerebral ischemia in the rat. Brain Res. 1993;619(1–2):334–338. doi: 10.1016/0006-8993(93)91630-B. [DOI] [PubMed] [Google Scholar]
- Lanneau D, Wettstein G, Bonniaud P, Garrido C. Heat shock proteins: cell protection through protein triage. Sci World J. 2010;10:1543–1552. doi: 10.1100/tsw.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Yenari MA, Sun GH, Xu L, Emond MR, Cheng D, Steinberg GK, Giffard RG. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001;170(1):129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004;35(9):2195–2199. doi: 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- Lee BS, Woo CW, Kim ST, Kim KS. Long-term neuroprotective effect of postischemic hypothermia in a neonatal rat model of severe hypoxic ischemic encephalopathy: a comparative study on the duration and depth of hypothermia. Pediatr Res. 2010;68(4):303–308. doi: 10.1203/PDR.0b013e3181ef3007. [DOI] [PubMed] [Google Scholar]
- Li Q, Nakano Y, Shang J, Ohta Y, Sato K, Takemoto M, Hishikawa N, Yamashita T, Abe K. Temporal profiles of stress protein inductions after focal transient ischemia in mice brain. J Stroke Cerebrovasc Dis. 2016;25(10):2344–2351. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Liu AY, Bian H, Huang LE, Lee YK. Transient cold shock induces the heat shock response upon recovery at 37 degrees C in human cells. J Biol Chem. 1994;269(20):14768–14775. [PubMed] [Google Scholar]
- Lowenstein DH, Simon RP, Sharp FR. The pattern of 72-kDa heat shock protein-like immunoreactivity in the rat brain following flurothyl-induced status epilepticus. Brain Res. 1990;531(1–2):173–182. doi: 10.1016/0006-8993(90)90771-3. [DOI] [PubMed] [Google Scholar]
- Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81(2):355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25(7):899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in sterotaxic coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Plumier JC, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2(3):162–167. doi: 10.1379/1466-1268(1997)002<0162:TMETHI>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47(6):782–791. doi: 10.1002/1531-8249(200006)47:6<782::AID-ANA11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Robertson NJ, Tan S, Groenendaal F, van Bel F, Juul SE, Bennet L, Derrick M, Back SA, Valdez RC, Northington F, Gunn AJ, Mallard C. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr. 2012;160(4):544–552. doi: 10.1016/j.jpeds.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S. Hypoxic-ischemic encephalopathy and novel strategies for neuroprotection. Clin Perinatol. 2012;39(4):919–929. doi: 10.1016/j.clp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Lowenstein D, Simon R, Hisanaga K. Heat shock protein hsp72 induction in cortical and striatal astrocytes and neurons following infarction. J Cereb Blood Flow Metab. 1991;11(4):621–627. doi: 10.1038/jcbfm.1991.113. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kinouchi H, Koistinaho J, Chan PH, Sagar SM. HSP70 heat shock gene regulation during ischemia. Stroke. 1993;24(12 Suppl):I72–I75. [PubMed] [Google Scholar]
- Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20(7):1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Sonna LA, Fujita J, Gaffin SL, Lilly CM (2002) Invited review: effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92(4):1725–1742 [DOI] [PubMed]
- Sonna LA, Kuhlmeier MM, Carter HC, Hasday JD, Lilly CM, Fairchild KD. Effect of moderate hypothermia on gene expression by THP-1 cells: a DNA microarray study. Physiol Genomics. 2006;26(1):91–98. doi: 10.1152/physiolgenomics.00296.2005. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27: mechanisms of cellular protection against neuronal injury. Curr Mol Med. 2009;9(7):863–872. doi: 10.2174/156652409789105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Terao Y, Miyamoto S, Hirai K, Kamiguchi H, Ohta H, Shimojo M, Kiyota Y, Asahi S, Sakura Y, Shintani Y. Hypothermia enhances heat-shock protein 70 production in ischemic brains. Neuroreport. 2009;20(8):745–749. doi: 10.1097/WNR.0b013e32832a2f32. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Hong S, Matsumori Y, Shiina H, Kayama T, Ra S, Dillman WH, Liu J, Panter SS, Weinstein PR. Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome C release and subsequent DNA fragmentation after permanent focal ischemia. J Cereb Blood Flow Metab. 2003;23:718–727. doi: 10.1097/01.WCB.0000054756.97390.F7. [DOI] [PubMed] [Google Scholar]
- van der Weerd L, Lythgoe MF, Badin RA, Valentim LM, Akbar MT, de Belleroche JS, Latchman DS, Gadian DG. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia—an MRI study. Exp Neurol. 2005;195(1):257–266. doi: 10.1016/j.expneurol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- van der Weerd L, Tariq Akbar M, Aron Badin R, Valentim LM, Thomas DL, Wells DJ, Latchman DS, Gadian DG, Lythgoe MF, de Belleroche JS. Overexpression of heat shock protein 27 reduces cortical damage after cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(4):849–856. doi: 10.1038/jcbfm.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Ander BP, Liao IH, Hansen JE, Kim C, Clements D, Weisbart RH, Nishimura RN, Sharp FR. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41(3):538–543. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]