Abstract
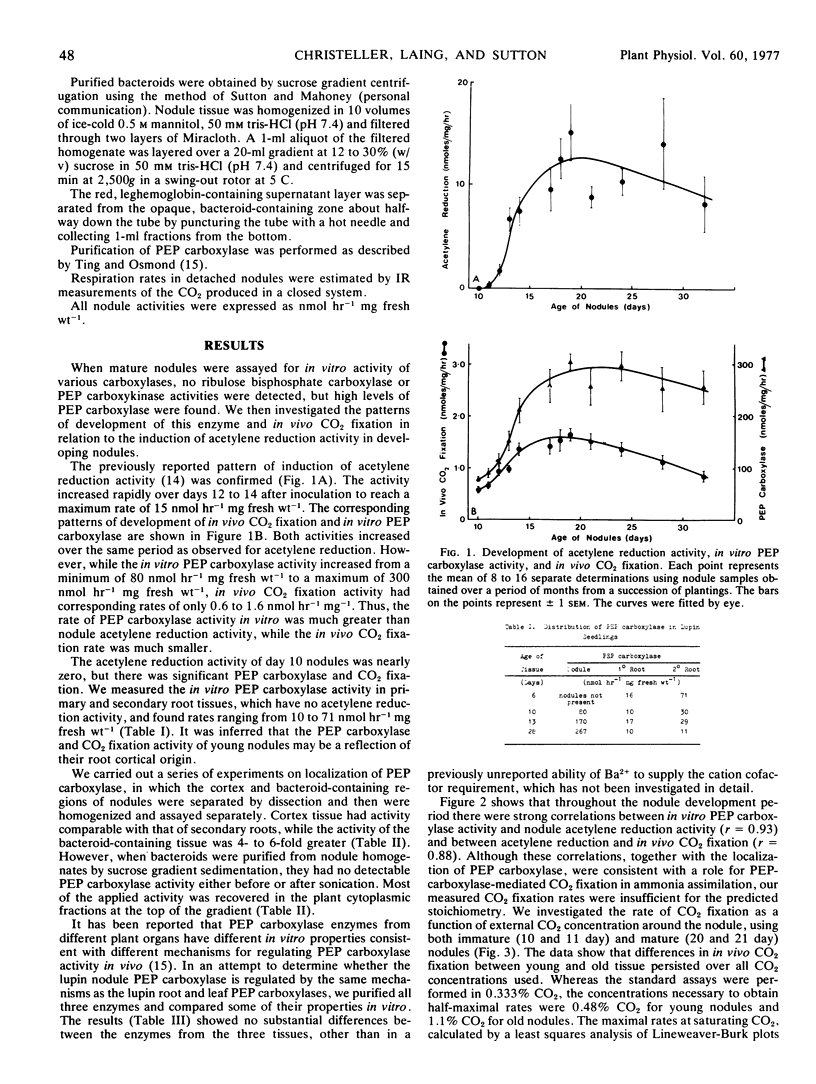
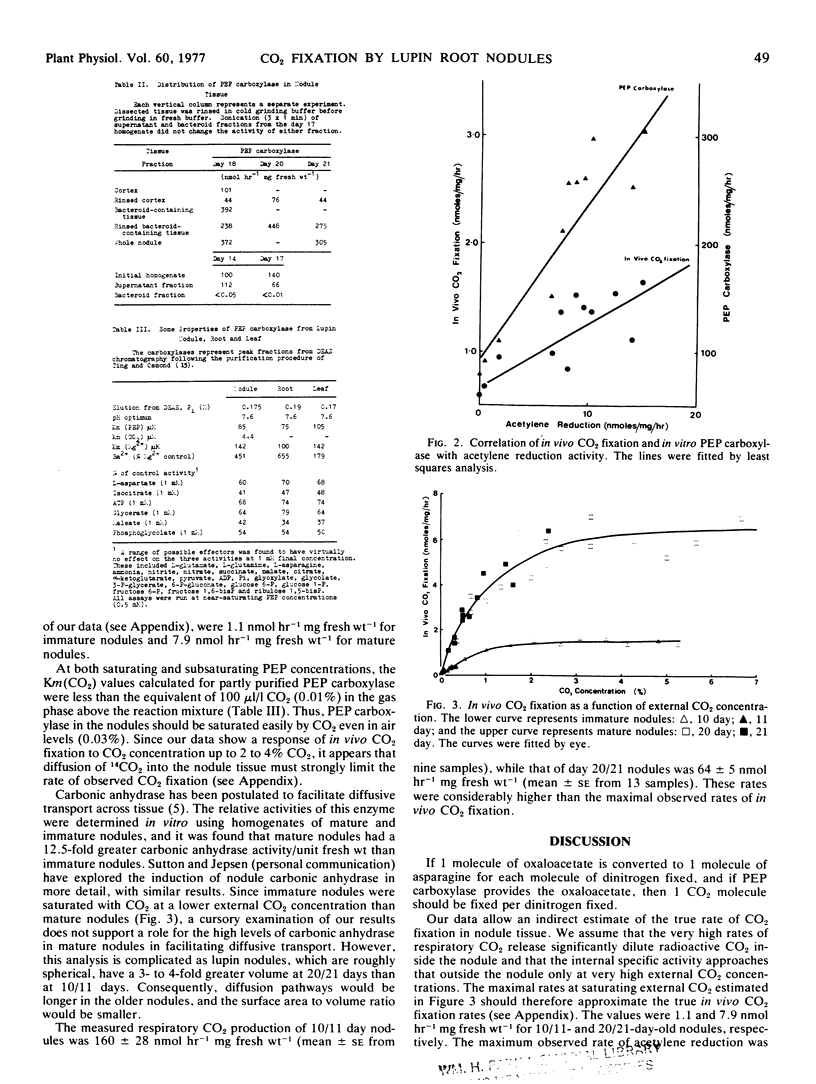
In vivo CO2 fixation and in vitro phosphoenolpyruvate (PEP) carboxylase levels have been measured in lupin (Lupinus angustifolius L.) root nodules of various ages. Both activities were greater in nodule tissue than in either primary or secondary root tissue, and increased about 3-fold with the onset of N2 fixation. PEP carboxylase activity was predominantly located in the bacteroid-containing zone of mature nodules, but purified bacteroids contained no activity. Partially purified PEP carboxylases from nodules, roots, and leaves were identical in a number of kinetic parameters. Both in vivo CO2 fixation activity and in vitro PEP carboxylase activity were significantly correlated with nodule acetylene reduction activity during nodule development. The maximum rate of in vivo CO2 fixation in mature nodules was 7.9 nmol hour−1 mg fresh weight−1, similar to rates of N2 fixation and reported values for amino acid translocation.
The results suggest that the oxaloacetete used as the primary “carbon skeleton” acceptor for ammonia assimilation and amino acid synthesis in lupin nodules is provided via the PEP carboxylase reaction rather than through the tricarboxylic acid cycle. The source of PEP is presumably glycolysis, while the major source of CO2 is inferred to be respiration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christeller J. T., Laing W. A. Isotope Discrimination by Ribulose 1,5-Diphosphate Carboxylase: No Effect of Temperature or HCO(3) Concentration. Plant Physiol. 1976 Apr;57(4):580–582. doi: 10.1104/pp.57.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner J., Ting I. P. CO(2) Metabolism in Corn Roots. II. Intracellular Distribution of Enzymes. Plant Physiol. 1967 May;42(5):719–724. doi: 10.1104/pp.42.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. An assat for PEP carboxykinase in crude tissue extracts. Anal Biochem. 1973 Mar;52(1):280–285. doi: 10.1016/0003-2697(73)90350-3. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- Robertson J. G., Warburton M. P., Farnden K. J. Induction of glutamate synthase during nodule development in lupin. FEBS Lett. 1975 Jul 15;55(1):33–37. doi: 10.1016/0014-5793(75)80950-1. [DOI] [PubMed] [Google Scholar]
- Sutton W. D., Jepsen N. M. Studies with detached lupin root nodules in culture: I. Maintenance and induction of acetylene reduction activity. Plant Physiol. 1975 Nov;56(5):665–670. doi: 10.1104/pp.56.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiol. 1973 Mar;51(3):448–453. doi: 10.1104/pp.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. A. Pyruvate carboxylation and plant metabolism. Biol Rev Camb Philos Soc. 1962 May;37:215–256. doi: 10.1111/j.1469-185x.1962.tb01611.x. [DOI] [PubMed] [Google Scholar]


