Abstract
The effect of dinitramine, a selective herbicide, on the plasma membrane of the soybean (Glycine max L.) root was studied. Used as marker systems to observe the herbicide effect were two plasma-membrane-specific enzymes, pH 6.5 ATPase and glucan synthetase.
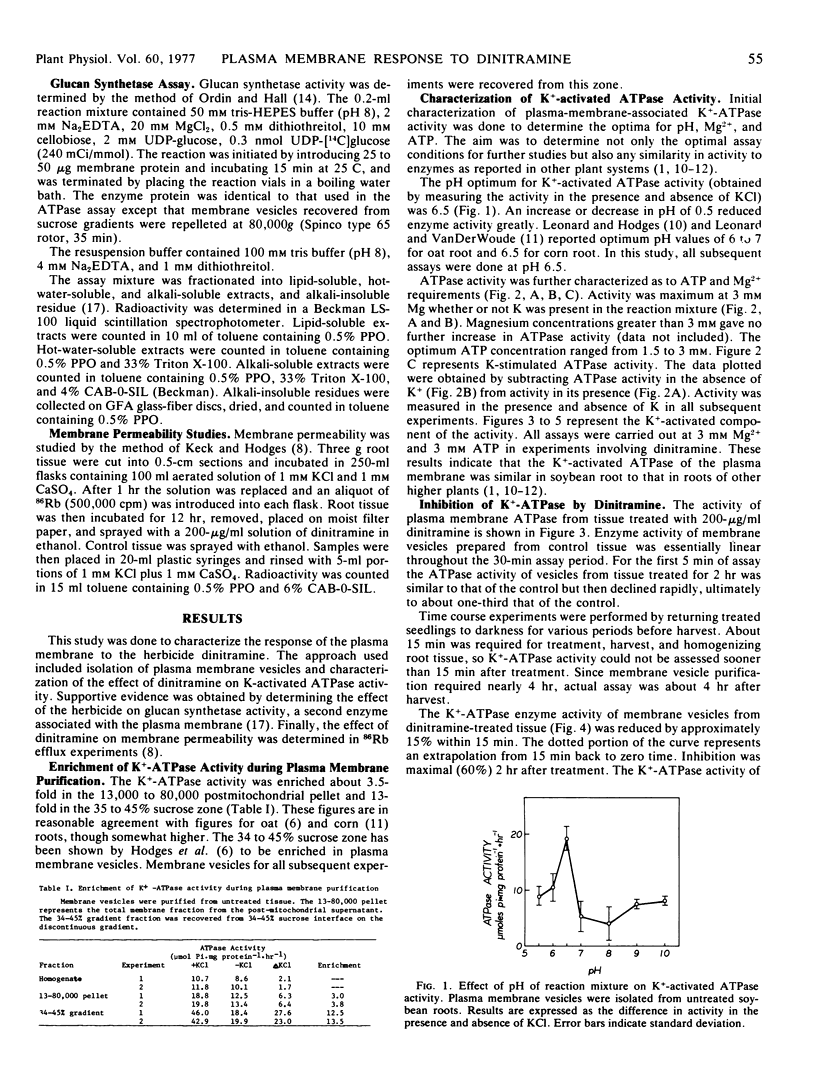
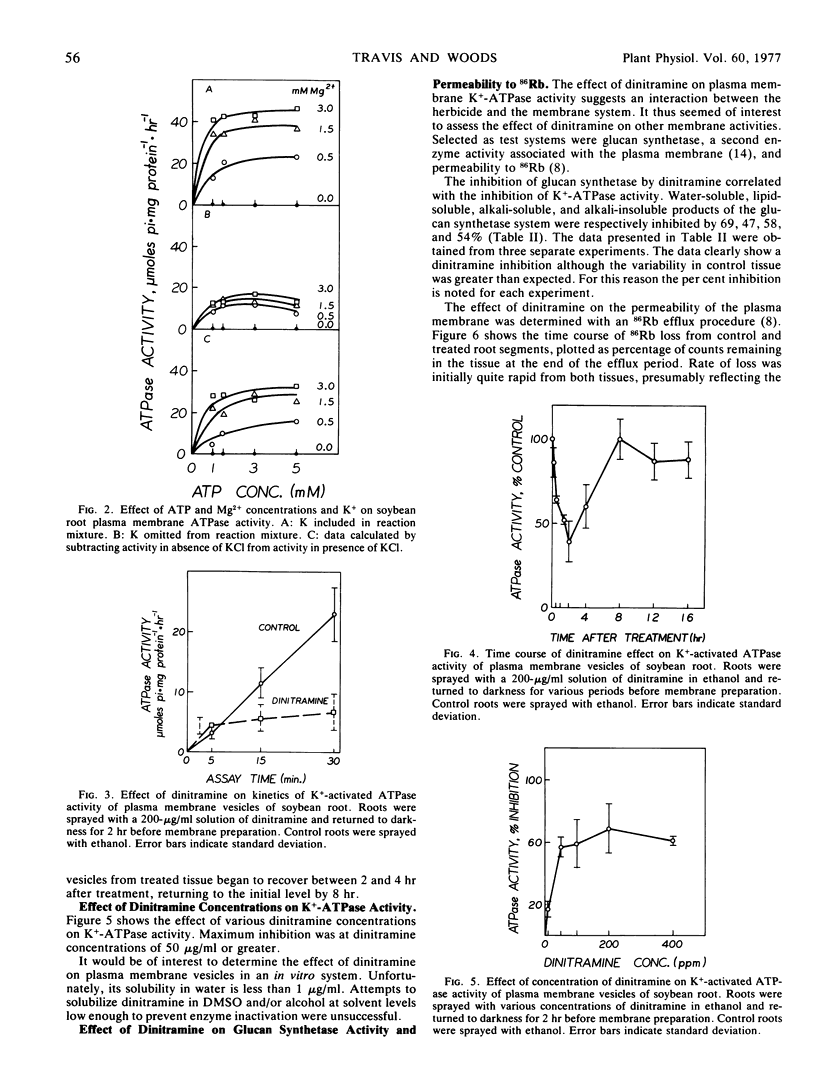
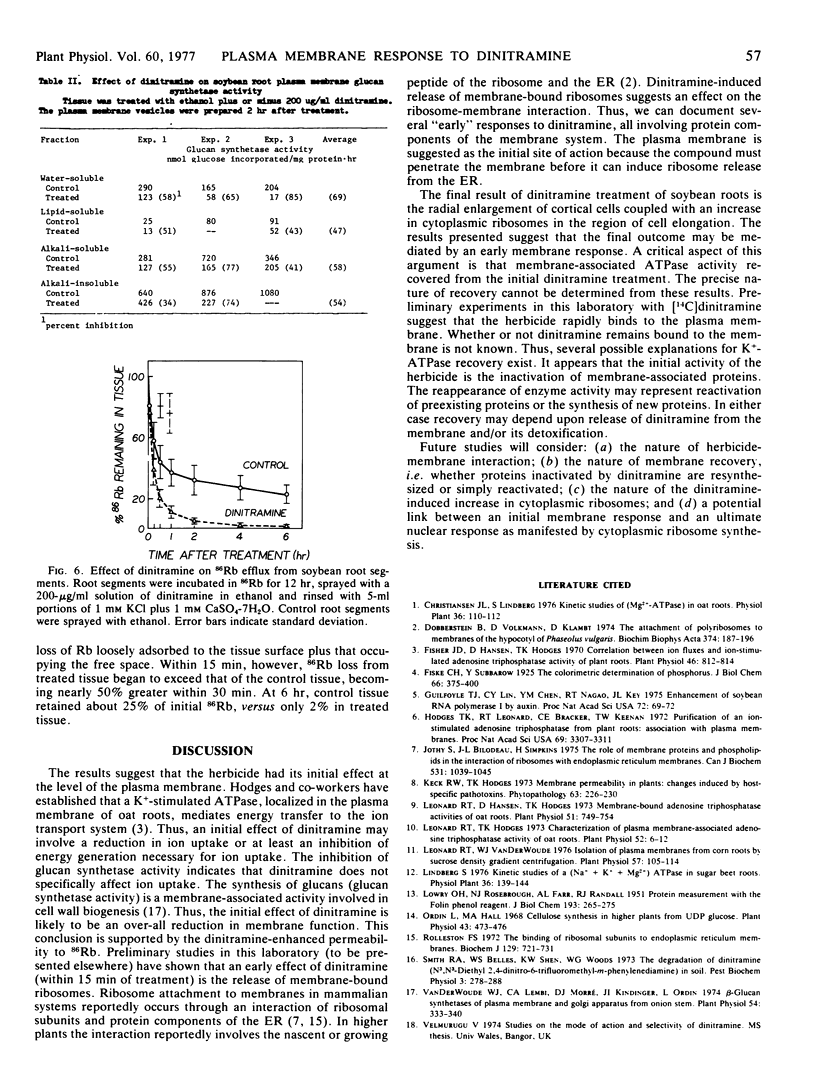
The activity of pH 6.5 ATPase decreased significantly in membrane vesicles prepared from roots harvested 15 minutes after treatment with dinitramine. Maximum inhibition occurred in roots harvested 2 hours after treatment. Glucan synthetase activity decreased similarly within 2 hours of treatment.
Membrane permeability to 86Rb was rapidly increased by dinitramine.
The activity of pH 6.5 ATPase returned to the control level within 8 hours of treatment with dinitramine.
These results show dinitramine's initial site of action to be the plasma membrane, producing an over-all reduction in membrane function through inactivation of membrane-associated proteins.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dobberstein B., Volkmann D., Klämbt D. The attachment of polyribosomes to membranes of the hypocotyl of Phaseolus vulgaris. Biochim Biophys Acta. 1974 Dec 6;374(2):187–196. doi: 10.1016/0005-2787(74)90362-1. [DOI] [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle T. J., Lin C. Y., Chen Y. M., Nagao R. T., Key J. L. Enhancement of soybean RNA polymerase I by auxin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):69–72. doi: 10.1073/pnas.72.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothy S., Bilodeau J. L., Simpkins H. The role of membrane proteins and phospholipids in the interaction of ribosomes with endoplasmic reticulum membranes. Can J Biochem. 1975 Sep;53(9):1039–1045. doi: 10.1139/o75-143. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Hall M. A. Cellulose synthesis in higher plants from UDP glucose. Plant Physiol. 1968 Mar;43(3):473–476. doi: 10.1104/pp.43.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S. The binding of ribosomal subunits to endoplasmic reticulum membranes. Biochem J. 1972 Sep;129(3):721–731. doi: 10.1042/bj1290721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]


