Abstract
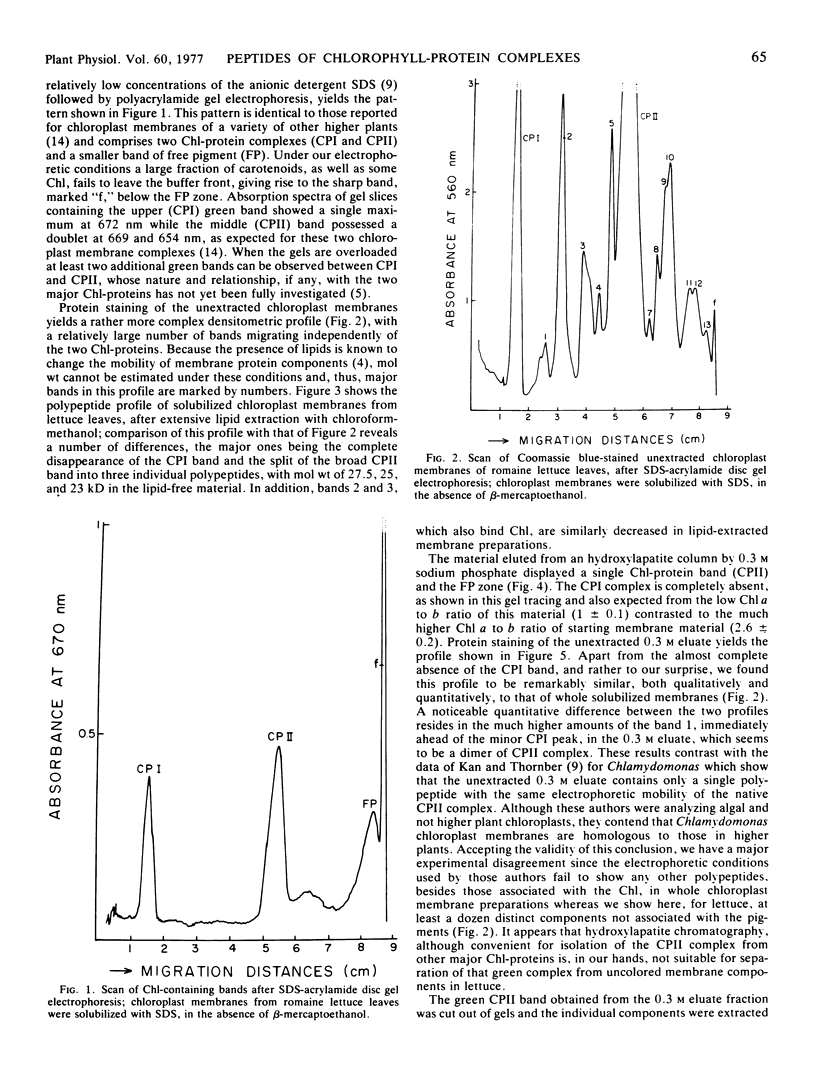
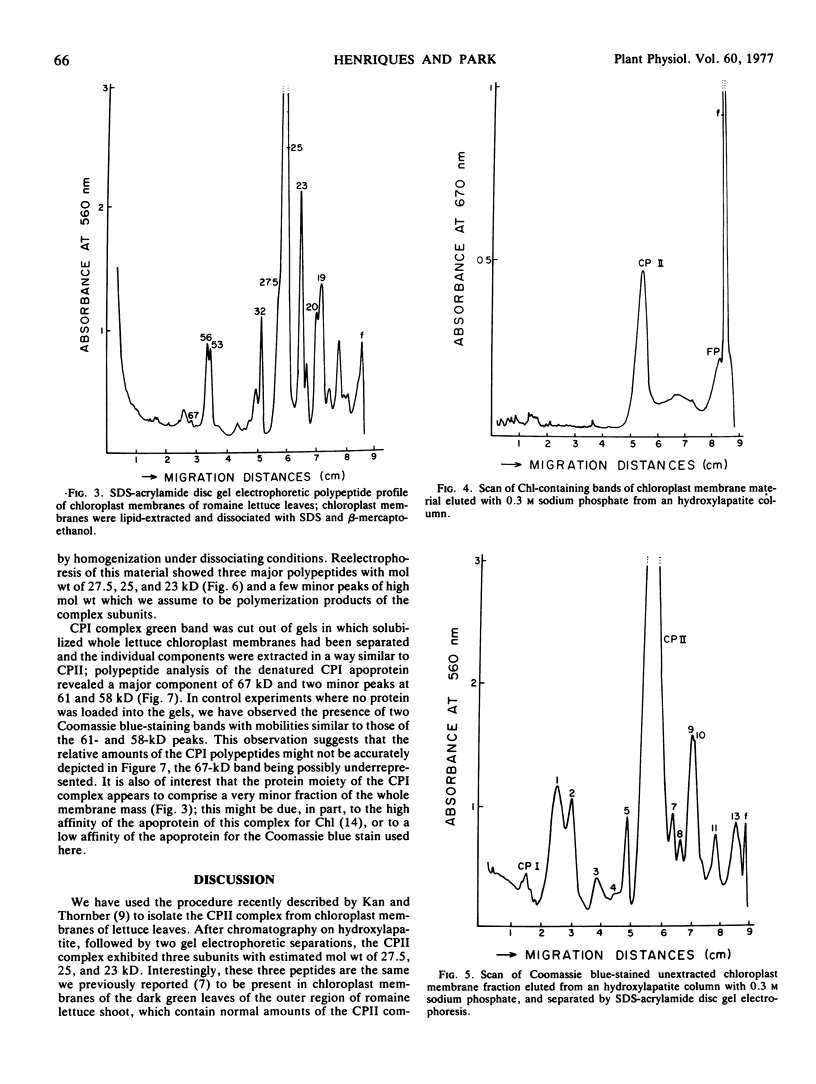
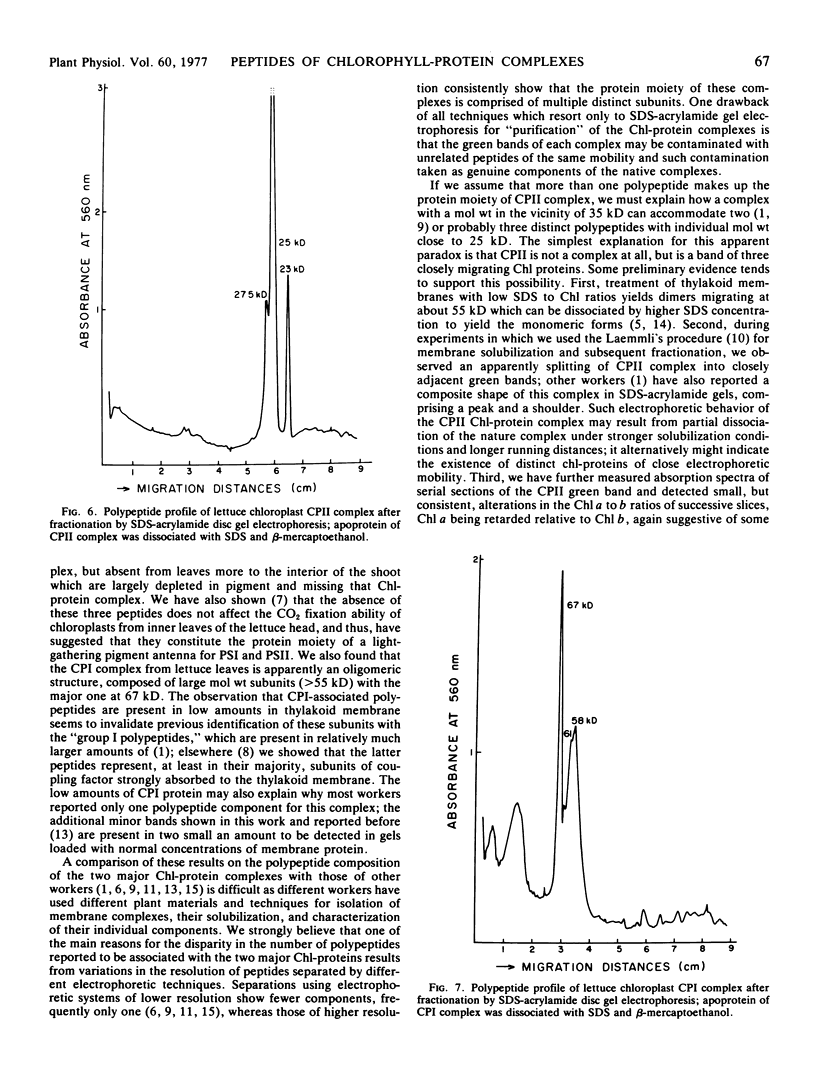
The protein moiety of the two major chlorophyll-protein complexes associated with chloroplast membranes of outer, dark green leaves of a romaine lettuce shoot (Lactuca sativa L. var. Romana) has been analyzed by discontinuous sodium dodecyl sulfate-polyacrylamide disc gel electrophoresis. Complex II, also termed light-harvesting chlorophyll-protein complex, is shown to consist of a major polypeptide of 25 kilodaltons (kD) and two minor ones of 27.5 and 23 kD. The 25 kD subunit is the single largest polypeptide component of the chloroplast membranes, accounting for about 25% of their total protein. Complex I contains only high molecular weight subunits, the major one being at 67 kD, these subunits representing only a small percentage of the chloroplast membrane total protein.
These data, suggesting an oligomeric nature for the apoprotein of these two chlorophyll-protein complexes, are difficult to reconcile with the estimated molecular weights of the native complexes and raise some intriguing questions as to the types of interactions among the components of these major lipoproteins of the photosynthetic membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Energy transfer between photosystem II and photosystem I in chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):72–85. doi: 10.1016/0005-2728(75)90190-5. [DOI] [PubMed] [Google Scholar]
- Fessenden-Raden J. M. Effect of fatty acids on the movement and staining of membrane proteins in polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1347–1353. doi: 10.1016/s0006-291x(72)80123-2. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Development of the photosynthetic unit in lettuce. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4560–4564. doi: 10.1073/pnas.73.12.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Identification of chloroplast membrane peptides with subunits of coupling factor and ribulose-1,5 diphosphate carboxylase. Arch Biochem Biophys. 1976 Oct;176(2):472–478. doi: 10.1016/0003-9861(76)90190-9. [DOI] [PubMed] [Google Scholar]
- Kan K. S., Thornber J. P. The Light-harvesting Chlorophyll a/b-Protein Complex of Chlamydomonas reinhardii. Plant Physiol. 1976 Jan;57(1):47–52. doi: 10.1104/pp.57.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Machold O. On the molecular nature of chloroplast thylakoid membranes. Biochim Biophys Acta. 1975 Apr 8;382(4):494–505. doi: 10.1016/0005-2736(75)90217-5. [DOI] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


