Abstract
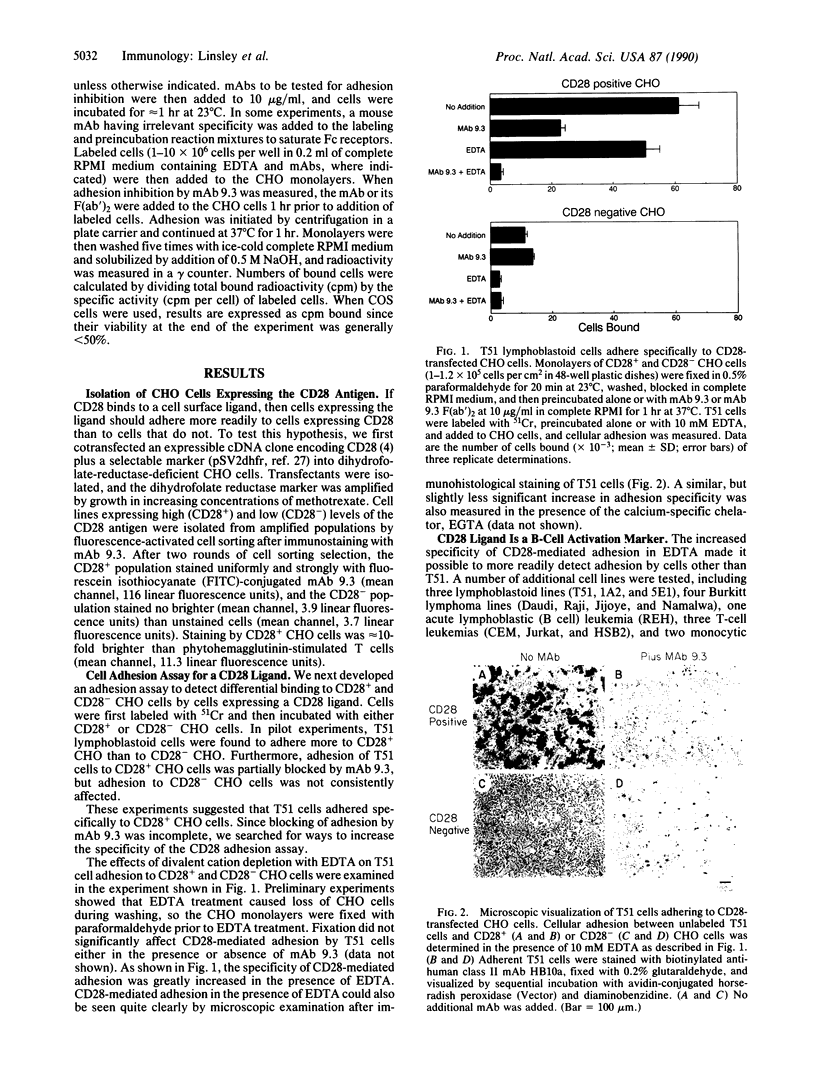
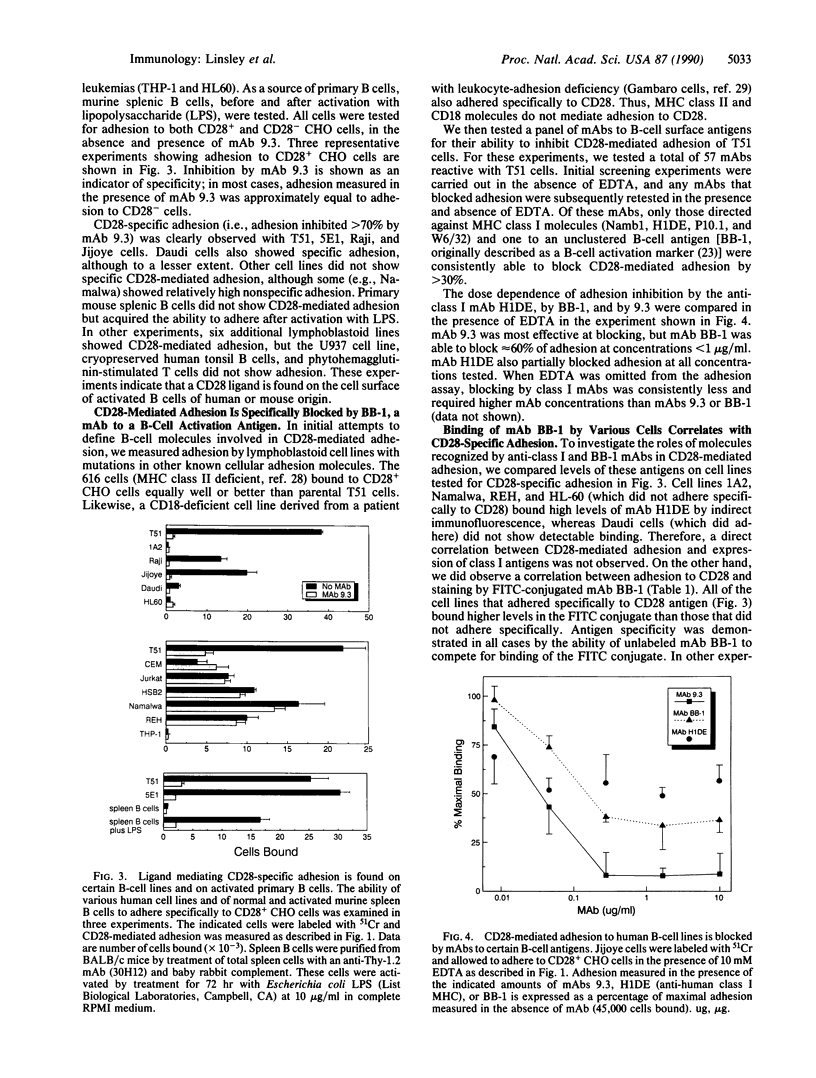
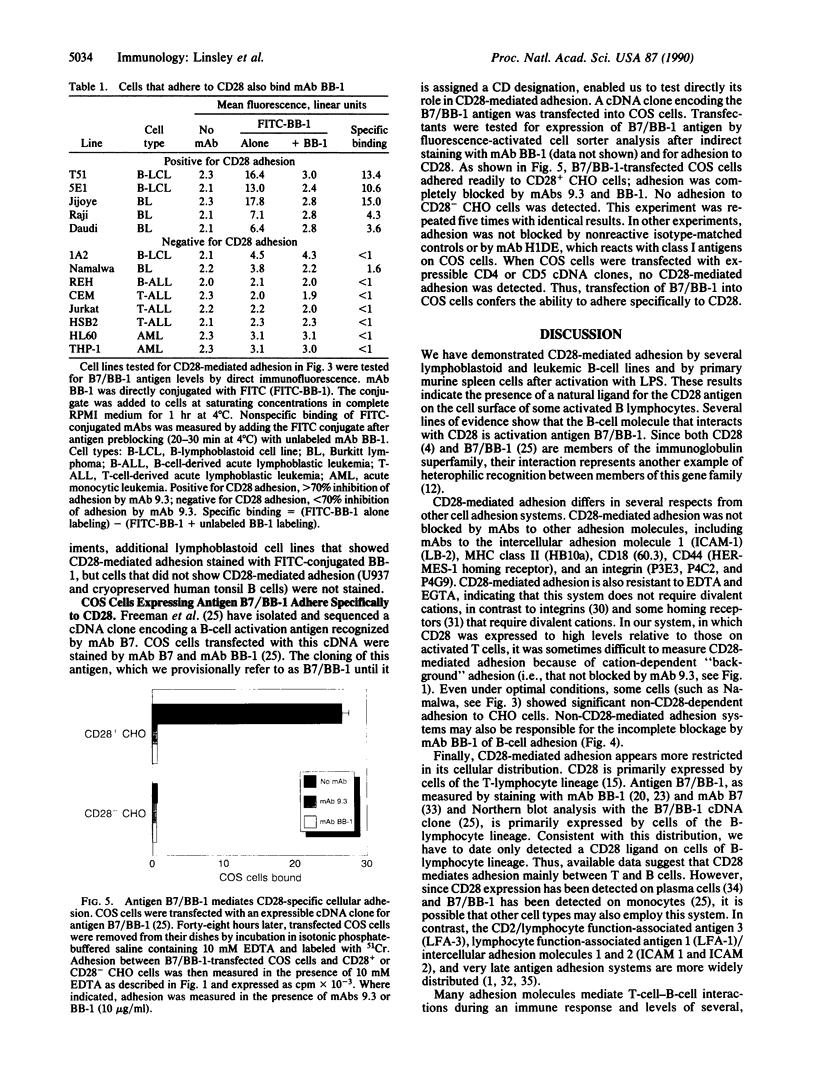
Studies using monoclonal antibodies (mAbs) have implicated the homodimeric glycoprotein CD28 as an important regulator of human T-cell activation, in part by posttranscriptional control of cytokine mRNA levels. Although the CD28 antigen has functional and structural characteristics of a receptor, a natural ligand for this molecule has not been identified. Here we show that the CD28 antigen, expressed in Chinese hamster ovary (CHO) cells, mediated specific intercellular adhesion with human lymphoblastoid and leukemic B-cell lines and with activated primary murine B cells. CD28-mediated adhesion was not dependent upon divalent cations. Several mAbs were identified that inhibited CD28-mediated adhesion, including mAb BB-1 against the B-cell activation antigen B7/BB-1 and some mAbs against major histocompatibility complex class I antigens. B7/BB-1 expression correlated closely with CD28-mediated adhesion, but class I expression did not. Transfected COS cells expressing the B7/BB-1 antigen adhered to CD28+ CHO cells; this adhesion was blocked by mAbs to CD28 and B7/BB-1. The specific recognition by CD28 of the B-cell activation antigen B7/BB-1 represents a heterophilic interaction between members of the immunoglobulin superfamily that may serve to regulate T-cell cytokine levels at sites of B-cell activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty P. G., Ochs H. D., Harlan J. M., Price T. H., Rosen H., Taylor R. F., Hansen J. A., Klebanoff S. J. Absence of monoclonal-antibody-defined protein complex in boy with abnormal leucocyte function. Lancet. 1984 Mar 10;1(8376):535–537. doi: 10.1016/s0140-6736(84)90933-4. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A., Holly R. C., Dinndorf P. A., Shu G. Polypeptides on human B lymphocytes associated with cell activation. Hum Immunol. 1986 May;16(1):100–113. doi: 10.1016/0198-8859(86)90039-x. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Hansen J. A., Good R. A., Gupta S. Monoclonal antibody analysis of human T lymphocyte subpopulations exhibiting autologous mixed lymphocyte reaction. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5096–5098. doi: 10.1073/pnas.78.8.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Mohagheghpour N., Hansen J. A., Engleman E. G. Alloantigen-specific cytotoxic and suppressor T lymphocytes are derived from phenotypically distinct precursors. J Immunol. 1983 Nov;131(5):2296–2300. [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Segil J. M., Lee G., Whitman J. F., Nadler L. M. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989 Oct 15;143(8):2714–2722. [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland L. K., Norris N. A., Grosmaire L. S., Ferrone S., Gladstone P., Ledbetter J. A. Signal transduction in lymphocyte activation through crosslinking of HLA class I molecules. Hum Immunol. 1989 Aug;25(4):269–289. doi: 10.1016/0198-8859(89)90089-x. [DOI] [PubMed] [Google Scholar]
- Gladstone, Pious D. Stable variants affecting B cell alloantigens in human lymphoid cells. Nature. 1978 Feb 2;271(5644):459–461. doi: 10.1038/271459a0. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz C. M., Unanue E. R. Regulation of antigen-presentation-I. IFN-gamma induces antigen-presenting properties on B cells. J Immunol. 1988 Dec 15;141(12):4083–4088. [PubMed] [Google Scholar]
- Hemler M. E. Adhesive protein receptors on hematopoietic cells. Immunol Today. 1988 Apr;9(4):109–113. doi: 10.1016/0167-5699(88)91280-7. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Kakiuchi T., Chesnut R. W., Grey H. M. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983 Jul;131(1):109–114. [PubMed] [Google Scholar]
- Kishimoto T. K., Larson R. S., Corbi A. L., Dustin M. L., Staunton D. E., Springer T. A. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Moretta A., Messner H. A., Moretta L., Croce C. M. Tp44 molecules involved in antigen-independent T cell activation are expressed on human plasma cells. J Immunol. 1987 Jun 15;138(12):4128–4132. [PubMed] [Google Scholar]
- Krieger J. I., Grammer S. F., Grey H. M., Chesnut R. W. Antigen presentation by splenic B cells: resting B cells are ineffective, whereas activated B cells are effective accessory cells for T cell responses. J Immunol. 1985 Nov;135(5):2937–2945. [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human b-cell differentiation. I. Analysis of immunoglobulin heavy chain switching using monoclonal anti-immunoglobulin M, G, and A antibodies and pokeweed mitogen-induced plasma cell differentiation. J Exp Med. 1982 Mar 1;155(3):839–851. doi: 10.1084/jem.155.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesslauer W., Koning F., Ottenhoff T., Giphart M., Goulmy E., van Rood J. J. T90/44 (9.3 antigen). A cell surface molecule with a function in human T cell activation. Eur J Immunol. 1986 Oct;16(10):1289–1296. doi: 10.1002/eji.1830161017. [DOI] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- McKenzie D. Alloantigen presentation by B cells. Requirement for IL-1 and IL-6. J Immunol. 1988 Nov 1;141(9):2907–2911. [PubMed] [Google Scholar]
- Norment A. M., Salter R. D., Parham P., Engelhard V. H., Littman D. R. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988 Nov 3;336(6194):79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Shimizu Y. Two molecular pathways of human T cell adhesion: establishment of receptor-ligand relationship. Curr Opin Immunol. 1988 Sep-Oct;1(1):92–97. doi: 10.1016/0952-7915(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L. A., Ledbetter J. A., Lee K., June C. H., Thompson C. B. CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J Immunol. 1990 Mar 1;144(5):1646–1653. [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]






