Abstract
Despite the diverse physiological activities of androgens and glucocorticoids, the corresponding receptors are very close members of the nuclear-receptor super family. Their action mechanisms show striking similarities, since both receptors recognize very similar DNA-response elements and recruit the same coactivators to their target genes. The specificity of the responses lies mainly in the tissue-specific expression of the receptors and in their ligand specificity. In cells, where both receptors are expressed, the mechanisms leading to the difference in target genes are less obvious. They lie in part in subtle variations of the DNA-binding sites, in cooperativity with other transcription factors and in differential allosteric signals from the DNA and ligand to other receptor domains. We will highlight the different suggestions that might explain the DNA sequence selectivity and will compare the possible allosteric routes between the response elements and the different functions in the transactivation process. The interplay of androgen and glucocorticoid receptors is also highly relevant in clinical settings, where both receptors are therapeutically targeted. We will discuss the possibility that the glucocorticoid and androgen receptors can play partially redundant roles in castration-resistant prostate cancer.
Keywords: Androgen receptor, Glucocorticoid receptor, DNA binding, DNA-response element, Prostate Cancer
Introduction
The physiological roles of androgens and glucocorticoids are very different, and this is reflected in their clinical applications. Androgens are the male sex steroids mainly involved in development and maintenance of reproductive organs and spermatogenesis. Clinically, they might provide benefit as hormone replacement therapy for hypogonadal men or in cases of severe cachexia or osteoporosis [1, 2]. On the other hand, in the therapy of metastatic castration-resistant prostate cancer, androgen action is blocked by androgen deprivation, androgen synthesis inhibitors, and/or the use of androgen receptor (AR) antagonists [3]. Glucocorticoids, however, are mainly controlling inflammation and metabolism which has led to their wide-spread use to treat inflammatory and immunologic disorders [4].
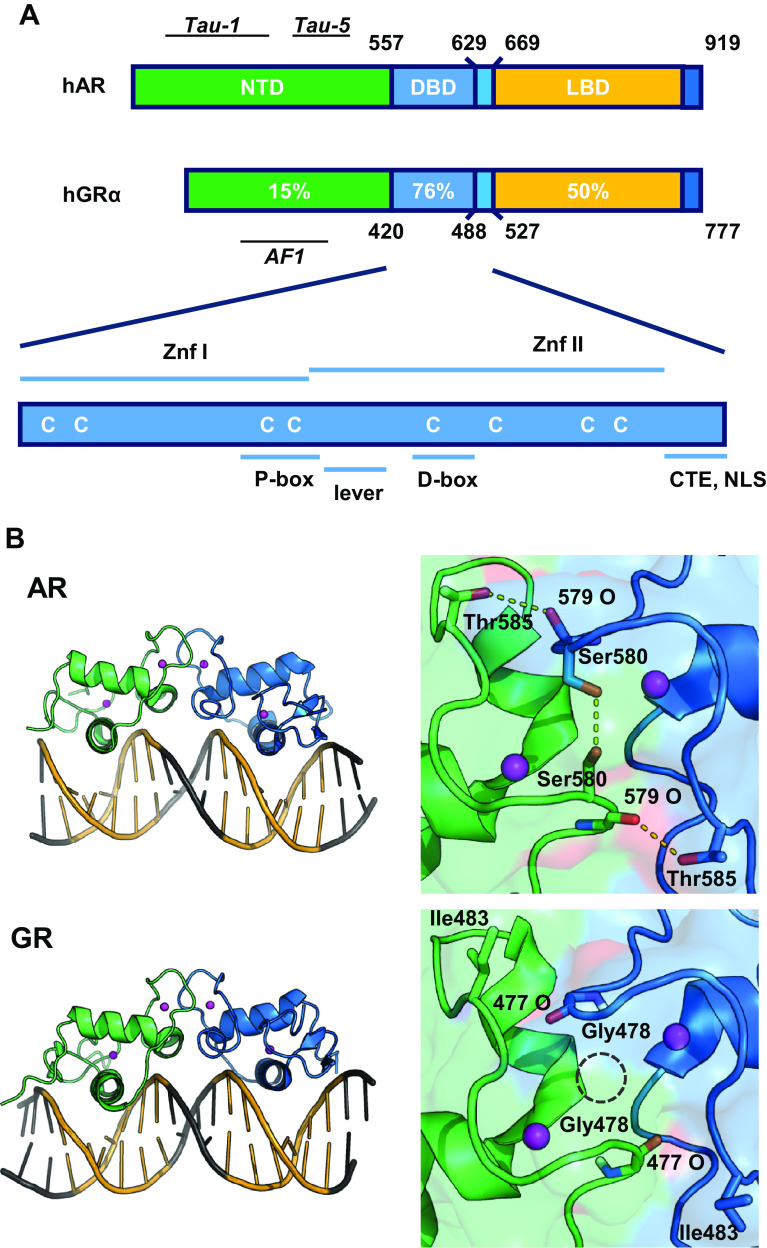
Androgen and glucocorticoid effects are mediated by their corresponding receptors, the AR and the glucocorticoid receptor (GR), which are both nuclear receptors. Nuclear receptors are ligand-inducible transcription factors; they have a centrally located DNA-binding domain (DBD) which is connected via a hinge region to a carboxyterminal ligand-binding domain (LBD) and an amino-terminal activation function (NTD) (Fig. 1a). The DBD exists of two zinc-coordinating modules and forms the signature domain of the nuclear-receptor family. Because of their essential roles in target gene selection, both DBD and hinge region as well as the response elements will be discussed in detail.
Fig. 1.
a Comparison of the domain structure of the androgen and glucocorticoid receptor (AR and GR), with indication of the different domains and level of conservation. NTD amino-terminal domain, DBD DNA-binding domain, LBD ligand-binding domain; Znf Zinc finger, CTE carboxyterminal extension, NLS nuclear localization signal. b Structure of the DNA-binding domains of the AR and GR bound to DNA (left panels). At the right, details of the D-boxes with dotted lines indicating AR-specific H-bonds and the GR specific glycine hole
(adapted from [12])
The LBDs of all nuclear receptors are folded in a typical 3-layered, 12-helical structure with a ligand-binding cavity [5]. While the overall structure of the LBD is conserved, receptor-specific residues delineating the ligand-binding cavity determine the ligand specificity of the receptors. The binding of the cognate ligand is proposed to induce a repositioning of the carboxyterminal helix (H12) of the LBD, thus forming an activation function (called AF2) which is a docking site for α-helical LxxLL motifs that are found in most coactivator complexes [6].
The NTD of the nuclear receptors is very diverse both in length and sequence. In addition, between the NTDs of AR and GR, there is little or no conservation, although both have transcription activating properties (called AF1). A comparison between the NTD of AR and GR has been extensively reviewed elsewhere [7–9].
Here, we will compare how the AR and GR interact with DNA and chromatin; how they evoke tissue-specific transcriptional responses and in how far they have interchangeable functions, for instance, in prostate cancer [10, 11].
Comparing the DNA-binding domains of AR and GR
AR and GR are steroid receptors which form a specific subfamily of the nuclear receptors. Steroid receptors can be divided based on their sequence specificity into two groups: the estrogen receptors (ER) and ER-like receptors on one hand and the glucocorticoid, mineralocorticoid (MR), progesterone (PR), and androgen receptors on the other hand. The latter are also called the oxosteroid receptors.
The structures of the DNA-binding domains of nuclear receptors were solved by X-ray crystallography [12, 13] and later refined by NMR [14]. The DNA-binding domains of all steroid receptors are very similar (Fig. 1a). Part of the first zinc finger (P-box) folds into an α-helix which fits in the major groove of the DNA thus making sequence-specific contacts with 5′-AGAACA-3′-like or 5′-GGTACA-3′-like motifs [15]. Part of the second zinc finger (D-box) is involved in receptor dimerization. Because of this specific D-box dimerization, the DNA-recognition helices of the two DBDs are positioned at a very specific distance relative to one another. This structural characteristic determines that all steroid receptors recognize bipartite DNA elements with exactly three nucleotide spacers, called inverted repeat with 3-nucleotide spacer (IR3). There is an important, strong cooperativity between the two monomers when they bind to DNA [15].
A detailed comparison of the DBDs from AR and GR reveals many structural similarities, but also some remarkable differences. The amino acids in the DNA-binding helix, which make the sequence-specific contacts with the bases, are identical in GR and AR, but small nuances in the three-dimensional structure of the α-helix indicate a slightly stronger affinity of the AR-DBD for its 5′-AGAACA-3′ motif [16]. For the AR-DBD, there is only one crystal structure available (Fig. 1b), but for the GR-DBD, a series of NMR structures with different GREs and receptor isoforms were reported [17, 18]. This revealed a bidirectional allosteric signaling role for the so-called ‘lever arm’, transmitting signals from the DNA reading head and the DBD dimerization surface to other functional domains of the receptor. As a consequence, GREs that differ in one base only differentially affect GR transactivating properties [17]. It is interesting to note that the sequence of the lever arm is different between GR and AR which implies different receptor-specific allosteric pathways.
The amino acids of the second zinc finger which constitute the dimerization surface of the DBDs are also conserved between the oxosteroid receptors (Fig. 2b). Nevertheless, there are two AR-specific residues, Ser598 (Gly in GR, PR, and MR) and Thr603 (Ile in GR, PR, and MR) that contribute to the stronger DNA-dependent dimerization for AR. The Ser598 of AR not only increases the van der Waals forces, but also adds two hydrogen bonds to the interface. In case of a glycine at this position, a molecular cavity is apparent between the two DBDs (Fig. 1b). Moreover, Thr603 in the AR-DBD can form additional hydrogen bonds which cannot be formed by the corresponding isoleucine in the GR, PR, or MR (Fig. 1b) [12, 16].
Fig. 2.
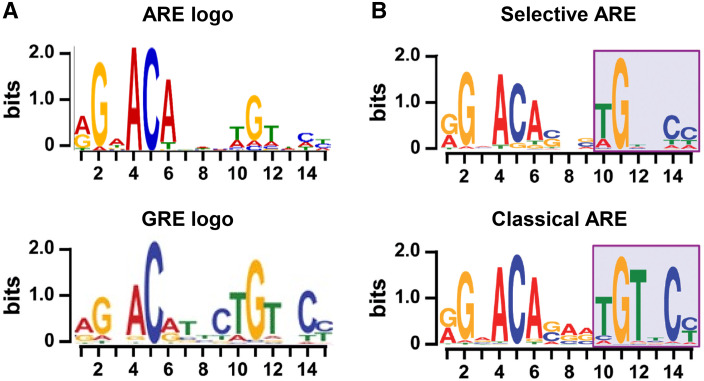
a Logo presentation of top-enriched ARE motifs provided by Cheung based on [99] (top) and top-enriched GRE motifs from [35] (bottom). b Logo presentation of top-enriched ARE motifs specific for the SPARKI model, considered to be selAREs (top) or for classical AREs (bottom).
Adapted from [48]
In conclusion, although the two zinc fingers of the AR and GR-DBDs differ only in 12 out of 65 residues and even when most of the substitutions are conservative, small variations in structure seem to have functional consequences. These subtle differences in the way steroid receptors bind DNA correlated with slightly different consensus binding sites and overlapping but distinct sequence specificities between oxosteroid receptors, which will be discussed further [19, 20]. Surprisingly, the differences in P-box sequence between AR and ER, which control a different set of target genes in vivo, are insufficient to impose gene selectivity in vitro. Indeed, the P-box of the ER-DBD makes sequence-specific contacts with the 5′-AGGTCA-3′ hexamer half sites of the estrogen response elements (ERE), while the oxosteroid P-box recognizes their cognate 5′-AGAACA-3′ hexamer. However, despite these differences, the AR is able to bind EREs and this has been proposed to be a mechanisms by which it interferes with ER functioning in breast cancer [21]. Observations like these clearly demonstrate that the DNA binding as such cannot explain the specificity of the physiological steroid responses. What the role of the other receptor domains could be during the selection of the target enhancers is being discussed in the following sections.
Comparing androgen and glucocorticoid response elements
The lack of receptor specificity of the first identified response elements for glucocorticoids, progestagens, mineralocorticoids, and androgens has been confusing. Indeed, in binding and transactivation assays, all glucocorticoid response elements (GRE) are recognized by AR, MR, and PR and vice versa; many androgen response elements (AREs) are recognized by the other oxosteroid receptors [22–24]. In addition, in more recent chromatin immunoprecipitation assays (ChIP-seq), the in silico derived consensus sequences for AR and GR binding motifs were quite similar (Fig. 2a). How can receptors with such diverse physiological roles act through DNA elements with so similar sequences? A large part of the answer must lie in the cell-specific chromatin environment [25]. For GR, for example, only a 5% overlap was seen between its binding sites in mammary versus pituitary cell types indicating a crucial role for epigenetic chromatin factors in intracellular receptor-DNA binding [26]. Cell-specific transcription factor occupancy of enhancers and promotors can also contribute to receptor selectivity. An example for this is the pioneering role of FoxA1 which specifies AR DNA binding in LNCaP cells [27]. However, what happens when cells express both AR and GR and when both ligands are present? In LNCaP cells that express both AR (endogenously) and GR (exogenously), or VCaP cells that express both receptors (endogenously), there is a large overlap between AR and GR binding sites indicating that the receptors might bind the same DNA elements [27, 28]. These common binding sites are located in active enhancers near genes that are responsive to both hormones. Because the activity of some of these genes is correlated with oncogenic processes, this supports the hypothesis that re-expression of GR in castration-resistant prostate cancer could explain the progression into an AR-independent form of the disease [10, 11].
AR and GR both bind to the AGAACA consensus sequence organized as an inverted repeat with a 3n spacer
The first systematic discovery of genomic AR binding sites was based on ChIP-on-chip and ChIP-seq experiments on AR-positive prostate cancer cell lines. While the earlier, limited ‘old school’ biochemical analyses indicated that AR binds DNA motifs that are organized as 5′-AGAACA-3′-like repeats, initial in silico analyses of the ARBS lead to the idea that the AR might also bind monomeric motifs or dimeric motifs with variable spacing and orientations [29–31]. However, later mutational analyses of such putative alternative AREs strongly indicated that the AREs are always dimeric in nature with an exact 3 nucleotide spacer [24, 32]. A study on DNA specificity of human transcription factors that used high-throughput SELEX for determining binding sites also pointed out the dimeric nature of the binding motif of the AR with 5′ GTACA 3′ as the consensus half site [33]. Chen et al. described in another study that the sequence specificity of the AR depended on the ligand. In LNCaP cells, agonist bound AR binds the classical inverted repeat-like elements, while in the genomic binding sites for antagonist-bound AR, elements which resemble a 5′-CnnG-3′ repeat with a 5 nucleotide spacer are enriched [34].
While the data for monomeric binding are less convincing for AR, monomeric binding for the GR has been reported to some of its enhancers [35]. Moreover, ChIP-exo data, which give a more detailed indication of the exact borders of the receptor-binding motifs, revealed mainly dimeric binding sites for GR and a redistribution to monomeric sites when the D-box of the GR is mutated [36, 37].
It should be noted that in this review, we do not discuss the possibility of indirect DNA binding, which has been well documented, certainly in case of the GR. Indeed, such GR tethering to DNA via other transcription factors could involve monomeric receptors and will result in receptor-specific effects on gene activation and/or repression [38–40]. In how far such monomeric GR might play a role in castration-resistant prostate cancer has not been resolved yet.
Despite the high similarity between AREs and GREs (Fig. 2a), we and others identified differential receptor recognition that could offer an alternative explanation for receptor specificity. A subset of AREs turns out not to be recognized by GR. When cloned upstream of a reporter gene, they confer responsiveness to androgens and progestins but not to mineralocorticoids or glucocorticoids [12, 41]. In vitro DNA-binding assays showed that AR and PR, but not MR or GR, bind these selective AREs (selARE) with high affinity. Moreover, the isolated GR-DBD binds these selAREs as monomers or as non-cooperative dimers [42, 43]. So what makes an ARE selective for AR?
Discovery of selective AREs and the proposed differential AR binding mode
A comparison of the sequences of the first selAREs with that of the first known classical AREs led us to propose that the selAREs could be organized as direct repeats, rather than inverted repeats of 5′-AGAACA-3′-like hexamers [22, 24]. This was further corroborated by the observation that any synthetic direct repeat was able to confer androgen but not glucocorticoid responsiveness to reporter genes. Moreover, when mutations reduce the direct repeat-like nature of selective AREs, they gained responsiveness to glucocorticoids [19]. Does this mean that selAREs are bound by AR dimers in a head-to-tail conformation, much like many of the non-steroid receptors? [44]. This possibility was suggested by the fact that swapping of the dimerization interface between the DBDs of AR and GR also swapped the selectivity: an AR-DBD with the second zinc finger module of the GR no longer binds selective AREs, but still binds classical AREs [43, 45]. Vice versa, a GR-DBD with the second zinc finger of the AR gains affinity for selective AREs. However, the crystal structure of the AR-DBD on a direct repeat element shows a clear inverted protein dimer (head-to-head conformation) [12]. Therefore, unlike what has been reported for several other nuclear receptors, direct repeat binding does not mean head-to-tail dimerization in case of the AR [46].
To answer the question how common selective AREs are and whether this type of selectivity has any in vivo relevance, we developed the SPARKI mouse model. In this model, the exon encoding the second zinc finger of the AR was replaced for the corresponding exon of the GR gene. The resulting mutant AR can still bind to classical AREs, but no longer binds to or activates via selAREs; hence the name specificity affecting AR knockin (SPARKI) [45]. The SPARKI male mice develop a phenotype resembling partial androgen insensitivity [47]. Loss of binding to selAREs was first demonstrated by the loss of responsiveness in testes and epididymis of a subset of the androgen-regulated genes that subsequently were shown to have selAREs in their enhancers/promoters [45, 47]. More recent AR ChIP-seq on prostate and epididymis of SPARKI versus wild (WT) type mice also revealed distinctive patterns of chromatin binding by the WT-AR versus the SPARKI-AR [48]. While in wild-type epididymal tissue, 10 009 ARBS were picked up, only 6446 binding events were detected in the SPARKI tissue. This loss of AR binding events again correlated well with loss of androgen responsiveness of nearby genes as demonstrated in transcriptome analysis. Moreover, most of the SPARKI ARBS were also bound by AR in wild-type tissue and correlated with the vicinity of androgen-responsive genes, indicating that the SPARKI mutation led to the loss of a specific function of the AR and not in the complete inactivation of AR DNA binding.
The evaluation of the AR binding sequences in SPARKI and WT tissues allowed for the construction of a consensus sequence for selAREs (AR binding lost in SPARKI) and for classical AREs (AR binding retained in SPARKI and present in WT) (Fig. 2b). These two consensus sequences were similar but distinct [48]. The classical ARE consensus is virtually identical to the GRE consensus taken from Schiller [35]. The consensus of the selective elements is also very similar, except for a loss of thymine conservation at position 12 (Fig. 2b). Clearly, these similarities left little room for the hypothesis that selective AREs have a direct repeat nature.
Therefore, how can we explain the variation in sequence preference between AR and GR? For both receptors, it was postulated that the affinity for the upstream, more conserved 5′-AGAACA-3′ hexamer is high and binding to this hexamer results in a DNA-dependent dimerization with a second monomer that will bind hexamers that can diverge more from 5′-AGAACA-3′ [14–16]. It is tempting to speculate that the dimerization interface in the second zinc finger, which is stronger for the AR (Fig. 1b), allows more divergent downstream hexamers. This hypothesis is not confirmed by mutational analyses in which the Serine and Threonine in AR were swapped for the GR residues and vice versa, and the Glycine and Isoleucine in the GR were swapped for the AR residues in the GR [49].
Unfortunately, in the only crystal structure of the AR-DBD bound to DNA that has been published until now [12], the motif is organized as a direct repeat (based on the earlier report) with an adenine at position 12. Therefore, the final explanation for the lower stringency of the AR versus the GR or for the enrichment of the thymine at this position remains to be elucidated. Possibly, the difference in sequence specificity could be explained by alternative DNA or chromatin interactions contributed by the CTE or by receptor-specific allosteric interactions with other receptor domains or coactivators. This will be discussed in the following.
Hinge region
Despite their name, hinge regions are more than the flexible connection between the DBDs and the LBDs. For the AR-, PR-, GR-, and ER-DBDs, it is clear that residues immediately carboxyterminal of the second zinc finger are involved in DNA binding (Fig. 1a) [50, 51]. Indeed, the addition of the CTEs to the DBD increased the binding affinity for the DNA-response elements [43]. While sequence specificity of the full size receptors is not affected by mutations or deletions in the CTE, clearly, the affinity for DNA was [52, 53]. For other nuclear receptors, the interactions of similar carboxyterminal extensions with the DNA immediately adjacent to the hexamers have been well documented [54]. For AR and GR, however, the possible contacts with the DNA still need to be elucidated because of the unstructured nature of the CTE in the thus far known crystals. Moreover, for both AR and GR, the CTE coincides with a nuclear localization signal known to interact with importin-α [55]. This is not the only function of the CTE, since the AR (629)RKLKK(633) motif was shown to be involved in intranuclear mobility, control of the N/C interactions (see in the following), and even the transactivation properties of the AR [52, 53]. Of course, these different functions are executed at different timepoints and at different locations in the cell involving different interaction partners. As an additional layer of complexity, these functions seem to be differentially affected by posttranslational modifications, such as the acetylation of the Lysines of the (629)RKLKK(633) motif [56–58].
Allosteric signaling between the DNA elements and the other receptor domains
Like for most proteins, allostery plays an integrative role in the functioning of nuclear receptors. For the ligand-binding domains of NR, there are clear allosteric pathways identified between the ligand-binding pockets and the activation functions, where coregulators interact [59]. Many data also indicate allosteric communications between the DNA-binding domain and the DNA elements (reviewed in [60]). While intra-domain allostery within the DBD and LBD has been examined in great detail, the allostery between different domains is much harder to study. The best studied is the allostery from the GR-DBD, where the so-called lever arm situated between the two zinc finger modules plays a clear role in the transmission of sequence-specific signals from the DNA to coactivator recruitment by the LBD [17, 61]. This is further illustrated by the effect of a splice variant of the GR which has a single amino acid added to the lever arm and which dramatically affects the differential coregulator recruitment to the GR [18].
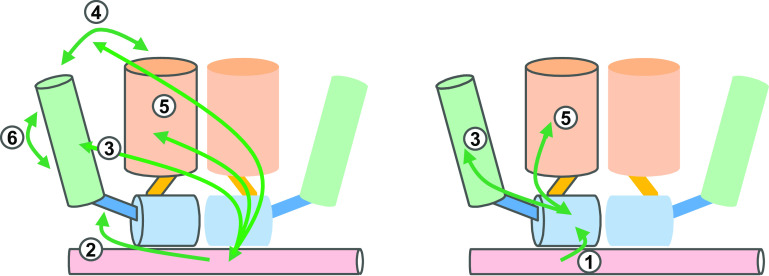
Physiological evidence for allosteric effects in the AR comes from the functional analysis of AR-NTD mutations, and detected in androgen insensitivity patients that affect the functioning of AR in an ARE-specific way [62]. In addition, the deletion of the (23)FQNLF(27) motif which is involved in NTD-LBD communications in the AR has an effect on transactivation via classical AREs, but has little effect on reporters based on selAREs [63]. Moreover, the deletion of the polyglutamine tract or mutations in the sumoylation sites in the NTD have an effect on AR transactivation via classical elements, but not on selAREs [64]. Clearly, we need more detailed three-dimensional structures of the full-length androgen or glucocorticoid receptors, such as the ones described for other NRs [46, 65, 66]. Possible allosteric pathways between different domains for which there is some experimental, albeit sometimes circumstantial evidence are given in Fig. 3.
Fig. 3.
Schematic presentation of the allosteric signaling pathways between different domains of the AR or GR. Color code: light red DNA; light blue DBD; blue hinge; green NTD; orange LBD. 1 Signaling can take place between the DNA to the DNA-binding domain [17]; 2 between the DNA to the hinge [53]; 3 from the DNA to the amino-terminal domain [52, 63]; 4 from the DNA to the N/C interactions [63]; 5 from the DNA-binding domain to the ligand-binding domain [100]; and 6 between Tau-1 and Tau-5 [101]. For reasons of simplification, only allosteric pathways within one monomer are shown
Role of lncRNA in receptor functioning
The recent discovery of many thousands long noncoding RNA-encoding genes in the human genome will affect the study of virtually all biological processes [67]. These lncRNAs bind to protein-like transcription factors and coactivators and have been proposed to act as complex-building scaffolds. How many of them interact with nuclear receptors in general and steroid receptors more specifically, for example, remains to be determined. However, even before the term was coined, lncRNAs were known to interact functionally with the AR-NTD. This was first demonstrated for the steroid receptor RNA activator (SRA) by the group of O’Malley [68]. The structure–function analyses of SRA as well as the interacting proteins are still being identified [69, 70]. More recently, other lncRNAs, such as PRNCR1 and PCGEM1, have been proposed to affect AR functioning via its amino-terminal domain [71].
Another action mechanism was reported for ‘growth arrest-specific transcript’ called gas5. GR was shown to bind to a motif in this lncRNA, and this was proposed to squelch the receptor away from the genome [72]. The receptor-binding RNA motif in gas5 is a double-stranded fragment which resembles a 5′-AGAACA-3′-like monomeric DNA motif, hence called GRE mimic. In vitro, this RNA motif can titrate MR, PR, and AR activity in cellular overexpression experiments [73]. It will be exciting to learn how gas5 is implicated in the action mechanisms of these steroid receptors in normal physiology and in disease mechanisms. Because of the possible role of the NTDs, it is likely that here too, receptor specific mechanisms will exist.
In conclusion, over the coming years, we can expect a dramatic increase in our insights on how lncRNAs affect NR biology in many different physiological processes and disease mechanisms.
Cooperativity with other transcription factors
The first AREs in eukaryotic genes were described for the androgen-dependent, prostate-specific C3 component of the prostatic binding protein [74, 75], the prostate-specific antigen [76], the probasin [77], and sex-limited protein genes [78]. For all these, it was immediately apparent that the AREs were part of complex enhancers to which several other transcription factors, such as NF1, Oct1, SP1, and GATA, need to bind to result in their full activity [78–82]. Indeed, mutation analyses of the individual binding sites within these complex enhancers illustrated the cooperative nature of the binding of transcription factors. Clearly, the AR is the ligand-induced factor which turns on the enhancers, but they themselves also depend on the binding of other enabling transcription factors to induce transcription of a nearby gene. ChIP-seq data on series of transcription factors in cell lines and more recently also on tissue confirmed that: (1) steroid receptors involve other transcription factors to activate their target genes [25, 29]; (2) transcription factors recruit histone mark writing, reading, and erasing coregulators; and (3) the co-regulating transcription factors are involved in the tissue specificity of the responses [48, 83]. The details on how different transcription factors cooperate during the transactivation via enhancers and chromatin histone modifications are being elucidated further. FoxA1 seems to act upstream of GATA2 as a pioneer factor for the AR [84]. However, the same transcription factor can have different roles depending on the enhancers under study (reviewed for AR in prostate cancer in [25, 85]).
Despite near identity of the DBDs, there are large differences between AR and GR in mobility reflecting kinetics of chromatin binding as measured by fluorescence recovery after photobleaching. These differences of residence at the MMTV enhancer array are compound effects of DNA binding as well as differential effects of other binding transcription factors, histon modifiers, and chromatin remodeling complexes [86, 87].
Should we target the GR and AR simultaneously in castration-resistant prostate cancer
While AR can be targeted in castration-resistant prostate cancer with ADT or anti-androgens, glucocorticoids can be used to relieve pain or suppress androgens. A first clinical observation relevant for this discussion is that AR-targeting therapies can lead to the appearance of mutations that change ligand specificity of the AR and allows glucocorticoids to act as agonists for the AR [88].
In addition, the high similarity between the DBDs of AR and GR as well as their response elements led to the hypothesis that the receptors could be interchangeable [89]. In normal prostate epithelial cells, AR but not GR is expressed, so this is not relevant. During ADT treatment, however, GR can be upregulated and thus be co-expressed with the AR [11]. It was postulated, therefore, that in ADT or under anti-androgen therapy, the GR might take over the role of (inhibited) AR and thus lead to castration resistance or resistance against the latest AR-targeting therapies [10]. Clinically, this is an important question, since inhibitors of androgen synthesis, which are used to treat castration-resistant prostate cancer, are usually supplemented with glucocorticoids or glucocorticoid precursors to prevent side effects [90].
In prostate cancer cell lines, many genes that are androgen responsive can also respond to glucocorticoids, provided that the GR is expressed [41]. In such cells, the responses to androgens and glucocorticoids largely overlap, and overexpression of GR is correlated with a loss of anti-androgen control of proliferation [10]. Jaaskelainen et al. and Isikbay et al. showed that GR and AR both can activate anti-apoptotic genes, so when AR is inactivated by anti-androgens, the GR can still upregulate these genes [11, 91]. However, this seems in discordance with clinical practice, where glucocorticoids have been used in PCa treatment for many years. Their suppressive effects on serum androgen levels, their clinical benefit on PSA levels, and negative effects on tumor volume have been well established [92, 93]. Moreover, their inhibitory role on prostate cancer cell proliferation as well as angiogenesis has been documented in preclinical models [94, 95]. Therefore, while the possibility of GR taking over the survival role of the AR has been shown in preclinical work, at the moment, there is little evidence that this would be a major problem in the clinic. Nevertheless, there is need for well-controlled clinical studies to verify whether glucocorticoids could have adverse effects, maybe on a specific subtype of castration-resistant prostate cancer, which express GR. In the meantime, studying the role of AR and GR and their functional interactions in prostate cancer remains of major importance when developing and implementing new anti-androgen therapies [96].
Future prospects
Over the last years, major steps forward have been taken in our knowledge of the transcription activation process. Surprisingly, we are still far from understanding the tissue-specific actions of AR and GR, two very similar steroid receptors with very divergent biological roles. Indeed, although general roadmaps of the activation are well described, now, we need to search for the details that explain the different rules of engagement of these two receptors.
The description of structures of full size receptors or larger receptor fragments bound to DNA, ligand, and coregulator peptides continues to reveal more details on the allosteric pathways within the receptors [44, 97]. The maturing of a number of technologies, such as ChIP-exo to better define the DNA-binding events [36], RIME to identify protein–protein interactions at the level of chromatin [98], and CRISPR/Cas9 to mutate enhancers or to reactivate genes expressing specific transcription factors or coregulators, now allows for the testing of the hypotheses on the allosteric signals of specific DNA elements on receptor functioning.
Acknowledgements
The work was supported by the KU Leuven (GOA/15/017), the Research Foundation Flanders (FWO: G.0858.11, G.0684.12 N and G.0830.13 N), the Belgian Federal Government (National Cancer Plan KPC_29_023), and a grant from Kom op tegen Kanker.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER, 3rd, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Borjesson AE, Ohlsson C. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–960. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134(1):54–67. doi: 10.1016/j.pharmthera.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387(6634):733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, McEwan IJ. Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocr Rev. 2012;33(2):271–299. doi: 10.1210/er.2011-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavery DN, McEwan IJ. Structural characterization of the native NH2-terminal transactivation domain of the human androgen receptor: a collapsed disordered conformation underlies structural plasticity and protein-induced folding. BioChemistry. 2008;47(11):3360–3369. doi: 10.1021/bi702221e. [DOI] [PubMed] [Google Scholar]
- 9.McEwan IJ, Lavery D, Fischer K, Watt K. Natural disordered sequences in the amino terminal domain of nuclear receptors: lessons from the androgen and glucocorticoid receptors. Nucl Recept Signal. 2007;5:e001. doi: 10.1621/nrs.05001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C, Zheng D, Sawyers CL. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, Conzen SD, Szmulewitz RZ. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5(2):72–89. doi: 10.1007/s12672-014-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci USA. 2004;101(14):4758–4763. doi: 10.1073/pnas.0401123101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, Sigler PB. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- 14.van Tilborg MA, Bonvin AM, Hard K, Davis AL, Maler B, Boelens R, Yamamoto KR, Kaptein R. Structure refinement of the glucocorticoid receptor-DNA binding domain from NMR data by relaxation matrix calculations. J Mol Biol. 1995;247(4):689–700. doi: 10.1006/jmbi.1995.0173. [DOI] [PubMed] [Google Scholar]
- 15.Zilliacus J, Wright AP, Carlstedt-Duke J, Gustafsson JA. Structural determinants of DNA-binding specificity by steroid receptors. Mol Endocrinol (Baltimore, Md) 1995;9(4):389–400. doi: 10.1210/mend.9.4.7659083. [DOI] [PubMed] [Google Scholar]
- 16.Claessens F, Gewirth DT. DNA recognition by nuclear receptors. Essays Biochem. 2004;40:59–72. doi: 10.1042/bse0400059. [DOI] [PubMed] [Google Scholar]
- 17.Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324(5925):407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas-Chollier M, Watson LC, Cooper SB, Pufall MA, Liu JS, Borzym K, Vingron M, Yamamoto KR, Meijsing SH. A naturally occurring insertion of a single amino acid rewires transcriptional regulation by glucocorticoid receptor isoforms. Proc Natl Acad Sci USA. 2013;110(44):17826–17831. doi: 10.1073/pnas.1316235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verrijdt G, Schoenmakers E, Haelens A, Peeters B, Verhoeven G, Rombauts W, Claessens F. Change of specificity mutations in androgen-selective enhancers. Evidence for a role of differential DNA binding by the androgen receptor. J Biol Chem. 2000;275(16):12298–12305. doi: 10.1074/jbc.275.16.12298. [DOI] [PubMed] [Google Scholar]
- 20.Haelens A, Verrijdt G, Callewaert L, Peeters B, Rombauts W, Claessens F. Androgen-receptor-specific DNA binding to an element in the first exon of the human secretory component gene. Biochem J. 2001;353(Pt 3):611–620. doi: 10.1042/bj3530611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69(15):6131–6140. doi: 10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- 22.Claessens F, Verrijdt G, Schoenmakers E, Haelens A, Peeters B, Verhoeven G, Rombauts W. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J Steroid Biochem Mol Biol. 2001;76(1–5):23–30. doi: 10.1016/S0960-0760(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 23.De Vos P, Claessens F, Peeters B, Rombauts W, Heyns W, Verhoeven G. Interaction of androgen and glucocorticoid receptor DNA-binding domains with their response elements. Mol Cell Endocrinol. 1993;90(2):R11–R16. doi: 10.1016/0303-7207(93)90160-L. [DOI] [PubMed] [Google Scholar]
- 24.Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F. The rules of DNA recognition by the androgen receptor. Mol Endocrinol (Baltimore, Md) 2010;24(5):898–913. doi: 10.1210/me.2009-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer. 2012;12(6):381–385. doi: 10.1038/nrc3263. [DOI] [PubMed] [Google Scholar]
- 26.John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43(3):264–268. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, Janne OA. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73(5):1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 28.Cleutjens CB, Steketee K, van Eekelen CC, van der Korput JA, Brinkmann AO, Trapman J. Both androgen receptor and glucocorticoid receptor are able to induce prostate-specific antigen expression, but differ in their growth-stimulating properties of LNCaP cells. Endocrinology. 1997;138(12):5293–5300. doi: 10.1210/endo.138.12.5564. [DOI] [PubMed] [Google Scholar]
- 29.Wang QB, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27(3):380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21(16):2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8(9):871–878. doi: 10.1038/sj.embor.7401046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinckemalie L, Spans L, Dubois V, Laurent M, Helsen C, Joniau S, Claessens F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol Endocrinol (Baltimore, Md) 2013;27(12):2028–2040. doi: 10.1210/me.2013-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jolma A, Yan J, Whitington T, Toivonen J, Nitta KR, Rastas P, Morgunova E, Enge M, Taipale M, Wei G, Palin K, Vaquerizas JM, Vincentelli R, Luscombe NM, Hughes TR, Lemaire P, Ukkonen E, Kivioja T, Taipale J. DNA-binding specificities of human transcription factors. Cell. 2013;152(1–2):327–339. doi: 10.1016/j.cell.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Lan X, Thomas-Ahner JM, Wu D, Liu X, Ye Z, Wang L, Sunkel B, Grenade C, Chen J, Zynger DL, Yan PS, Huang J, Nephew KP, Huang TH, Lin S, Clinton SK, Li W, Jin VX, Wang Q. Agonist and antagonist switch DNA motifs recognized by human androgen receptor in prostate cancer. EMBO J. 2015;34(4):502–516. doi: 10.15252/embj.201490306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiller BJ, Chodankar R, Watson LC, Stallcup MR, Yamamoto KR. Glucocorticoid receptor binds half sites as a monomer and regulates specific target genes. Genome Biol. 2014;15(7):418. doi: 10.1186/s13059-014-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starick SR, Ibn-Salem J, Jurk M, Hernandez C, Love MI, Chung HR, Vingron M, Thomas-Chollier M, Meijsing SH. ChIP-exo signal associated with DNA-binding motifs provides insight into the genomic binding of the glucocorticoid receptor and cooperating transcription factors. Genome Res. 2015;25(6):825–835. doi: 10.1101/gr.185157.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim HW, Uhlenhaut NH, Rauch A, Weiner J, Hubner S, Hubner N, Won KJ, Lazar MA, Tuckermann J, Steger DJ. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Res. 2015;25(6):836–844. doi: 10.1101/gr.188581.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoneveld OJ, Gaemers IC, Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. 2004;1680(2):114–128. doi: 10.1016/j.bbaexp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, Beck IM, De Bosscher K. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol Cell Endocrinol. 2013;380(1–2):41–54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 40.De Bosscher K, Beck IM, Dejager L, Bougarne N, Gaigneaux A, Chateauvieux S, Ratman D, Bracke M, Tavernier J, Vanden Berghe W, Libert C, Diederich M, Haegeman G. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-kappaB and AP-1. Cell Mol Life Sci. 2014;71(1):143–163. doi: 10.1007/s00018-013-1367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steketee K, Ziel-van der Made AC, van der Korput HA, Houtsmuller AB, Trapman J. A bioinformatics-based functional analysis shows that the specifically androgen-regulated gene SARG contains an active direct repeat androgen response element in the first intron. J Mol Endocrinol. 2004;33(2):477–491. doi: 10.1677/jme.1.01478. [DOI] [PubMed] [Google Scholar]
- 42.Haelens A, Verrijdt G, Callewaert L, Christiaens V, Schauwaers K, Peeters B, Rombauts W, Claessens F. DNA recognition by the androgen receptor: evidence for an alternative DNA-dependent dimerization, and an active role of sequences flanking the response element on transactivation. Biochem J. 2003;369(Pt 1):141–151. doi: 10.1042/bj20020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoenmakers E, Alen P, Verrijdt G, Peeters B, Verhoeven G, Rombauts W, Claessens F. Differential DNA binding by the androgen and glucocorticoid receptors involves the second Zn-finger and a C-terminal extension of the DNA-binding domains. Biochem J. 1999;341(Pt 3):515–521. doi: 10.1042/bj3410515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rastinejad F, Huang P, Chandra V, Khorasanizadeh S. Understanding nuclear receptor form and function using structural biology. J Mol Endocrinol. 2013;51(3):T1–T21. doi: 10.1530/JME-13-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, Moehren U, Swinnen JV, Verhoeven G, Verrijdt G, Claessens F. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci USA. 2007;104(12):4961–4966. doi: 10.1073/pnas.0610814104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helsen C, Claessens F. Looking at nuclear receptors from a new angle. Mol Cell Endocrinol. 2014;382(1):97–106. doi: 10.1016/j.mce.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Kerkhofs S, Dubois V, De Gendt K, Helsen C, Clinckemalie L, Spans L, Schuit F, Boonen S, Vanderschueren D, Saunders PT, Verhoeven G, Claessens F. A role for selective androgen response elements in the development of the epididymis and the androgen control of the 5alpha reductase II gene. FASEB J Off Publ Fed Am Soc Exp Biol. 2012;26(10):4360–4372. doi: 10.1096/fj.11-202283. [DOI] [PubMed] [Google Scholar]
- 48.Sahu B, Pihlajamaa P, Dubois V, Kerkhofs S, Claessens F, Janne OA. Androgen receptor uses relaxed response element stringency for selective chromatin binding and transcriptional regulation in vivo. Nucleic Acids Res. 2014;42(7):4230–4240. doi: 10.1093/nar/gkt1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verrijdt G, Tanner T, Moehren U, Callewaert L, Haelens A, Claessens F. The androgen receptor DNA-binding domain determines androgen selectivity of transcriptional response. Biochem Soc Trans. 2006;34(Pt 6):1089–1094. doi: 10.1042/BST0341089. [DOI] [PubMed] [Google Scholar]
- 50.Clinckemalie L, Vanderschueren D, Boonen S, Claessens F. The hinge region in androgen receptor control. Mol Cell Endocrinol. 2012;358(1):1–8. doi: 10.1016/j.mce.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Burns KA, Li Y, Liu L, Korach KS. Research resource: comparison of gene profiles from wild-type ERalpha and ERalpha hinge region mutants. Mol Endocrinol (Baltimore, Md) 2014;28(8):1352–1361. doi: 10.1210/me.2014-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res. 2007;67(9):4514–4523. doi: 10.1158/0008-5472.CAN-06-1701. [DOI] [PubMed] [Google Scholar]
- 53.Tanner TM, Denayer S, Geverts B, Van Tilborgh N, Kerkhofs S, Helsen C, Spans L, Dubois V, Houtsmuller AB, Claessens F, Haelens A. A 629RKLKK633 motif in the hinge region controls the androgen receptor at multiple levels. Cell Mol Life Sci. 2010;67(11):1919–1927. doi: 10.1007/s00018-010-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khorasanizadeh S, Rastinejad F. Nuclear-receptor interactions on DNA-response elements. Trends Biochem Sci. 2001;26(6):384–390. doi: 10.1016/S0968-0004(01)01800-X. [DOI] [PubMed] [Google Scholar]
- 55.Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121(Pt 7):957–968. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- 56.Wang C, Powell MJ, Popov VM, Pestell RG. Acetylation in nuclear receptor signaling and the role of sirtuins. Mol Endocrinol (Baltimore, Md) 2008;22(3):539–545. doi: 10.1210/me.2007-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaughan L, Logan IR, Cook S, Neal DE, Robson CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277(29):25904–25913. doi: 10.1074/jbc.M203423200. [DOI] [PubMed] [Google Scholar]
- 58.Gaughan L, Logan IR, Neal DE, Robson CN. Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 2005;33(1):13–26. doi: 10.1093/nar/gki141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackinnon JA, Gallastegui N, Osguthorpe DJ, Hagler AT, Estebanez-Perpina E. Allosteric mechanisms of nuclear receptors: insights from computational simulations. Mol Cell Endocrinol. 2014;393(1–2):75–82. doi: 10.1016/j.mce.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Lefstin JA, Yamamoto KR. Allosteric effects of DNA on transcriptional regulators. Nature. 1998;392(6679):885–888. doi: 10.1038/31860. [DOI] [PubMed] [Google Scholar]
- 61.Watson LC, Kuchenbecker KM, Schiller BJ, Gross JD, Pufall MA, Yamamoto KR. The glucocorticoid receptor dimer interface allosterically transmits sequence-specific DNA signals. Nat Struct Mol Biol. 2013;20(7):876–883. doi: 10.1038/nsmb.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tadokoro-Cuccaro R, Davies J, Mongan NP, Bunch T, Brown RS, Audi L, Watt K, McEwan IJ, Hughes IA. Promoter-dependent activity on androgen receptor N-terminal domain mutations in androgen insensitivity syndrome. Sex Dev. 2014;8(6):339–349. doi: 10.1159/000369266. [DOI] [PubMed] [Google Scholar]
- 63.Callewaert L, Verrijdt G, Christiaens V, Haelens A, Claessens F. Dual function of an amino-terminal amphipatic helix in androgen receptor-mediated transactivation through specific and nonspecific response elements. J Biol Chem. 2003;278(10):8212–8218. doi: 10.1074/jbc.M210744200. [DOI] [PubMed] [Google Scholar]
- 64.Callewaert L, Christiaens V, Haelens A, Verrijdt G, Verhoeven G, Claessens F. Implications of a polyglutamine tract in the function of the human androgen receptor. Biochem Biophys Res Commun. 2003;306(1):46–52. doi: 10.1016/S0006-291X(03)00902-1. [DOI] [PubMed] [Google Scholar]
- 65.Rastinejad F, Ollendorff V, Polikarpov I. Nuclear receptor full-length architectures: confronting myth and illusion with high resolution. Trends Biochem Sci. 2015;40(1):16–24. doi: 10.1016/j.tibs.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Billas I, Moras D. Allosteric controls of nuclear receptor function in the regulation of transcription. J Mol Biol. 2013;425(13):2317–2329. doi: 10.1016/j.jmb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ, O’Malley BW. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97(1):17–27. doi: 10.1016/S0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 69.Lanz RB, Razani B, Goldberg AD, O’Malley BW. Distinct RNA motifs are important for coactivation of steroid hormone receptors by steroid receptor RNA activator (SRA) Proc Natl Acad Sci USA. 2002;99(25):16081–16086. doi: 10.1073/pnas.192571399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Redfern AD, Colley SM, Beveridge DJ, Ikeda N, Epis MR, Li X, Foulds CE, Stuart LM, Barker A, Russell VJ, Ramsay K, Kobelke SJ, Li X, Hatchell EC, Payne C, Giles KM, Messineo A, Gatignol A, Lanz RB, O’Malley BW, Leedman PJ. RNA-induced silencing complex (RISC) Proteins PACT, TRBP, and Dicer are SRA binding nuclear receptor coregulators. Proc Natl Acad Sci USA. 2013;110(16):6536–6541. doi: 10.1073/pnas.1301620110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang L, Lin C, Jin C, Yang JC, Tanasa B, Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, Evans CP, Rosenfeld MG. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500(7464):598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3(107):ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hudson WH, Pickard MR, de Vera IM, Kuiper EG, Mourtada-Maarabouni M, Conn GL, Kojetin DJ, Williams GT, Ortlund EA. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nature Commun. 2014;5:5395. doi: 10.1038/ncomms6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Claessens F, Celis L, Peeters B, Heyns W, Verhoeven G, Rombauts W. Functional characterization of an androgen response element in the first intron of the C3(1) gene of prostatic binding protein. Biochem Biophys Res Commun. 1989;164(2):833–840. doi: 10.1016/0006-291X(89)91534-9. [DOI] [PubMed] [Google Scholar]
- 75.Claessens F, Celis L, De Vos P, Peeters B, Heyns W, Verhoeven G, Rombauts W. Intronic androgen response elements of prostatic binding protein genes. Biochem Biophys Res Commun. 1993;191(2):688–694. doi: 10.1006/bbrc.1993.1272. [DOI] [PubMed] [Google Scholar]
- 76.Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271(11):6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- 77.Rennie PS, Bruchovsky N, Leco KJ, Sheppard PC, McQueen SA, Cheng H, Snoek R, Hamel A, Bock ME, MacDonald BS, et al. Characterization of two cis-acting DNA elements involved in the androgen regulation of the probasin gene. Mol Endocrinol (Baltimore, Md) 1993;7(1):23–36. doi: 10.1210/mend.7.1.8446105. [DOI] [PubMed] [Google Scholar]
- 78.Adler AJ, Scheller A, Robins DM. The stringency and magnitude of androgen-specific gene activation are combinatorial functions of receptor and nonreceptor binding site sequences. Mol Cell Biol. 1993;13(10):6326–6335. doi: 10.1128/MCB.13.10.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Celis L, Claessens F, Peeters B, Heyns W, Verhoeven G, Rombauts W. Proteins interacting with an androgen-responsive unit in the C3(1) gene intron. Mol Cell Endocrinol. 1993;94(2):165–172. doi: 10.1016/0303-7207(93)90165-G. [DOI] [PubMed] [Google Scholar]
- 80.Darne CH, Morel L, Claessens F, Manin M, Fabre S, Veyssiere G, Rombauts W, Jean CL. Ubiquitous transcription factors NF1 and Sp1 are involved in the androgen activation of the mouse vas deferens protein promoter. Mol Cell Endocrinol. 1997;132(1–2):13–23. doi: 10.1016/S0303-7207(97)00116-0. [DOI] [PubMed] [Google Scholar]
- 81.Adler AJ, Scheller A, Hoffman Y, Robins DM. Multiple components of a complex androgen-dependent enhancer. Mol Endocrinol (Baltimore, Md) 1991;5(11):1587–1596. doi: 10.1210/mend-5-11-1587. [DOI] [PubMed] [Google Scholar]
- 82.Verrijdt G, Haelens A, Claessens F. Selective DNA recognition by the androgen receptor as a mechanism for hormone-specific regulation of gene expression. Mol Genet Metab. 2003;78(3):175–185. doi: 10.1016/S1096-7192(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 83.Pihlajamaa P, Sahu B, Janne OA. Determinants of Receptor- and Tissue-Specific Actions in Androgen Signaling. Endocr Rev. 2015;36(4):357–384. doi: 10.1210/er.2015-1034. [DOI] [PubMed] [Google Scholar]
- 84.Zhao JC, Fong KW, Jin HJ, Yang YA, Kim J, Yu J. FOXA1 acts upstream of GATA2 and AR in hormonal regulation of gene expression. Oncogene. 2016 doi: 10.1038/onc.2015.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mills IG. Maintaining and reprogramming genomic androgen receptor activity in prostate cancer. Nat Rev Cancer. 2014;14(3):187–198. doi: 10.1038/nrc3678. [DOI] [PubMed] [Google Scholar]
- 86.Tesikova M, Dezitter X, Nenseth HZ, Klokk TI, Mueller F, Hager GL, Saatcioglu F. Divergent binding and transactivation by two related steroid receptors at the same response element. J Biol Chem. 2016 doi: 10.1074/jbc.M115.684480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nenseth HZ, Dezitter X, Tesikova M, Mueller F, Klokk TI, Hager GL, Saatcioglu F. Distinctly different dynamics and kinetics of two steroid receptors at the same response elements in living cells. PLoS One. 2014;9(8):e105204. doi: 10.1371/journal.pone.0105204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 89.Cato AC, Weinmann J, Mink S, Ponta H, Henderson D, Sonnenberg A. The regulation of expression of mouse mammary tumor virus DNA by steroid hormones and growth factors. J Steroid Biochem. 1989;34(1–6):139–143. doi: 10.1016/0022-4731(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 90.Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab. 2010;21(5):315–324. doi: 10.1016/j.tem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaaskelainen T, Makkonen H, Palvimo JJ. Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr Opin Pharmacol. 2011;11(4):326–331. doi: 10.1016/j.coph.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Storlie JA, Buckner JC, Wiseman GA, Burch PA, Hartmann LC, Richardson RL. Prostate specific antigen levels and clinical response to low dose dexamethasone for hormone-refractory metastatic prostate carcinoma. Cancer. 1995;76(1):96–100. doi: 10.1002/1097-0142(19950701)76:1<96::AID-CNCR2820760114>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 93.Morioka M, Kobayashi T, Furukawa Y, Jo Y, Shinkai M, Matsuki T, Yamamoto T, Tanaka H. Prostate-specific antigen levels and prognosis in patients with hormone-refractory prostate cancer treated with low-dose dexamethasone. Urol Int. 2002;68(1):10–15. doi: 10.1159/000048411. [DOI] [PubMed] [Google Scholar]
- 94.Yano A, Fujii Y, Iwai A, Kawakami S, Kageyama Y, Kihara K. Glucocorticoids suppress tumor lymphangiogenesis of prostate cancer cells. Clin Cancer Res. 2006;12(20 Pt 1):6012–6017. doi: 10.1158/1078-0432.CCR-06-0749. [DOI] [PubMed] [Google Scholar]
- 95.Yemelyanov A, Czwornog J, Chebotaev D, Karseladze A, Kulevitch E, Yang X, Budunova I. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2007;26(13):1885–1896. doi: 10.1038/sj.onc.1209991. [DOI] [PubMed] [Google Scholar]
- 96.Narayanan S, Srinivas S, Feldman D. Androgen-glucocorticoid interactions in the era of novel prostate cancer therapy. Nat Rev Urol. 2016;13(1):47–60. doi: 10.1038/nrurol.2015.254. [DOI] [PubMed] [Google Scholar]
- 97.Yi P, Wang Z, Feng Q, Pintilie GD, Foulds CE, Lanz RB, Ludtke SJ, Schmid MF, Chiu W, O’Malley BW. Structure of a biologically active estrogen receptor-coactivator complex on DNA. Mol Cell. 2015;57(6):1047–1058. doi: 10.1016/j.molcel.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohammed H, D’Santos C, Serandour AA, Ali HR, Brown GD, Atkins A, Rueda OM, Holmes KA, Theodorou V, Robinson JL, Zwart W, Saadi A, Ross-Innes CS, Chin SF, Menon S, Stingl J, Palmieri C, Caldas C, Carroll JS. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013;3(2):342–349. doi: 10.1016/j.celrep.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tan PY, Chang CW, Chng KR, Wansa KD, Sung WK, Cheung E. Integration of regulatory networks by NKX3-1 promotes androgen-dependent prostate cancer survival. Mol Cell Biol. 2012;32(2):399–414. doi: 10.1128/MCB.05958-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Helsen C, Dubois V, Verfaillie A, Young J, Trekels M, Vancraenenbroeck R, De Maeyer M, Claessens F. Evidence for DNA-binding domain–ligand-binding domain communications in the androgen receptor. Mol Cell Biol. 2012;32(15):3033–3043. doi: 10.1128/MCB.00151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Callewaert L, Van Tilborgh N, Claessens F. Interplay between two hormone-independent activation domains in the androgen receptor. Cancer Res. 2006;66(1):543–553. doi: 10.1158/0008-5472.CAN-05-2389. [DOI] [PubMed] [Google Scholar]