Abstract
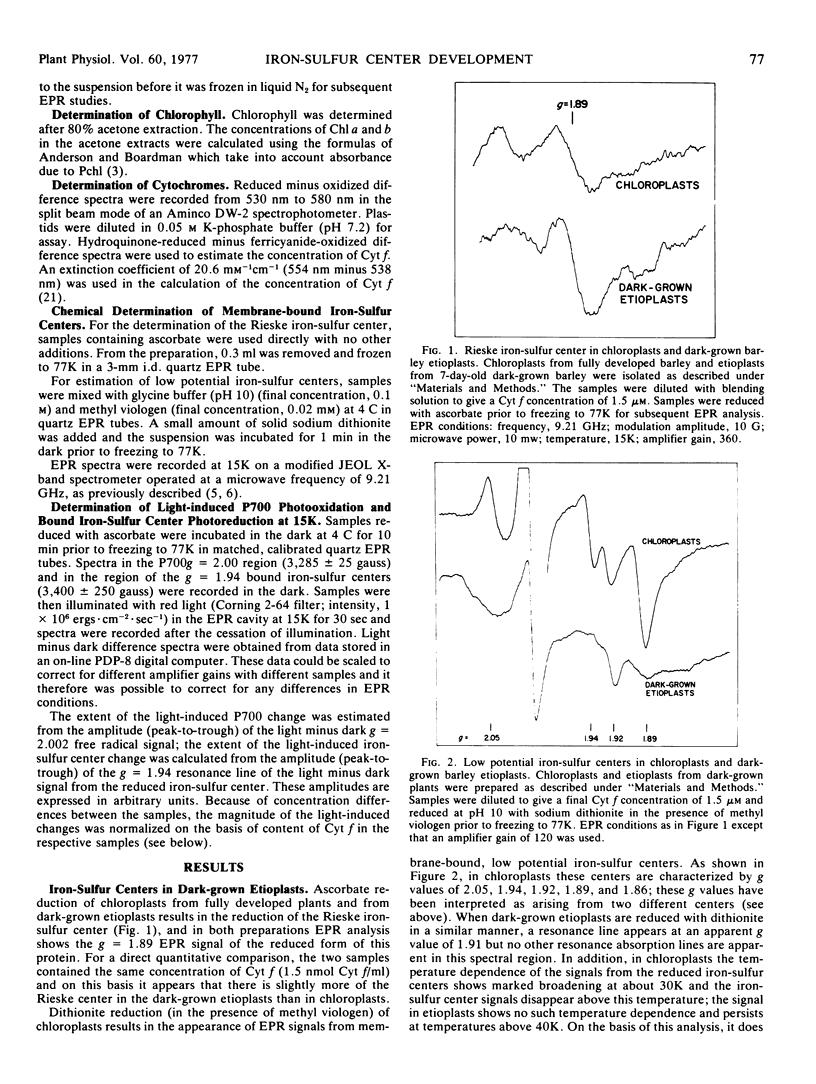
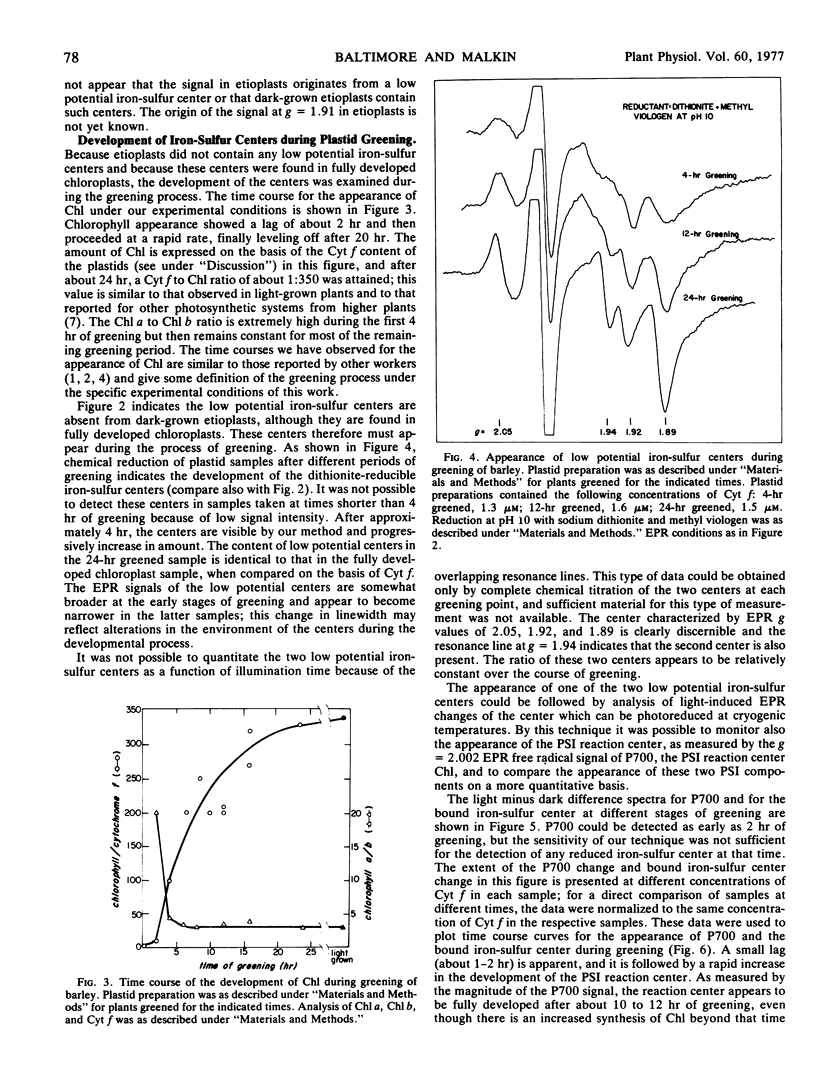
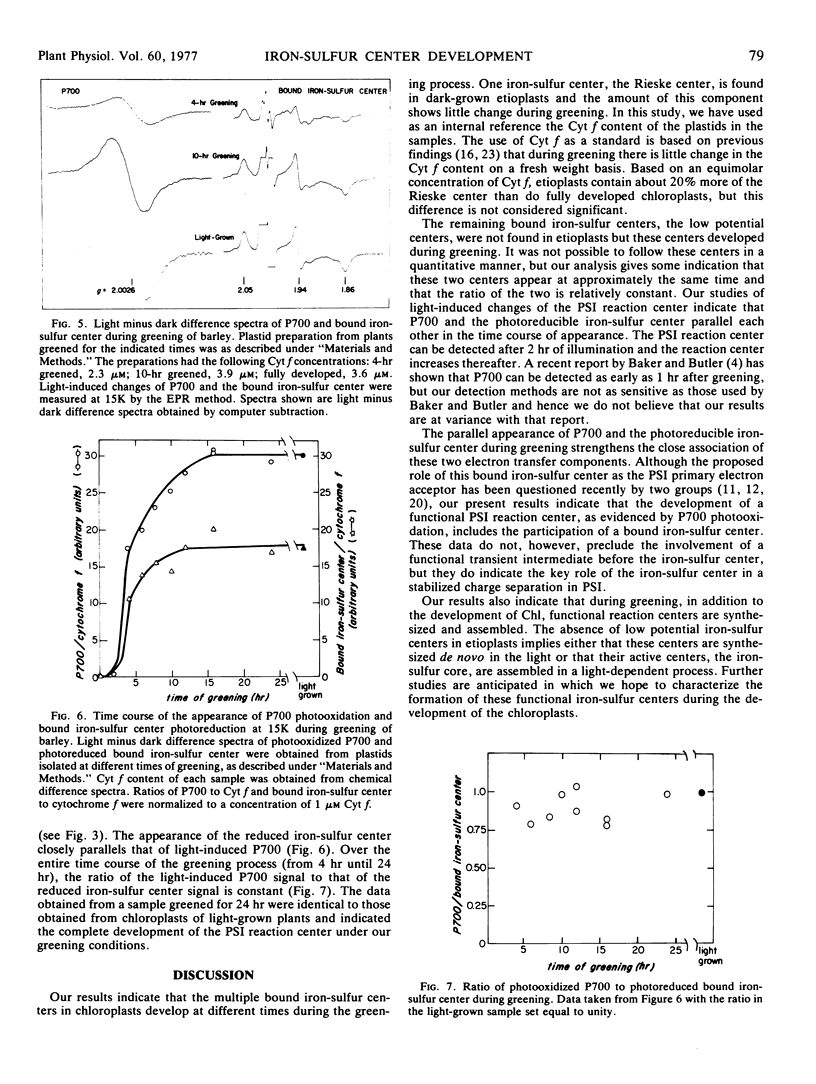
Dark-grown barley (Hordeum vulgare) etioplasts were examined for their content of membrane-bound iron-sulfur centers by electron paramagnetic resonance spectroscopy at 15K. They were found to contain the high potential iron-sulfur center characterized (in the reduced state) by an electron paramagnetic resonance g value of 1.89 (the “Rieske” center) but did not contain any low potential iron-sulfur centers. Per mole of cytochrome f, dark-grown etioplasts and fully developed chloroplasts had the same content of the Rieske center. During greening of etioplasts under continuous light, low potential bound iron-sulfur centers appear. In addition, the photosystem I reaction center, as measured by the photooxidation of P700 at 15K, also became functional; during greening the appearance of a photoreducible low potential iron-sulfur center paralleled the appearance of P700 photoactivity.
These findings indicate the close association of the low potential iron-sulfur centers with the photosystem I reaction center; they also support the concept that the development of stable charge separation in the photosystem I reaction center requires, in addition to P700, a low potential iron-sulfur center.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberte R. S., Thornber J. P., Naylor A. W. Biosynthesis of the photosystem I chlorophyll-protein complex in greening leaves of higher plants. Proc Natl Acad Sci U S A. 1973 Jan;70(1):134–137. doi: 10.1073/pnas.70.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R., Butler W. L. Development of the Primary Photochemical Apparatus of Photosynthesis during Greening of Etiolated Bean Leaves. Plant Physiol. 1976 Oct;58(4):526–529. doi: 10.1104/pp.58.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Correlation of reaction-center chlorophyll (P-700) oxidation and bound iron-sulfur protein photoreduction in chloroplast photosystem I at low temperatures. Biochim Biophys Acta. 1976 Jun 8;430(3):538–547. doi: 10.1016/0005-2728(76)90029-3. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Quantitative EPR studies of the primary reaction of photosystem I in chloroplasts. Biochim Biophys Acta. 1972 Dec 14;283(3):456–468. doi: 10.1016/0005-2728(72)90262-9. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Anderson J. M. Fractionation of the photochemical systems of photosynthesis. II. Cytochrome and carotenoid contents of particles isolated from spinach chloroplasts. Biochim Biophys Acta. 1967 Jul 5;143(1):187–203. doi: 10.1016/0005-2728(67)90120-x. [DOI] [PubMed] [Google Scholar]
- Cammack R., Evans M. C. E.P.R. spectra of iron-sulphur proteins in dimethylsulphoxide solutions: evidence that chloroplast photosystem I particles contain 4Fe-4S centres. Biochem Biophys Res Commun. 1975 Nov 17;67(2):544–549. doi: 10.1016/0006-291x(75)90846-3. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Cammack R. Determination of the oxidation-reduction potential of the bound iron-sulphur proteins of the primary electron acceptor complex of photosystem I in spinach chloroplasts. FEBS Lett. 1974 Dec 1;49(1):111–114. doi: 10.1016/0014-5793(74)80644-7. [DOI] [PubMed] [Google Scholar]
- Haslett B. G., Cammack R. The development of plastocyanin in greening bean leaves. Biochem J. 1974 Dec;144(3):567–572. doi: 10.1042/bj1440567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett B. G., Cammack R., Whatley F. R. Quantitative studies on ferredoxin in greening bean leaves. Biochem J. 1973 Nov;136(3):697–703. doi: 10.1042/bj1360697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen K. W., Boardman N. K. Development of Photochemical Activity and the Appearance of the High Potential Form of Cytochrome b-559 in Greening Barley Seedlings. Plant Physiol. 1973 Jun;51(6):1117–1126. doi: 10.1104/pp.51.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B., Hansen R. E., Beinert H. Oxidation-reduction potentials of bound iron-sulfur proteins of photosystem I. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2941–2945. doi: 10.1073/pnas.70.10.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin R., Aparicio P. J. Identification of a g equals 1.90 high-potential iron-sulfur protein in chloroplasts. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1157–1160. doi: 10.1016/0006-291x(75)90690-7. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcintosh A. R., Bolton J. R. Electron spin resonance spectrum of species "X" which may function as the primary electron acceptor in photosystem I of green plant photosynthesis. Biochim Biophys Acta. 1976 Jun 8;430(3):555–559. [PubMed] [Google Scholar]
- Nelson N., Neumann J. Isolation of a cytochrome b 6 -f particle from chloroplasts. J Biol Chem. 1972 Mar 25;247(6):1817–1824. [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieske J. S. Composition, structure, and function of complex III of the respiratory chain. Biochim Biophys Acta. 1976 Sep 27;456(2):195–247. doi: 10.1016/0304-4173(76)90012-4. [DOI] [PubMed] [Google Scholar]


