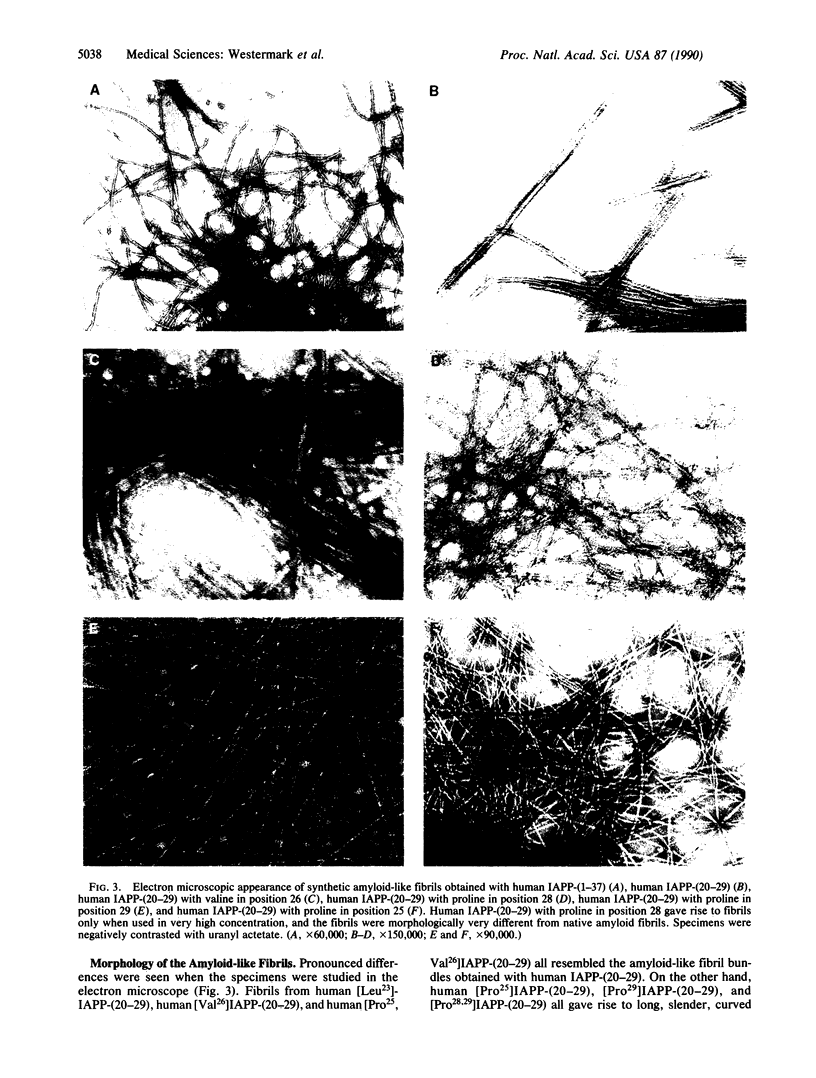
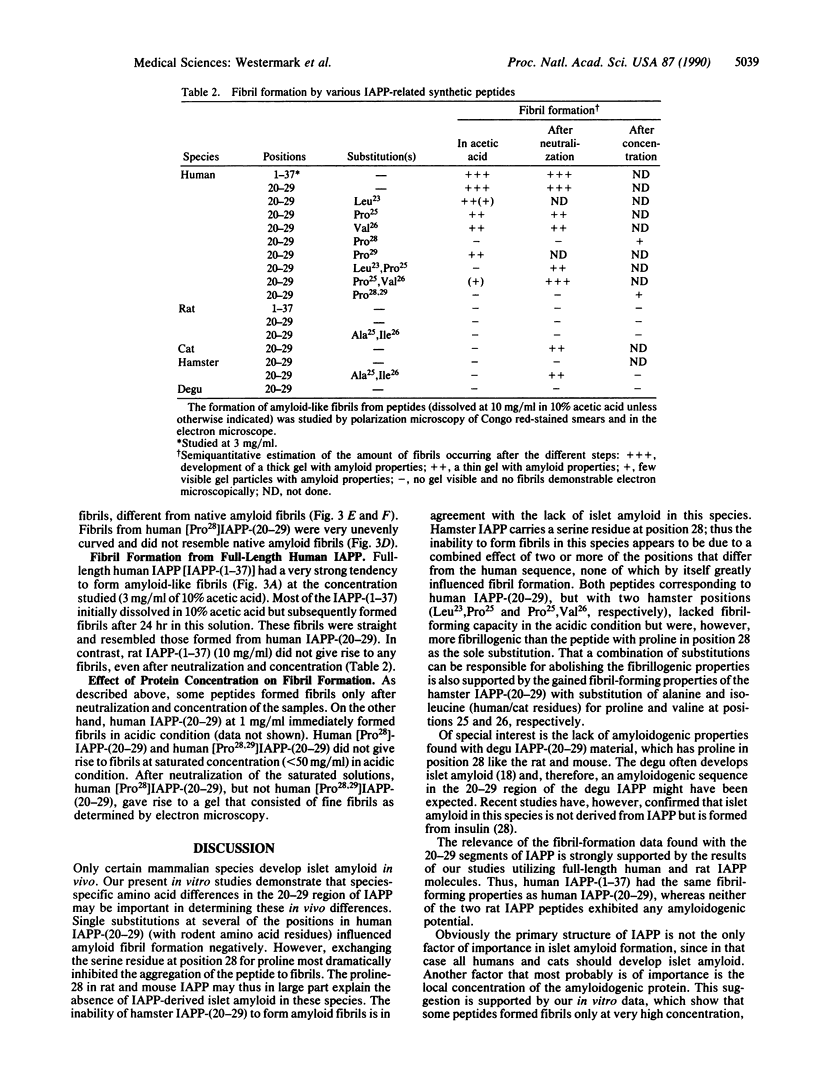
Abstract
Islet amyloid polypeptide (IAPP), a putative polypeptide hormone, is a product of pancreatic beta-cells and the major constituent of the amyloid deposits seen mainly in islets of type 2 diabetic humans and diabetic cats. The connection between IAPP amyloid formation and diabetes is unknown, but a limited segment of the IAPP molecule, positions 20-29, seems responsible for the aggregation to fibrils. Differences in the amino acid sequence of this region probably determine whether or not islet amyloid can develop in a particular species. Amyloid fibril formation can be mimicked in vitro with the aid of synthetic peptides. With this technique we show that peptides corresponding to IAPP positions 20-29 of human and cat, species that develop IAPP-derived islet amyloid, form amyloid-like fibrils in vitro. The corresponding IAPP segment from three rodent species that do not develop IAPP-derived amyloid did not give rise to fibrils. Substitution of the human IAPP-(20-29) decapeptide with one or two amino acid residues from species without islet amyloid generally reduced the capacity to form fibrils. We conclude that the sequence Ala-Ile-Leu-Ser-Ser, corresponding to positions 25-29 of human IAPP, is strongly amyloidogenic and that a proline-for-serine substitution in position 28, as in several rodents, almost completely inhibits formation of amyloid fibrils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsholtz C., Christmanson L., Engström U., Rorsman F., Jordan K., O'Brien T. D., Murtaugh M., Johnson K. H., Westermark P. Structure of cat islet amyloid polypeptide and identification of amino acid residues of potential significance for islet amyloid formation. Diabetes. 1990 Jan;39(1):118–122. doi: 10.2337/diacare.39.1.118. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Christmansson L., Engström U., Rorsman F., Svensson V., Johnson K. H., Westermark P. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989 Jul 17;251(1-2):261–264. doi: 10.1016/0014-5793(89)81467-x. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Svensson V., Rorsman F., Engström U., Westermark G. T., Wilander E., Johnson K., Westermark P. Islet amyloid polypeptide (IAPP):cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Exp Cell Res. 1989 Aug;183(2):484–493. doi: 10.1016/0014-4827(89)90407-2. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clark A., Edwards C. A., Ostle L. R., Sutton R., Rothbard J. B., Morris J. F., Turner R. C. Localisation of islet amyloid peptide in lipofuscin bodies and secretory granules of human B-cells and in islets of type-2 diabetic subjects. Cell Tissue Res. 1989 Jul;257(1):179–185. doi: 10.1007/BF00221649. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Leighton B., Dimitriadis G. D., Parry-Billings M., Kowalchuk J. M., Howland K., Rothbard J. B., Willis A. C., Reid K. B. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Bladen H. A., Linke R. P., Termine J. D. Beta-pleated sheet fibrils. A comparison of native amyloid with synthetic protein fibrils. J Histochem Cytochem. 1974 Dec;22(12):1141–1158. doi: 10.1177/22.12.1141. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Wiley C. A. Amyloid fibrils formed from a segment of the pancreatic islet amyloid protein. Biochem Biophys Res Commun. 1988 Sep 15;155(2):608–614. doi: 10.1016/s0006-291x(88)80538-2. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr Insular amyloidosis and diabetes mellitus in Macaca nigra. Diabetes. 1978 Apr;27(4):357–364. doi: 10.2337/diab.27.4.357. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Hayden D. W., Jordan K., Ghobrial H. K., Mahoney W. C., Westermark P. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol. 1988 Jan;130(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Jordan K., Betsholtz C., Westermark P. The putative hormone islet amyloid polypeptide (IAPP) induces impaired glucose tolerance in cats. Biochem Biophys Res Commun. 1990 Mar 16;167(2):507–513. doi: 10.1016/0006-291x(90)92053-3. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Jordan K., Westermark P. Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol. 1989 Aug;135(2):245–250. [PMC free article] [PubMed] [Google Scholar]
- Leffert J. D., Newgard C. B., Okamoto H., Milburn J. L., Luskey K. L. Rat amylin: cloning and tissue-specific expression in pancreatic islets. Proc Natl Acad Sci U S A. 1989 May;86(9):3127–3130. doi: 10.1073/pnas.86.9.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukinius A., Wilander E., Westermark G. T., Engström U., Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989 Apr;32(4):240–244. doi: 10.1007/BF00285291. [DOI] [PubMed] [Google Scholar]
- Mosselman S., Höppener J. W., Zandberg J., van Mansfeld A. D., Geurts van Kessel A. H., Lips C. J., Jansz H. S. Islet amyloid polypeptide: identification and chromosomal localization of the human gene. FEBS Lett. 1988 Nov 7;239(2):227–232. doi: 10.1016/0014-5793(88)80922-0. [DOI] [PubMed] [Google Scholar]
- Nishi M., Chan S. J., Nagamatsu S., Bell G. I., Steiner D. F. Conservation of the sequence of islet amyloid polypeptide in five mammals is consistent with its putative role as an islet hormone. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5738–5742. doi: 10.1073/pnas.86.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M., Sanke T., Nagamatsu S., Bell G. I., Steiner D. F. Islet amyloid polypeptide. A new beta cell secretory product related to islet amyloid deposits. J Biol Chem. 1990 Mar 15;265(8):4173–4176. [PubMed] [Google Scholar]
- Rose G. D. Prediction of chain turns in globular proteins on a hydrophobic basis. Nature. 1978 Apr 13;272(5654):586–590. doi: 10.1038/272586a0. [DOI] [PubMed] [Google Scholar]
- Sanke T., Bell G. I., Sample C., Rubenstein A. H., Steiner D. F. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988 Nov 25;263(33):17243–17246. [PubMed] [Google Scholar]
- Spear G. S., Caple M. V., Sutherland L. R. The pancreas in the degu. Exp Mol Pathol. 1984 Jun;40(3):295–310. doi: 10.1016/0014-4800(84)90047-9. [DOI] [PubMed] [Google Scholar]
- Westermark P., Engström U., Westermark G. T., Johnson K. H., Permerth J., Betsholtz C. Islet amyloid polypeptide (IAPP) and pro-IAPP immunoreactivity in human islets of Langerhans. Diabetes Res Clin Pract. 1989 Sep 18;7(3):219–226. doi: 10.1016/0168-8227(89)90008-9. [DOI] [PubMed] [Google Scholar]
- Westermark P. Fine structure of islets of Langerhans in insular amyloidosis. Virchows Arch A Pathol Pathol Anat. 1973 Mar 20;359(1):1–18. doi: 10.1007/BF00549079. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., O'Brien T. D., Hayden D. W., Johnson K. H. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol. 1987 Jun;127(3):414–417. [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986 Nov 14;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978 Nov;15(5):417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- Yano B. L., Hayden D. W., Johnson K. H. Feline insular amyloid: association with diabetes mellitus. Vet Pathol. 1981 Sep;18(5):621–627. doi: 10.1177/030098588101800507. [DOI] [PubMed] [Google Scholar]