Abstract
Corneal collagen has a number of properties that allow it to fulfil its role as the main structural component within the tissue. Fibrils are narrow, uniform in diameter and precisely organised. These properties are vital to maintain transparency and to provide the biomechanical prerequisites necessary to sustain shape and provide strength. This review describes the structure and arrangement of corneal collagen from the nanoscopic to the macroscopic level, and how this relates to the maintenance of the form and transparency of the cornea.
Keywords: Collagen, Cornea, Structure, Transparency
The cornea
The cornea is the transparent window that forms part of the outer tunic of the eye. It is the primary refractive element of the eye’s optical system and is a remarkable tissue in that it combines the strength required to fulfil its role of producing a tough container for the inner contents of the eye with precise curvature and a high level of transparency to visible wavelengths. This is achieved primarily by the unique structural properties of its main dry constituent, collagen.
The human cornea is about 0.5 mm thick at the centre, increasing somewhat towards the periphery. Beneath the covering tear film is a coating of epithelial cells, five or six layers deep, followed by a very thin collagenous layer called Bowman’s membrane. The bulk of the tissue is the stroma, about 250 lamellae of collagen-rich connective tissue. Fibrils in one lamella make varying angles with those in adjacent lamellae such that, within the plane of the cornea, fibrils occur in all azimuthal directions. Each lamella contains long collagen fibrils, which run parallel to one another (Fig. 1). The fibrils are spaced apart by a ground substance that is essentially a hydrated gel of proteoglycans (proteins containing long sulphated polysaccharide chains). The most posterior lamellae are bounded by another connective tissue layer, Descemet’s membrane, which is attached to the posterior stroma by dense fibres 22.3 nm thick (Binder et al. 1991). This forms the support for the innermost surface of the cornea, a single layer of cells called the endothelium.
Fig. 1.
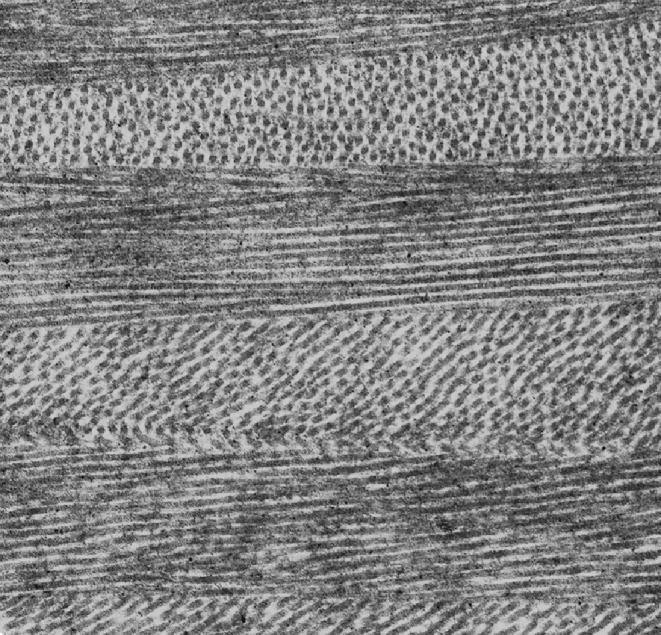
Ultrastructure of the corneal stroma showing the orientation of collagen fibrils within lamellae. Magnification 20K (courtesy of Dr. R. Young)
Corneal collagen
The collagen in the corneal stroma has been of special interest because it has two properties not generally found in other connective tissues, not even in the sclera (the so-called “white” of the eye):(1) the fibrils have narrow, relatively uniform diameters; (2) the fibrils seem to be arranged with a high degree of lateral order. Understanding these two properties has been the key to explaining the cornea’s optical and biomechanical properties (Meek and Boote 2004).
Collagen in the stroma is predominantly type I, with smaller amounts of types V, VI, XII, XIII, XIV and XXIV (Ihanamäki et al. 2004). The presence of type III collagen within the normal corneal stroma is disputed. Types I and V collagen are found together within heterotypic fibrils, and type V is buried beneath the surface with only the non-helical terminal extensions protruding. Type VI forms beaded filaments throughout the stroma, randomly arranged in a meshwork. This collagen probably stabilises the collagen fibrillar array, but it may also prolong the life of corneal cells by preventing anti-beta 1 integrin-induced apoptosis (Howell and Doane 1998). Type XII has been localised to areas where the stroma interfaces with Bowman’s and Descemet’s membranes (Gordon et al. 1996). At the Bowman’s interface, long isoform type XII collagen is aligned on the surface of type I collagen fibrils in a head-to-tail pattern, and this arrangement is thought to confer additional stability to the region (Wessel et al. 1997). Type XIII has been located in the posterior stroma, though its function is not known (Sandberg-Lall et al. 2000), whereas type XIV was found in the avian stroma associated with the surfaces of type I/V collagen fibrils (Ricard-Blum and Ruggiero 2005) where it is thought to play a role in compaction of the cornea, which is critical for transparency (Gordon et al. 1996). Finally, type XXIV collagen has been found in the developing murine cornea in conjunction with type I collagen, where it may play a role in the early regulation of fibril diameters (Koch et al. 2003).
Collagen fibrils
Along the corneal collagen fibril major axis, molecules are arranged with the usual collagen quarter stagger (Meek and Holmes 1983), leading to an axial periodicity of 65 nm (Meek et al. 1981). In the human cornea, each fibril in cross-section contains about 300 molecules (Meek and Leonard 1993), of which about 30 are type V (Holmes and Kadler 2004). The intermolecular Bragg spacing in wide angle X-ray diffraction patterns (1.6 nm) suggests that corneal fibrils are more hydrated than those from the sclera or tendon (Meek et al. 1991); this would reduce light scatter by lowering the refractive index mis-match between the fibrils and the ground substance. The exact lateral packing of the molecules is not known, and it has often been described as liquid-like (Wess 2008) or pseudo-hexagonal (Maroudas et al. 1991; Leonard and Meek 1997). Molecules are stabilised by intermolecular covalent crosslinks. In the mature cornea, cross-links are different to those found in other tissues, and their locations suggest that a proportion of adjacent molecules are in register (Yamauchi et al. 1996). Typical values for several structural and optical properties of corneal collagen fibrils are listed in Table 1.
Table 1.
Some properties of human corneal collagen fibrils
| Property | Value | Data source |
|---|---|---|
| Molecular Bragg spacing | 1.63 nm | Leonard and Meek 1997 |
| Fibril diameter | 31–34 nm | Meek and Leonard 1993; Daxer et al. 1998; Boote et al. 2003 |
| Interfibrillar Bragg spacing | 55–57 nm | Leonard and Meek 1997; Boote et al. 2003; |
| Fibril cross-section number density | 353-559 fibrils/μm2 | Freund et al. 1995 |
| Fibril area fraction | 0.3–0.4 | Meek and Leonard 1993 Freund et al. 1995 |
| Collagen fibril axial D-period | 65 nm | Meek et al. 1981 |
| Collagen fibril refractive index | 1.411–1.454 | Leonard and Meek 1997; Worthington 1984 |
| Fibril scattering cross-section per unit length (at 500 nm) | 3 × 10-2 Å | Freund et al. 1995 |
| Form birefringence of collagen fibrils | 0.0028–0.0042 | Naylor 1953; Worthington 1984 |
| Young’s modulus of fibrils | 1.0 GPa | Pinsky and Datye 1991 |
Values reflect the ranges quoted in the literature
The presence of subdomains of structure within corneal collagen fibrils, as in other type I collagen-containing tissues, is of interest since it may reveal important information about the overall mechanical properties of fibrils by preventing crack propagation, as well as relating to the nucleation and growth processes of fibril formation (Wess 2008). The basic building block is probably a microfibril containing five molecules with a right-handed supertwist, as in other collagens (Orgel et al. 2006). Holmes et al. (2001) used automated electron tomography to determine corneal collagen fibril structure and described microfibrils approximately 4 nm in diameter tilted approximately 15° to the fibril long axis, the tilt probably leading to the shortening of the axial periodicity from the 67 nm value found in tendons. Their data suggests that the tilt angle is constant regardless of depth within the fibrils, and that microfibrils exhibit regions of order and disorder along their length. When these microfibrils then assemble to form fibrils, there appear to be substantial variations in lateral size, both between fibrils and along individual fibrils, and Holmes and Kadler (2004) highlighted that lateral size is not controlled at the molecular level as in other molecules such as the thick filaments in striated muscles. Moreover, careful electron microscope measurements of fibril diameters from different connective tissues (Parry and Craig 1979) and from corneas from a number of species (Craig and Parry 1981) showed evidence for the existence of an 8-nm-diameter intermediate structure. This has also been reported in rabbit scar collagen (McCally et al. 2007) and suggests that fibrils grow by addition of discrete concentric radial shells (Hulmes et al. 1995; Ottani et al. 2002).
There may be other intermediate levels of organisation between molecule and fibril in the cornea (Ottani et al. 2002). Freeze-fracture and -etching (Marchini et al. 1986; Hirsch et al. 2001) has revealed fibril sub-structures greater than 4 nm wide, tilted with respect to the fibril axis by 16-17° (Fig. 2). A number of other techniques have also confirmed the presence of sub-fibrillar structures in collagen fibrils (Baselt et al. 1993; Yamamoto et al. 2000; Yamauchi et al. 1996), but whether or not these can exist as separate entities has yet to be established.
Fig. 2.
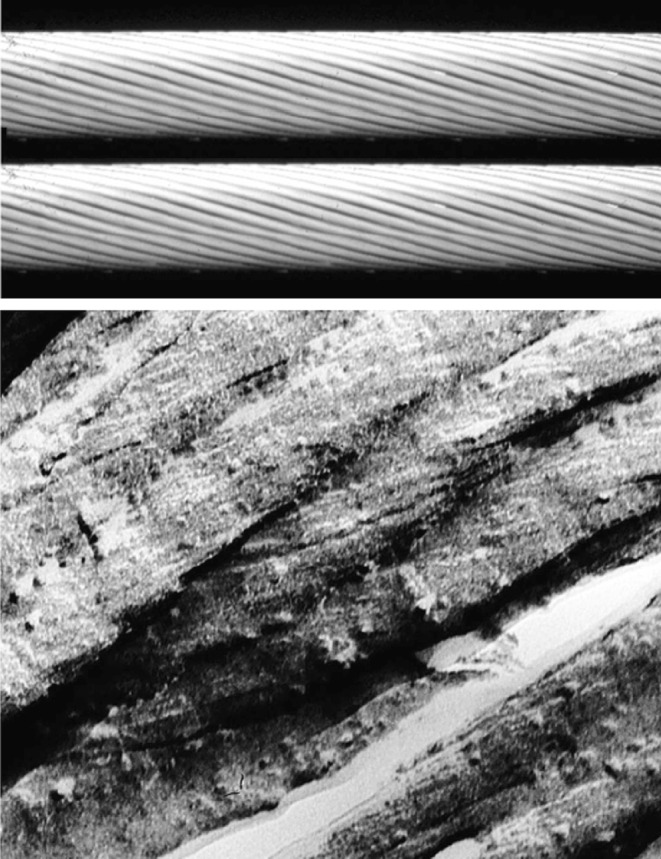
(Above) Molecular model of the sub-fibrillar helicoidal arrangement in corneal collagen. (Below) Freeze-fracture micrograph of hydrated bovine corneal collagen fibrils. Magnification 160K (Ottani et al. 2002, reproduced with permission of the copyright holder)
In the electron microscope, low-temperature electron microscope procedures revealed that corneal collagen fibrils have an average diameter of about 38 nm (Craig et al. 1986), a figure that is in good agreement with -ray scattering data (Meek and Leonard 1993). Other techniques have revealed larger values (Itoh et al. 1981; Marchini et al. 1986; Meller et al. 1997; Yamamoto et al. 2002) but these are probably artefacts due to sample preparation methods. Diameters have been reported to vary with depth in the cornea (Freund et al. 1995), though recent work indicates possible intra- and inter-sample variation, even between adjacent lamellae (Doughty and Bergmanson 2006). Diameters also increase with age (Daxer et al. 1998). The mechanism of these changes at the molecular and microfibrillar level are still not known, although age-related changes may involve non-enzymatic crosslinking, which is thought to increase the average spacing between adjacent collagen molecules (Malik et al. 1992).
Corneal collagen fibril diameters show considerable variation between species (Craig and Parry 1981; Meek and Leonard 1993) as do the centre-to-centre interfibrillar spacings (Gyi et al. 1988), although the fact that narrower fibrils are also more closely packed leads to a constant collagen volume fraction of about 28% (Meek and Leonard 1993). Diameters also increase from central to peripheral cornea in most species, although in this case the volume fraction changes (Boote et al. 2003). These differences probably relate to the biomechanical properties of the corneas (Parry et al. 1978; Jayasuriya et al. 2003; Meek 2008). The fibril volume fraction has a significant effect on the mechanical strength of a connective tissue (Boote et al. 2003; Goh et al. 2008). Parry (1988) pointed out that a tissue’s creep-inhibition ability, flexibility and ability to resist crack propagation are directly related to the percentage of smaller diameter fibrils, but tensile strength is greater in wider fibrils. It is possible that the higher packing density of narrow fibrils is necessary for maintaining corneal strength. For example, the central human cornea is thinner than the peripheral cornea, so the smaller, more closely packed fibrils may allow the central cornea to maintain strength while allowing the deformation that may occur, for example, during eye-rubbing. The fact that narrower fibrils are more closely spaced may also be related to optical properties of the cornea (Cox et al. 1970; Benedek 1971).
Regulators of fibril diameter and spacing
A number of different mechanisms have been suggested as regulators of collagen fibril growth in the cornea. It was noted some time ago that connective tissues show a strong negative correlation between fibril diameters and the levels of hydroxylysine-linked mono- and di-saccharides (Harding et al. 1980). The close association of types I and V within heterotypic corneal fibrils is thought to be a factor limiting the ultimate diameter of the fibrils (Birk 2001). In type V collagen the N-propeptide is not cleaved as in type I collagen and it therefore has be accommodated, apparently by allowing it to project to the fibril surface. The presence of these globular domains may prevent accretion of further molecules on the fibril surface (Linsenmayer et al. 1993; Birk 2001).
Proteoglycans have also been implicated in the control of fibril diameter. Lumican, keratocan, mimecan and decorin associate with the surface of corneal collagen fibrils and when these are removed in vitro, the fibrils aggregate (Meek and Holmes 1983). Lumican-deficient mouse corneas show a wider range of fibril diameters, which is suggestive of fibril aggregation or fusion (Chakravarti et al. 1988; Quantock et al. 2001). Corneas with pathological proteoglycan processing also show abnormal fibril diameters, also suggestive of fibril fusion (Quantock et al. 1993; Huang et al. 1996; Rawe et al. 1997). It could be, therefore, that different mechanisms are involved in diameter control at different hierarchical levels.
Not only is the precise nature of collagen fibrillar packing in the cornea unknown, but we also do not understand the factors that regulate this organisation. The proteoglycans surrounding the fibrils are major constituents of the interfibrillar space, and these undergo several changes during development that have been correlated with fibrillar compaction (Quantock and Young 2008). Furthermore, their absence in knock-out mouse corneas is accompanied by a loss of fibril order (Chakravarti et al. 1988; Meek et al. 2003b). The enzymatic sulphation of keratan sulphate glycosaminoglycan chains has been identified as the key requirement for collagen matrix organization (Hayashida et al. 2006). Using cationic dyes, it has been shown that corneal proteoglycans associate non-covalently with collagen fibrils at specific axial locations (Scott and Haigh 1985; Meek et al. 1986; Miyagawa et al. 2001), and this staining technique has been the basis of a three-dimensional (3-D) model in which proteoglycans connect next nearest-neighbour fibrils by groups of six proteoglycans, attached orthogonal to the circumference of the fibrils (Müller et al. 2004). However, 3-D electron micrograph reconstructions by our group showed that there are no systematic six-fold interactions between collagen and proteoglycans in the hexagonal array. Instead, proteoglycans form long electron-dense strands that hold two or more collagen fibrils together. Since these strands are oriented in different directions, proteoglycan bridges are formed between all adjacent collagen fibrils and give rise to the observed 3-D corneal fibrillar packing (Fig. 3). It is believed that different types of proteoglycans exert opposing forces on the collagen, with a repulsive force arising from osmotic pressure and an attractive force arising from the thermal motion of the proteoglycans (Knupp et al. 2009).
Fig. 3.
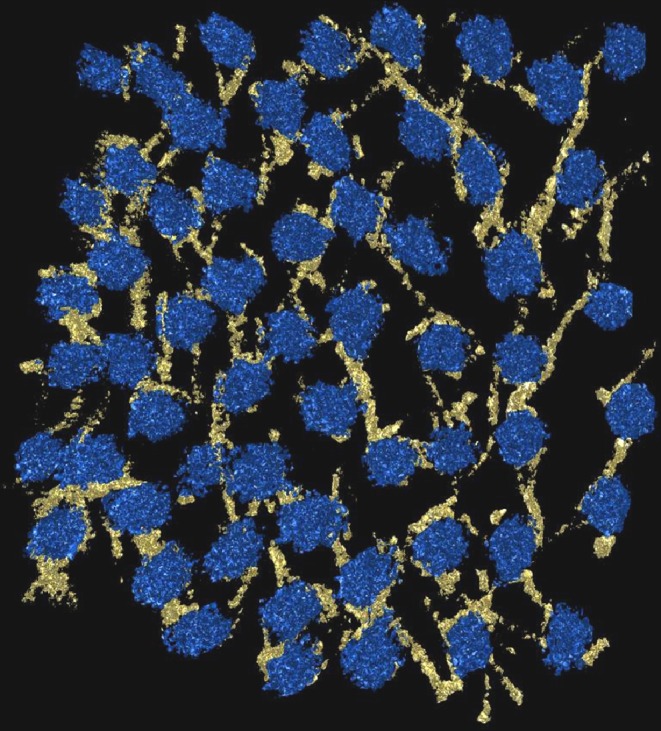
3-D electron microscope reconstruction of the arrangement of proteoglycans stained with Cuprolinic blue dye (yellow) and collagen fibrils in cross-section (blue) in the bovine cornea (courtesy of Dr. C. Knupp and Dr. P. Booth)
Other molecules and ions have also been implicated in maintaining the order within the arrangement of collagen fibrils. Dermatopontin (dpt) is an abundant component of the stromal extracellular matrix. From an investigation of dpt-null mouse corneas, it was apparent that dpt, particularly in the posterior stroma, plays a key role in fibrillar organisation, possibly by interacting with proteoglycans (Cooper et al. 2006). The ionic environment between the collagen fibrils is another contributing factor to the maintenance of fibrillar order. Transparency of the corneal stroma has been shown to be crucially dependent on the concentration of the ambient ion (Kostyuk et al. 2002), and Regini et al. (2004) showed that increasing the ionic strength (up to physiological levels) improves fibril ordering significantly, in agreement with the transparency data. It is believed that as the NaCl concentration is increased, chloride ions increasingly bind to the collagen fibrils (Elliott and Hodson 1998) and the fibrils become more and more electronegative, giving rise to extra repulsion between fibrils and leading to a higher level of ordering.
Corneal Lamellae
Corneal lamellae run like belts across the tissue and are typically up to 0.2 mm broad and 2 μm thick (Polack 1961; Komai and Ushiki 1991). In the human cornea there are distinct anatomical differences between the outermost and the deeper corneal lamellae. The anterior layers have a lamella density some 50% greater than those in the deep layers (Bergmanson et al. 2005); they frequently bifurcate and interweave (Radner et al. 1998) and are arranged in equal numbers within all orientations within the plane of the cornea. The posterior layers are more hydrated and are stacked rather like plywood, run from limbus to limbus and occur predominantly vertically and horizontally within the eye (Meek et al. 1987; Daxer and Fratzl 1997; Aghamohammadzadeh et al. 2004). The average arrangement of lamellae across the cornea (integrated thought the corneal thickness) is also not isotropic. In addition to the preferred orthogonal orientations in the pre-pupillary region, there appear to be invasive lamellae that enter and leave the tissue in the paracentral/peripheral region. At the limbus (where the cornea integrates with the adjacent sclera), there is a posteriorly located collagenous annulus (Newton and Meek 1998a, b), which is thought to maintain the change of curvature between the cornea and the rest of the eye (Boote et al. 2009). The arrangement of lamellae is symmetrical between left and right eyes (Boote et al. 2006), which may be of some clinical relevance as corneal grafts are carried out without concern for either orientation or left/right matching between donor and host.
There are interesting species differences in the organisation of the corneal lamellae. While all species studied to date show the presence of a circum-corneal collagen annulus, the vertical and horizontal central lamellae have only been observed in animals with high visual acuity. Other animals show either a uniaxial preferred orientation or a more circular orientation (Boote et al. 2004; Hayes et al. 2007). It is possible, therefore, that vertical and horizontal lamellae have evolved to withstand the forces of the extraocular muscles and allow greater precision of eye movement in certain species.
The substructure of the corneal lamellae has been extensively studied using polarised light, confocal microscopy and both scanning and transmission electron microscopy (reviewed by Meek and Fullwood 2001). Recently, there has been renewed interest in corneal lamellar structure because of the development of two-photon confocal microscopy, particularly with the use of second harmonic generated images (Han et al. 2005) (Fig. 4). Using these techniques in vitro, Morishige et al. (2006) showed in three-dimensions how the anterior corneal lamellae do not run from limbus to limbus, but, rather, work their way forward and terminate within Bowman’s membrane, which thus forms an anchor for these lamellae. These interfaces have been studied at high resolution using transmission microscopy (Mathew et al. 2008). The interwoven nature of the anterior stroma and the anchoring of the lamellae within Bowman’s membrane are thought to be important for the maintenance of normal corneal curvature (Müller et al. 2001). In vivo confocal tomography recently revealed that the collagen organisation immediately below Bowman’s membrane contains polymorphic fibrillar structures that represent the termination of these anterior lamellae within Bowman’s membrane (Kobayashi 2008).
Fig. 4.
Collagenous lamellae at different depths at the limbus, imaged by second harmonic generated two-photon microscopy. The aligned collagen at greater depths is the limbal annulus (courtesy of Dr. C. Kamma-Lorger)
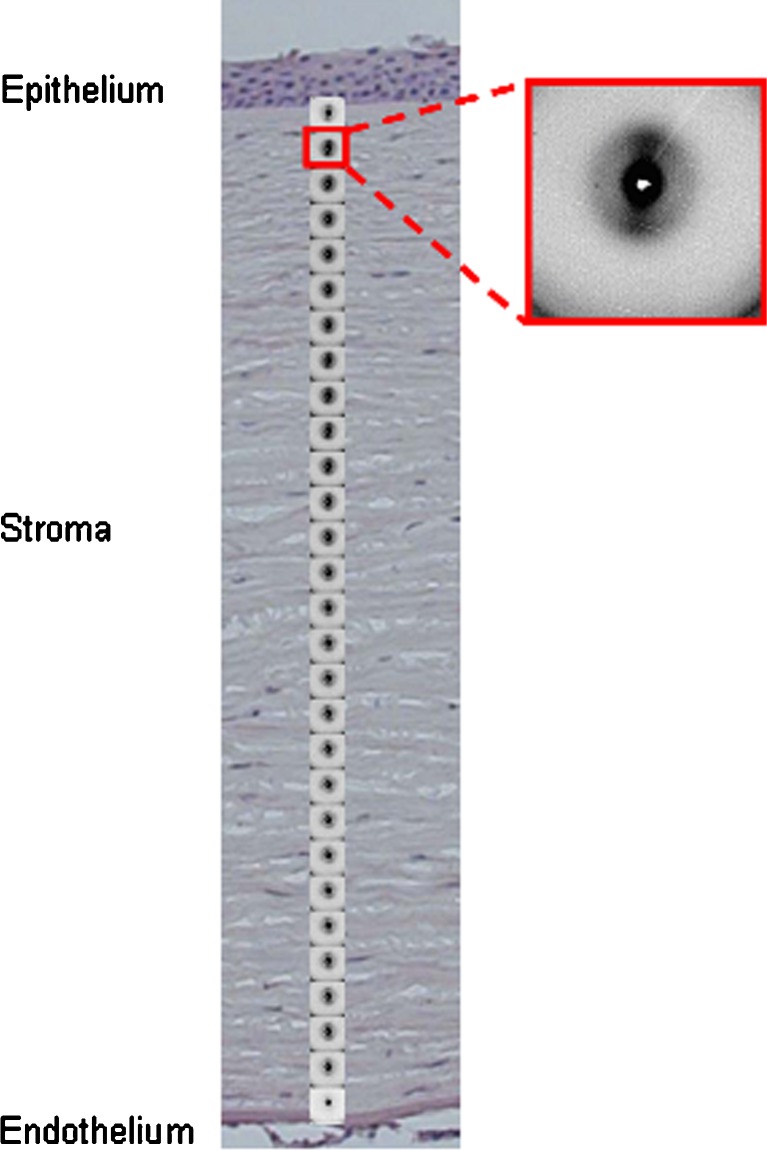
Recent developments in synchrotron radiation technology have also allowed the structure and organisation of corneal lamellae to be revisited. Using microfocus beams down to 5 nm in diameter, it has been possible to greatly increase the resolution previously available. By carefully examining the complex peripheral region of the cornea, Meek and Boote (2004) showed that, within a millimetre or so, overall lamella directions change continuously from being predominantly vertical and horizontal centrally to becoming a circular annulus at the limbus, and also that additional fibrils reinforce the two main meridians. The results obtained were consistent with the schematic shown in Fig. 5. Microfocus X-ray beams have also been used to examine strips of isolated corneal tissue edge-on, i.e. from anterior to posterior (Fig. 6). Using microfocus small angle scattering, Quantock et al. (2007) showed that there was no systematic change in average fibril diameter as a function of depth. These results seem to conflict with those from electron microscopy reported above (Freund et al. 1995) but are supported by more recent electron microscope measurements (Akhtar et al. 2008). With microfocus wide angle scattering, Kamma-Lorger et al. (2009) showed that the intermolecular spacing is relatively constant with tissue depth, indicating that there are no changes in the intrafibrillar water content, even when the bulk tissue hydration is changing. These new microfocus X-ray techniques thus hold some promise, particularly for studying the effects of treatments that may not penetrate the whole cornea, such as therapeutic corneal cross-linking (Wollensack et al. 2003).
Fig. 5.

Wide-angle X-ray scatter patterns taken across the cornea have allowed the preferential arrangement of the corneal lamellae to be mapped. Patterns at specific points around the limbus are shown here superimposed on a schematic of the changes in direction of the lamellae
Fig. 6.
Microfocus X-ray diffraction patterns taken through a thin strip of human cornea from anterior to posterior, shown superimposed on a histological section of a cornea
Transparency
The biophysical basis of why the cornea is transparent has intrigued scientists for many years. The collagen fibrils in the physiologically hydrated stroma have a refractive index of 1.41, whereas the material between has a refractive index of 1.36 (Leonard and Meek 1997) and, on the basis of this difference, the tissue should scatter a significant amount of the incident light (Meek et al. 2003a). In 1957, Maurice realised that the collagen fibrils in the corneal stroma do not act as independent scatterers and that the correlation in their relative positions leads to destructive interference of scattered light (Maurice 1957). In these early investigations, the assumption was that the fibrils possess long-range order. However, electron microscopy and X-ray diffraction have shown that there is no crystalline lattice but, rather, short-range order extending to about 120 nm (Sayers et al. 1982; Worthington and Inouye 1985). Fourier analysis of electron microscope images of corneal structure was consistent with this short-range order (Gisselberg et al. 1991).
Fibril packing models described as “liquid-like” or “short-range ordered” have been shown theoretically to produce a transparent cornea (Farrell and Hart 1969; Worthington 1984), but the processes responsible for the development and maintenance of such systems are difficult to define because they are intrinsically non-deterministic. Recently, there has been much interest in the special light-transmitting properties of a group of deterministic structures known as photonic crystals and quasicrystals (Vukusik and Sambles 2003; Prum and Torres 2004). In these structures, only certain frequencies of light are transmitted or reflected by a medium that consists of an array of two components with different dielectric constants, as is the case for the collagen fibrils and ground substance in the cornea. Photonic methods have been applied to the cornea (Ameen et al. 1998) but on the basis of a hexagonal arrangement of the collagen fibrils. Preliminary data suggest that the collagen fibrils in the cornea may be arranged as a photonic quasicrystal which shows long-range order but is predicted to display the same diffraction and light transmission properties as seen in the cornea (J. Doutch and KM Meek, unpublished).
Corneal swelling
It has long been known that a corneal button immersed in water or saline takes on considerable fluid and becomes opaque (Cogan and Kinsey 1942), and this has provided much insight into the biophysical properties of corneal collagen. Swelling takes place only in one direction (through the corneal thickness). The driving force for this swelling is a Donnan osmotic force generated by the high negative charge on the proteoglycans and also by chloride binding to proteins (Hodson et al. 1992). Maintenance of normal corneal thickness, and hence transparency, is provided in vivo by an outwardly directed electrogenic bicarbonate “pump” localised in the endothelial cells (Hodson and Miller 1976). When the cornea swells, electron microscopy shows that the collagen fibrils move apart, initially uniformly, but later irregularly, with the formation of collagen-free regions termed “lakes” (Kanai and Kaufman 1973). X-ray and neutron diffraction methods confirmed that interfibril spacing increases with hydration (Goodfellow et al. 1978; Elliott et al. 1982). They also showed that there is a linear relationship between (spacing)2 and hydration, the quadratic relationship arising because fibrils can only move apart in a plane perpendicular to the fibril major axis (Goodfellow et al. 1978). Furthermore, the spacing changes were the same regardless of the X-ray beam was perpendicular or parallel to the swelling. Since the cornea only swells in one direction, this suggests a certain amount of fluidity in fibril positioning once interfibrillar proteoglycan links are broken.
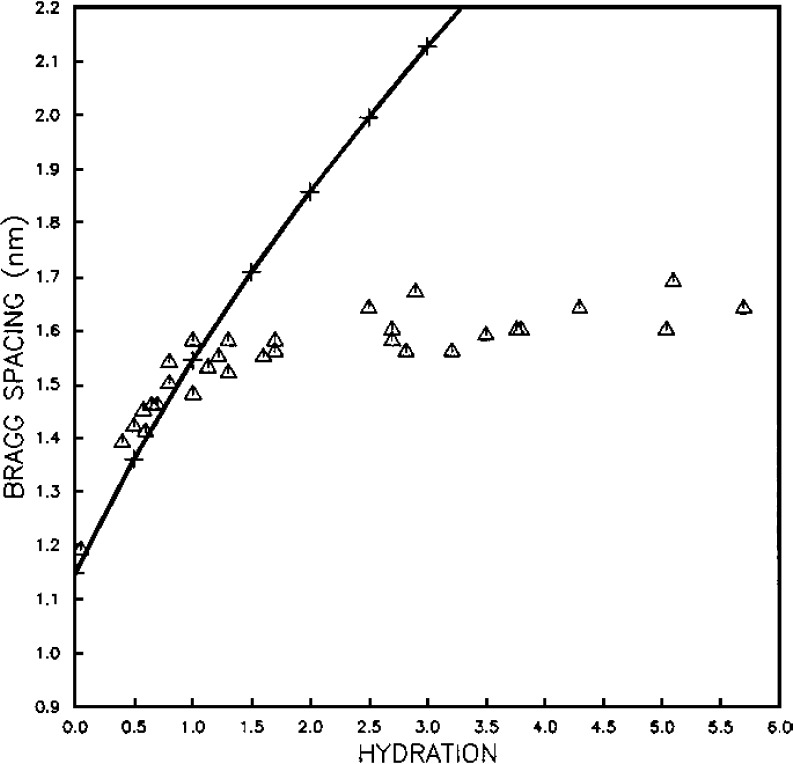
Subsequent X-ray diffraction studies monitored the effects of swelling on collagen in the cornea in more detail (Meek et al. 1991). Wide-angle diffraction showed that the Bragg spacing corresponding to the distance of closest approach of the collagen molecules (dry cornea) was 1.15 nm in the bovine cornea, increasing to 1.60 nm at physiological tissue hydration, which suggests that fibril diameters increased from 26 nm to 36 nm. Above this hydration, the intermolecular spacing (and hence the fibril diameter) changed very little, which is in agreement with earlier electron microscope (Payrau et al. 1967), birefringence (Maurice 1957) and diffusion (Maurice 1969) studies. A particularly interesting finding was that, as fibrils swell, their surfaces remain in contact until hydration H = 1 (hydration is defined as weight of water/dry weight of cornea). In other words, the initial water is distributed equally within and between the fibrils (Fig. 7), whereas after that, water is absorbed mainly by the matrix surrounding the fibrils. A similar two-stage process is observed when corneas dry: the initial water is lost from the interfibrillar space then, below H = 1, water is lost from within the fibrils themselves (Fratzl and Daxer 1993), suggesting that collagen fibrils in the corneal stroma are surrounded by an effectively cylindrical coating of proteoglycans, which appears as a 3-D fractal network. Understanding how water is compartmentalised within and between the collagen fibrils as the cornea swells has allowed us to quantify how, upon swelling, the refractive indices of the collagen fibrils and intefibrillar matrix change, and how this contributes to the light scattered by the cornea (Meek et al. 2003c).
Fig. 7.
Triangles show the variation of intermolecular Bragg spacing as a function of hydration for bovine cornea. Crosses show the variation of interfibrillar Bragg spacing as a function of hydration, normalised to the intermolecular data at H=0 (reproduced from Meek et al. (1991) with permission of the copyright holder)
Conclusions
The structure and organisation of corneal collagen is complex—and rather different to that found in other connective tissues. Although much is known about the formation and structure of corneal collagen, the mechanisms that give rise to helicoidal fibrils, the nature of the subfibrils and the diameter control mechanisms are still not fully understood. Similarly, the organisation of the fibrils and the way this is maintained require further study. These factors are vital for a full understanding of corneal transparency. New technologies are leading to a better appreciation of how higher level structural organisation supports the overall shape and strength of the tissue. This will be of some significance for understanding of corneal astigmatism and for devising methods for improving corneal refractive surgery.
Acknowledgements
The corneal research programme at Cardiff University is supported by the MRC, BBSRC, EPSRC, STFC and ESRF. Keith Meek is a Royal Society/Wolfson Merit Award holder. The author thanks Dr. C. Boote for helpful comments on the manuscript.
References
- Aghamohammadzadeh H, Newton RH, Meek KM. X-ray scattering used to map the preferred collagen orientation in the human cornea and limbus. Structure. 2004;12:249–256. doi: 10.1016/j.str.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Bron AJ, Salvi SM, Hawksworth NR, Tuft SJ, Meek KM. Ultrastructural analysis of collagen fibrils and proteoglycans in keratoconus. Acta Ophthalmol Scand. 2008;86:764–772. doi: 10.1111/j.1755-3768.2007.01142.x. [DOI] [PubMed] [Google Scholar]
- Ameen DB, Bishop MF, McMullen T. A lattice model for computing the transmissivity of the cornea and sclera. Biophys J. 1998;75:2520–2531. doi: 10.1016/S0006-3495(98)77697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselt DR, Revel JP, Baldeschweiler JD. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophys J. 1993;65:2644–2655. doi: 10.1016/S0006-3495(93)81329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- Bergmanson JP, Horne J, Doughty MJ, Garcia M, Gondo M. Assessment of the number of lamellae in the central region of the normal human corneal stroma at the resolution of the transmission electron microscope. Eye Contact Lens. 2005;31:281–287. doi: 10.1097/01.icl.0000165280.94927.0d. [DOI] [PubMed] [Google Scholar]
- Binder PS, Rock ME, Schmidt C, Anderson JA. High voltage electron microscopy of normal human cornea. Invest Ophthalmol Vis Sci. 1991;32:2234–2243. [PubMed] [Google Scholar]
- Birk DE. Type V collagen:heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Boote C, Dennis S, Newton RH, Puri H, Meek KM. Collagen fibrils appear more closely packed in the prepupillary cornea: optical and biomechanical implications. Invest Ophthalmol Vis Sci. 2003;44:2941–2948. doi: 10.1167/iovs.03-0131. [DOI] [PubMed] [Google Scholar]
- Boote C, Dennis S, Meek KM. Spatial mapping of collagen fibril organisation in primate cornea–an x-ray diffraction investigation. J Struct Biol. 2004;146:359–367. doi: 10.1016/j.jsb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Boote C, Hayes S, Abahussin M, Meek KM. Mapping collagen organization in the human cornea: left and right eyes are structurally distinct. Invest Ophthalmol Vis Sci. 2006;46:901–908. doi: 10.1167/iovs.05-0893. [DOI] [PubMed] [Google Scholar]
- Boote C, Hayes S, Young RD, Kamma-Lorger C, Hocking PM, Elsheikh A, Inglehearn CF, Ali M, Meek KM. Ultrastructural changes in the retinopathy, globe enlarged (rge) chick cornea. J Struct Biol. 2009;166(2):195–204. doi: 10.1016/j.jsb.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly:skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1988;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan DG, Kinsey VE. The cornea. 1. Transfer of water and sodium chloride by osmosis and diffusion through excised cornea. Arch Ophthalmol. 1942;27:466–482. [Google Scholar]
- Cooper LJ, Bentley AJ, Nieduszynski IA, Talabani S, Thomson A, Utani A, Shinkai H, Fullwood NJ, Brown GM. The role of dermatopontin in the stromal organisation of the cornea. Invest Ophthalmol Vis Sci. 2006;47:3303–3310. doi: 10.1167/iovs.05-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Farrell RA, Hart RW, Langham ME. The transparency of the mammalian cornea. J Physiol. 1970;210:601–616. doi: 10.1113/jphysiol.1970.sp009230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AS, Parry DAD. Collagen fibrils of the vertebrate cornea stroma. J Ultrastruct Res. 1981;74:232–239. doi: 10.1016/s0022-5320(81)80081-0. [DOI] [PubMed] [Google Scholar]
- Craig AS, Robertson JG, Parry DA. Preservation of corneal collagen fibril structure using low-temperature procedures for electron moicroscopy. J Ultrastruct Mol Struct Res. 1986;96:172–175. doi: 10.1016/0889-1605(86)90018-2. [DOI] [PubMed] [Google Scholar]
- Daxer A, Fratzl P. Collagen fibril organisation in the human corneal stroma and its implications in keratoconus. Invest Ophthalmol Vis Sci. 1997;38:121–129. [PubMed] [Google Scholar]
- Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma:structure and ageing. Invest Ophthalmol Vis Sci. 1998;39:644–647. [PubMed] [Google Scholar]
- Doughty MJ, Bergmanson JPG. Assessment of the apparent intra- and inter-sample variability in the collagen fibril diameter in the posterior corneal stroma of rabbits. A transmission electron microscope study. Ophthalmic Res. 2006;38:335–342. doi: 10.1159/000096228. [DOI] [PubMed] [Google Scholar]
- Elliott GF, Hodson SA. Cornea, and the swelling of polyelectrolyte gels of biological interest. Rep Prog Phys. 1998;61:1325–1365. [Google Scholar]
- Elliott GF, Sayers Z, Timmins PA. Neutron diffraction studies of the corneal stroma. J Mol Biol. 1982;155:389–393. doi: 10.1016/0022-2836(82)90011-0. [DOI] [PubMed] [Google Scholar]
- Farrell RA, Hart RW. On the theory of the spatial organisation of macromolecules in connective tissue. Bull Math Biophys. 1969;31:727–760. doi: 10.1007/BF02477784. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Daxer A. Structural transformation of collagen fibrils in corneal stroma during drying. An x-ray scattering study. Biophys J. 1993;64:1210–1214. doi: 10.1016/S0006-3495(93)81487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund DE, McCally RL, Farrell RA, Cristol SM, L’Hernault NL, Edelhauser HF. ultrastructure in anterior and posterior stroma of perfused human and rabbit corneas. Invest Ophthalmol Vis Sci. 1995;36:1508–1523. [PubMed] [Google Scholar]
- Gisselberg M, Clark JI, Vaezy S, Osgood TB. A quantitative evaluation of Fourier components in transparent and opaque calf cornea. Am J Anat. 1991;191:408–418. doi: 10.1002/aja.1001910408. [DOI] [PubMed] [Google Scholar]
- Goh KL, Holmes DF, Lu H-Y, Richardson S, Kadler KE, Purslow PP, Wess TJ. Ageing changes in the tensile properties of tendons: Influence of collagen fibril volume fraction. J Biomech Eng. 2008;130:1–8. doi: 10.1115/1.2898732. [DOI] [PubMed] [Google Scholar]
- Goodfellow JM, Elliott GF, Woolgar AE. X-ray diffraction studies of the corneal stroma. J Mol Biol. 1978;119:237–252. doi: 10.1016/0022-2836(78)90436-9. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Foley JW, Linsenmayer TF, Fitch JM. Temporal expression of types XII and XIV collagen mRNA and protein during avian corneal development. Dev Dyn. 1996;206:49–58. doi: 10.1002/(SICI)1097-0177(199605)206:1<49::AID-AJA5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gyi TJ, Meek KM, Elliott GF. Collagen interfibrillar distances in corneal stroma using synchrotron X-ray diffraction: a species study. Int J Biol Macromol. 1988;10:265–269. [Google Scholar]
- Han M, Giese G, Bille JF. Second harmonic generation imaging of collagen fibrils in cornea and sclera. Opt Express. 2005;13:5791–5797. doi: 10.1364/opex.13.005791. [DOI] [PubMed] [Google Scholar]
- Harding JJ, Crabbe MJC, Panjwani NA (1980) Corneal collagen. In: Robert L, Robert A (eds) Biochemistry of normal and pathological connective tissues. Colloques Internationaux du C.N.R.S. 287: 51–64
- Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, Kerr B, Hughes CE, Caterson B, Tanigami A, Nakayama J, Fukada MN, Tano Y, Nishida K, Quantock AJ. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O sulphotransferase. Proc Natl Acad Sci USA. 2006;103:13333–13338. doi: 10.1073/pnas.0605441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Boote C, Lewis J, Sheppard J, Abahussin M, Quantock AJ, Purslow C, Votruba M, Meek KM. Comparative study of fibrillar collagen arrangement in the corneas of primates and other mammals. Anat Rec. 2007;290:1542–1550. doi: 10.1002/ar.20613. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Prenant G, Renard G. The three-dimensional supramolecular organisation of the extracellular matrix in human and rabbit corneal stroma as revealed by ultrarapid-freezing and deep-etching methods. Exp Eye Res. 2001;72:123–135. doi: 10.1006/exer.2000.0935. [DOI] [PubMed] [Google Scholar]
- Hodson SA, Miller F. The bicarbonate ion pump in the endothelium which regulates the hydration of rabbit cornea. J Physiol. 1976;263:563–577. doi: 10.1113/jphysiol.1976.sp011645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson S, Kaila D, Hammond S, Rebello G, Al-Omari Y. Transient chloride binding as a contributory factor to corneal stromal swelling in the ox. J Physiol. 1992;450:89–103. doi: 10.1113/jphysiol.1992.sp019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DF, Kadler KE. The precision of lateral size control in the assembly of corneal collagen fibrils. J Mol Biol. 2005;345:773–784. doi: 10.1016/j.jmb.2004.10.078. [DOI] [PubMed] [Google Scholar]
- Holmes DF, Gilpin CJ, Baldock C, Ziese U, Koster AJ, Kadler KE. Corneal collagen fibril structure in three dimensions: structural insights into fibril assembly, mechanical properties, and tissue organization. Proc Natl Acad Sci USA. 2001;98:7307–7312. doi: 10.1073/pnas.111150598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SJ, Doane KJ. Type VI collagen increases cell survival and prevents anti-beta 1 integrin-mediated apoptosis. Exp Cell Res. 1998;241:230–241. doi: 10.1006/excr.1998.4051. [DOI] [PubMed] [Google Scholar]
- Huang Y, Bron AJ, Meek KM, Velodi A, McDonald B. Ultrastructural study of the cornea in a bone-marrow transplanted Hurler syndrome patient. Exp Eye Res. 1996;62:377–387. doi: 10.1006/exer.1996.0043. [DOI] [PubMed] [Google Scholar]
- Hulmes DJS, Wess TJ, Prockop DJ, Fratzl P. Radial packing, order, and disorder in collagen fibrils. Biophys J. 1995;68:1661–1670. doi: 10.1016/S0006-3495(95)80391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihanamäki T, Pelliniemi LJ, Vuorio E. Collagens and collagen-related matrix components in the human and mouse eye. Prog Retin Eye Res. 2004;23:403–434. doi: 10.1016/j.preteyeres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Itoh T, Klein L, Geil PH. Age dependence of collagen fibrils and subfibril diameters revealed by transverse freeze-fracture and -etching technique. J Microsc. 1981;125:343–357. [Google Scholar]
- Jayasuriya AC, Scheinbeim JI, Lubkin V, Bennett G, Kramer P. Piezoelectric and mechanical properties in bovine cornea. J Biomed Mater Res. 2003;66A:260–265. doi: 10.1002/jbm.a.10536. [DOI] [PubMed] [Google Scholar]
- Kamma-Lorger CS, Boote C, Young RD, Hayes S, Quantock AJ, Meek KM. Depth profile study of collagen molecular structure in normal human cornea. Acta Ophthalmol (Copenh) 2009;86:S243. [Google Scholar]
- Kanai A, Kaufman HE. Electron microscopic studies of swollen corneal stroma. Ann Ophthalmol. 1973;5:178–190. [PubMed] [Google Scholar]
- Knupp C, Lewis PN, Young, RD, Pinali, C, Meek, KM, Quantock AJ (2009) Three-dimensional electron tomography throws new light on the structure of the cornea. ARVO e-abstract 4532/D77. Available at www.iovs.org [DOI] [PubMed]
- Kobayashi A. In vivo laser confocal microscopic analysis of the interface between Bowman’s layer and the stroma of the cornea. Nippon Ganka Gakkai Zasshi. 2008;112:947–952. [PubMed] [Google Scholar]
- Koch M, Laub F, Zhou P, Hahn R, Tanaka S, Burgeson RE, Gerecke DR, Ramirez F, Gordon MK. Collagen XXIV, a vertebrate fibrillar collagen with structural features of invertebrate collagens: selective expression in developing cornea and bone. J Biol Chem. 2003;278:43236–43244. doi: 10.1074/jbc.M302112200. [DOI] [PubMed] [Google Scholar]
- Komai Y, Ushiki T. The three-dimensional organisation of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–2258. [PubMed] [Google Scholar]
- Kostyuk O, Nalovina O, Mubard TM, Regini JW, Meek KM, Quantock AJ, Elliott GF, Hodson SA. transparency of the bovine corneal stroma at physiological hydration and its dependence on concentration of the ambient ion. J Physiol. 2002;2:633–642. doi: 10.1113/jphysiol.2002.021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard DW, Meek KM. Refractive indices of the collagen fibrils and extrafibrillar material of the corneal stroma. Biophys J. 1997;72:1382–1387. doi: 10.1016/S0006-3495(97)78784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer TF, Gibney E, Igoe F, Gordon MK, Fitch JM, Fessler LI, Birk DE. Type V collagen: molecular structure and fibrillar organization of the chicken alpha-1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol. 1993;121:1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM. Ageing of the human corneal stroma: structural and biochemical changes. Biochim Biophys Acta. 1992;1138:222–228. doi: 10.1016/0925-4439(92)90041-k. [DOI] [PubMed] [Google Scholar]
- Marchini M, Morocutti M, Ruggeri A, Koch MHJ, Bigi A, Roveri N. Differences in the fibril structure of corneal and tendon collagen. An electron microscopy and x-ray diffraction investigation. Connect Tissue Res. 1986;15:269–281. doi: 10.3109/03008208609001985. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Wachtel E, Grushko G, Katz EP, Weinberg P. The effect of osmotic and mechanical pressures on water partitioning in articular cartilage. Biochim Biophys Acta. 1991;1073:285–294. doi: 10.1016/0304-4165(91)90133-2. [DOI] [PubMed] [Google Scholar]
- Mathew JH, Bergmanson JPG, Doughty MJ. Fine structure of the interface between the anterior limiting lamina and the anterior stromal fibrils of the human cornea. Invest Ophthalmol Vis Sci. 2008;49:3914–3918. doi: 10.1167/iovs.07-0707. [DOI] [PubMed] [Google Scholar]
- Maurice DM. The structure and transparency of the cornea. J Physiol. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DM. Cornea and Sclera. In: Davson H, editor. The eye. London: Academic Press; 1969. [Google Scholar]
- McCally RL, Freund DE, Zorn A, Bonney-Ray J, Grebe R, de la Cruz Z, Green WR. Light scattering and ultrastructure of healed penetrating corneal wounds. Invest Ophthalmol Vis Sci. 2007;48:157–165. doi: 10.1167/iovs.06-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM. The cornea and sclera. In: Fratzl P, editor. Collagen. Structure and biomechanics. New York: Springer Science +Business Media; 2008. pp. 359–396. [Google Scholar]
- Meek KM, Boote C. The organization of collagen in the corneal stroma. Exp Eye Res. 2004;78:503–512. doi: 10.1016/j.exer.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Meek KM, Fullwood NJ. Corneal and scleral collagens–a microscopists perspective. Micron. 2001;32:261–272. doi: 10.1016/s0968-4328(00)00041-x. [DOI] [PubMed] [Google Scholar]
- Meek KM, Holmes DF. Interpretation of the electron microscopical appearance of collagen fibrils from corneal stroma. Int J Biol Macromol. 1983;5:17–25. [Google Scholar]
- Meek KM, Leonard DW. The Ultrastructure of the corneal stroma: a comparative study. Biophys J. 1993;64:273–280. doi: 10.1016/S0006-3495(93)81364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Elliott GF, Sayers W, SB KM. Interpretation of the meridional X-ray diffraction pattern from collagen fibrils in corneal stroma. J Mol Biol. 1981;149:477–488. doi: 10.1016/0022-2836(81)90482-4. [DOI] [PubMed] [Google Scholar]
- Meek KM, Elliott GF, Nave C. A synchrotron x-ray diffraction study of bovine cornea stained with cupromeronic blue. Coll Res Rel. 1986;6:203–218. doi: 10.1016/s0174-173x(86)80026-7. [DOI] [PubMed] [Google Scholar]
- Meek KM, Blamires T, Elliott GF, Gyi T, Nave C. The organisation of collagen fibrils in the human corneal stroma: A synchrotron X-ray diffraction study. Curr Eye Res. 1987;6:841–846. doi: 10.3109/02713688709034853. [DOI] [PubMed] [Google Scholar]
- Meek KM, Fullwood NJ, Cooke PH, Elliott GF, Maurice DM, Quantock AJ, Wall RS, Worthington CR. Synchrotron X-ray diffraction studies of the cornea with implications for stromal hydration. Biophys J. 1991;60:467–474. doi: 10.1016/S0006-3495(91)82073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Leonard DW, Connon CJ, Dennis S, Meek KM. Transparency, swelling and scarring in the corneal stroma. Eye. 2003a;17:927–936. doi: 10.1038/sj.eye.6700574. [DOI] [PubMed] [Google Scholar]
- Meek KM, Quantock AJ, Boote C, Liu CY, Kao WW-Y. An x-ray scattering investigation of corneal structure in keratocan-deficient mice. Matrix Biol. 2003b;22:467–475. doi: 10.1016/s0945-053x(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Meek KM, Dennis S, Khan S. Changes in the refractive index of the stroma and its extrafibrillar matrix when the cornea swells. Biophys J. 2003c;85:2205–2212. doi: 10.1016/s0006-3495(03)74646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller D, Peters K, Meller K. Human cornea and sclera studied by atomic force microscopy. Cell Tissue Res. 1997;288:111–118. doi: 10.1007/s004410050798. [DOI] [PubMed] [Google Scholar]
- Miyagawa A, Kobayashi M, Fujita Y, Hamdy O, Hirano K, Nakamura M, Miyake Y. Surface ultrastructure of collagen fibrils and their association with proteoglycans in human cornea and sclera by atomic force microscopy and energy-filtering transmission electron microscopy. Cornea. 2001;20:651–656. doi: 10.1097/00003226-200108000-00019. [DOI] [PubMed] [Google Scholar]
- Morishige N, Petroll WM, Nishida T, Kenney MC, Jester JV. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J Cataract Refract Surg. 2006;32:1784–1791. doi: 10.1016/j.jcrs.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LJ, Pels E, Vrensen GFJM. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–443. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LJ, Pels E, Schurmans LRH, Vrensen GFJM. A new three-dimensional model of the organisation of proteoglycans and collagen fibrils in the human corneal stroma. Exp Eye Res. 2004;78:493–501. doi: 10.1016/s0014-4835(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Naylor EJ. Polarized light studies of corneal structure. Br J Ophthalmol. 1953;37:77–84. doi: 10.1136/bjo.37.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RH, Meek KM. Circum-corneal annulus of collagen fibrils in the human limbus. Invest Ophthalmol Vis Sci. 1998;39:1125–1134. [PubMed] [Google Scholar]
- Newton RH, Meek KM. The integration of the corneal and limbal fibrils in the human eye. Biophys J. 1998;75:2508–2512. doi: 10.1016/S0006-3495(98)77695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel JPRO, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci USA. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M. Hierarchical structures in fibrillar collagens. Micron. 2002;33:587–596. doi: 10.1016/s0968-4328(02)00033-1. [DOI] [PubMed] [Google Scholar]
- Parry DAD. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys Chem. 1988;29:195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- Parry DA, Craig AS. Electron microscope evidence for an 80A unit in collagen fibrils. Nature. 1979;282:213–215. doi: 10.1038/282213a0. [DOI] [PubMed] [Google Scholar]
- Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978;203:305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- Payrau P, Pouliquen Y, Faure JP, Offret G. La Transparence de la Cornée, la Mécanismes de ses Altérations. Paris: Masson et Cie; 1967. [Google Scholar]
- Pinsky P, Datye V. A microstructurally-based finite element model of the incised human cornea. J Biomech. 1991;10:907–922. doi: 10.1016/0021-9290(91)90169-n. [DOI] [PubMed] [Google Scholar]
- Polack FM. Morphology of the cornea. 1. Study with silver stains. Am J Ophthalmol. 1961;51:1051–1056. doi: 10.1016/0002-9394(61)91794-9. [DOI] [PubMed] [Google Scholar]
- Prum RO, Torres RH. Structural colouration of mammalian skin:convergent evolution of coherently scattering dermal collagen arrays. J Exp Biol. 2004;207:2157–2172. doi: 10.1242/jeb.00989. [DOI] [PubMed] [Google Scholar]
- Quantock AJ, Young RD. Development of the corneal stroma, and the collagen–proteoglycan associations that help define its structure and function. Dev Dyn. 2008;237:2607–2621. doi: 10.1002/dvdy.21579. [DOI] [PubMed] [Google Scholar]
- Quantock AJ, Meek KM, Fullwood NJ, Zabel RW. Scheie’s syndrome: the architecture of corneal collagen and distribution of corneal proteoglycans. Can J Ophthalmol. 1993;28:266–272. [PubMed] [Google Scholar]
- Quantock AJ, Meek KM, Chakravarti S. An x-ray diffraction investigation of corneal structure in lumican-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:1750–1756. [PubMed] [Google Scholar]
- Quantock AJ, Boote C, Young RD, Hayes S, Hidetoshi T, Kawasaki S, Ohta N, Iida T, Yagi N, Kinoshita S, Meek KM. Small-angle fibre diffraction studies of corneal matrix structure: a depth-profiled investigation of the human eye-bank cornea. J Appl Crystallography. 2007;40:s335–s340. [Google Scholar]
- Radner W, Zehetmayer M, Aufreiter R, Mallinger R. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea. 1998;17:537–543. doi: 10.1097/00003226-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Rawe IM, Leonard DW, Meek KM. X-ray diffraction and transmission electron microscopy of Morquio syndrome type A cornea: a structural analysis. Cornea. 1997;16:369–376. [PubMed] [Google Scholar]
- Regini JW, Elliott GF, Hodson SA. The ordering of corneal collagen fibrils with increasing ionic strength. J Mol Biol. 2004;336:179–186. doi: 10.1016/j.jmb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris) 2005;53:430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Sandberg-Lall M, Hagg PO, Wahlstrom I, Pihlajaniemi T. Type XIII collagen is widely expressed in the adult and developing human eye and accentuated in the ciliary muscle, the optic nerve and the neural retina. Exp Eye Res. 2000;70:401–410. doi: 10.1006/exer.1998.0826. [DOI] [PubMed] [Google Scholar]
- Sayers Z, Koch MHJ, Whitburn SB, Meek KM, Elliott GF, Harmsen A. Synchrotron x-ray diffraction study of corneal stroma. J Mol Biol. 1982;160:593–607. doi: 10.1016/0022-2836(82)90317-5. [DOI] [PubMed] [Google Scholar]
- Scott JE, Haigh M. ‘Small’-proteoglycan: collagen interactions: Keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the ‘a’ and ‘c’ bands. Biosci Rep. 1985;5:765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- Vukusik P, Sambles JR. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- Wess TJ. Collagen fibrillar structure and hierarchies. In: Fratzl P, editor. Collagen. Structure and mechanics. New York: Springer Science +Business Media; 2008. pp. 49–48. [Google Scholar]
- Wessel H, Anderson S, Fite D, Halvas E, Hempel J, SundarRaj N. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest Ophthalmol Vis Sci. 1997;38:2408–2422. [PubMed] [Google Scholar]
- Wollensack G, Spöerl E, Seiler T. Treatment of keratoconus by collagen crosslinking. Ophthalmologe. 2003;100:44–49. doi: 10.1007/s00347-002-0700-3. [DOI] [PubMed] [Google Scholar]
- Worthington CR. The structure of the cornea. Q Rev Biophys. 1984;17:423–451. doi: 10.1017/s003358350000487x. [DOI] [PubMed] [Google Scholar]
- Worthington CR, Inouye H. X-ray diffraction study of the cornea. Int J Biol Macromol. 1985;7:2–8. [Google Scholar]
- Yamamoto S, Hashizume H, Hitomi J, Shigeno M, Sawaguchi S, Abe H, Ushiki T. The subfibrillar arrangement of corneal and scleral collagens as revealed by scanning electron and atomic force microscopy. Arch Histol Cytol. 2000;63:127–135. doi: 10.1679/aohc.63.127. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Hitomi J, Sawaguchi S, Abe H, Shigeno M, Ushiki T. Observation of human corneal and scleral collagen fibrils by atomic force microscopy. Jpn J Ophthalmol. 2002;46:496–501. doi: 10.1016/s0021-5155(02)00558-0. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Chandler GS, Tanzawa H, Katz EP. Cross-linking and the molecular packing of corneal collagen. Biochem Biophys Res Commun. 1996;219:311–315. doi: 10.1006/bbrc.1996.0229. [DOI] [PubMed] [Google Scholar]