Abstract
Protein kinase C (PKC) is a family of serine/threonine protein kinases, and alterations have been found in PKC isoform expression and localization in the failing heart. These alterations in PKC activation levels influence the PKC-mediated phosphorylation status of cellular target proteins involved in Ca2+-handling and sarcomeric contraction. The differences observed in the effects due to PKC-mediated phosphorylation may underlie part of the contractile dysfunction observed in the failing heart. It is therefore important to establish the beneficial and detrimental effects of this kinase in the healthy and failing heart. The function of PKC has been studied intensively; however, the complexity of the regulation of this kinase makes the interpretation of the different effects difficult. The main focus of this review is the (patho)physiological impact of phosphorylation of sarcomeric proteins, myosin light chain-2, troponin I and T, desmin, myosin binding protein-C, and titin by PKC.
Keywords: Cardiac, Heart failure, Protein kinase C, Kinase, Phosphorylation, Sarcomeric proteins
Introduction
Protein kinase C (PKC) is a member of the serine/threonine protein kinase family and widely expressed in mammalian tissue. It is involved in numerous cellular processes, such as transcription of DNA, cell death, cell growth, Ca2+-handling, and also fine-tunes myofilament function. PKC isoforms have been implicated in several diseases, such as prostate and breast cancer, pulmonary disease, and heart failure (Belin et al. 2007; Konopatskaya and Poole 2010; Parker 2003; Parker and Murray-Rust 2004; Sumandea et al. 2004).
Phosphorylation of proteins involved in Ca2+-handling and myofilament proteins is an important regulator of myocardial performance. Kinase and phosphatase pathways are very complex and often intertwine, which makes it difficult to study the functional effects of one specific phosphoprotein, especially when it has multiple phosphorylation sites regulated by different kinases and phosphatases. It is therefore of great importance to investigate the complex pathways leading to protein (de)phosphorylation and to study the effects of PKC-mediated protein phosphorylation on contractile properties of the myocardium, both in health and disease (Agnetti et al. 2007).
Kinase-mediated regulation of myocardial performance: PKA and PKC
Catecholamine stimulation of the β-adrenergic receptors in the myocardium is important to adjust myocardial performance to meet increased demands of the heart as occurs upon increased stress (e.g., exercise). Stimulation of the β-adrenergic signaling pathway leads to an increased level of cyclic adenosine monophosphate (cAMP), which in turn activates protein kinase A (PKA). PKA is known to phosphorylate several Ca2+-handling and myofilament proteins and thereby regulates contractile function of the heart. For example, PKA-mediated phosphorylation of the ryanodine receptor (RyR2) located on the sarcoplasmatic reticulum (SR) increases the amount of calcium released by the RyR2 upon activation by calcium, which enters the cell via the L-type Ca2+-channels (i.e., PKA activation enhances the Ca2+-induced Ca2+-release). This increase in intracellular calcium enhances myocardial contractility and output of the heart (Lehnart et al. 2004). PKA-mediated phosphorylation of phospholamban increases re-uptake of calcium into the SR via the SR-Ca2+ATPase (SERCA2) and thereby exerts a positive lusitropic effect (i.e., enhanced relaxation). Accordingly, PKA-mediated phosphorylation of the myofilament protein troponin I (cTnI) at Ser23/24 (PKA sites on cTnI) decreases myofilament Ca2+-sensitivity and thereby contributes to an acceleration of the rate of cardiac relaxation (Gaponenko et al. 1999; Kentish et al. 2001; Layland et al. 2005; Solaro 2008). Phosphorylation of cardiac myosin binding protein-C (cMyBP-C) by PKA has been indicated to have a distinct role in the increase of the cross-bridge cycling kinetics (Stelzer et al. 2006), and PKA-mediated phosphorylation of the giant protein titin has been found to reduce passive stiffness (Granzier and Labeit 2004; Kruger and Linke 2006; van Heerebeek et al. 2006; Yamasaki et al. 2002).
Diverse changes in PKA-mediated phosphorylation in cardiac disease have been reported upon catecholamine overstimulation of the β-adrenergic receptors. The RyR2 receptors become hyperphosphorylated (Lehnart et al. 2004) and the myofilament proteins (cTnI, cMyBP-C, and titin) become hypophosphorylated (Hamdani et al. 2008; Solaro 2008; Solaro and de Tombe 2008), all of which seems to be detrimental for cardiac performance. There is also a leakage of calcium from the SR, thereby increasing the cytosolic Ca2+-levels during diastole, an enhanced myofilament Ca2+-sensitivity, and an increased passive stiffness that limits relaxation of the heart muscle.
Apart from β-adrenergic receptor-activated PKA, myocardial performance can be regulated by a different family of serine/threonine protein kinases, the PKC family. The regulatory role of PKC may be even larger during cardiac disease, as increased PKC activity and expression levels have been shown in the failing heart (Belin et al. 2007; Braz et al. 2004; Lamberts et al. 2007).
Activation and distribution of PKC isoforms
There are multiple known isozymes comprising the PKC family. These are divided into three subgroups that are structurally and functionally distinguished. The conventional PKC isoforms (PKCα, βI, βII, and γ) are diacylglycerol (DAG)-sensitive and Ca2+-responsive. The second group are the novel isoforms (PKC ε, ϕ, η and δ,) which are DAG-sensitive but Ca2+-insensitive. The third group comprises the atypical isoforms (PKC ζ and ι) which are not DAG- or Ca2+-sensitive (Cockcroft and Thomas 1992; Dempsey et al. 2000; Parker and Murray-Rust 2004). PKC can be regulated downstream of the multitude of receptors that couple to activation of phospholipase C, including Gq-coupled receptors (G-protein-coupled receptors) (Konopatskaya and Poole 2010; Parker and Murray-Rust 2004). Upon activation PKC has been shown to translocate to different sub-cellular sites through interactions with docking proteins.
There have been multiple studies exploring the expression and distribution of PKC isoforms in the healthy and failing heart; however, the exact regulation of the different PKC isoforms remains rather elusive. Differences in the expression and localization of PKC isoforms may be age- and species- dependent. Moreover, disease-induced changes in the PKC expression/activity profile may depend on the underlying cause and stage of the disease. In 1994, Rybin and Steinberg studied the PKC isoform expression pattern during cardiac development in the rat heart. Their results demonstrated the importance of age-dependence in PKC isoform expression in the heart. PKCα and PKCδ were observed in fetal and neonatal rat ventricular myocytes, but not in adult rat cardiomyocytes (Rybin and Steinberg 1994). In addition to age-dependency, there are also species differences in the expression of PKC isoforms in the heart. Bogoyevitch et al. (1993) showed that in the adult rat heart, PKCε is the most abundant isoform present, with endothelin-1 stimulation causing a rapid translocation and activation of PKCε in rat cardiomyocytes in vivo. In the human heart, Bowling et al. (1999) reported a significant increase in PKCβI, PKCβII, and PKCα in membrane fractions of tissue from failing hearts relative to non-failing hearts, but not in cytosolic fractions. In contrast, PKCε expression in the cytosol and membrane was not significantly different between failing and non-failing hearts (Bowling et al. 1999). PKCα, β, ε, ζ, and γ were found to be expressed in healthy rabbit hearts, but when heart failure was induced, western blotting showed a decreased PKC activity and expression of the PKC isoforms α, β1, γ, and ε (Rouet-Benzineb et al. 1996). Belin et al. (2007) studied PKCα quantity, activity, and signaling to myofilaments in rat with early and end-stage congestive heart failure and found that PKCα expression and activation was unaltered in early heart failure, but it did increase in end-stage heart failure.
PKC-mediated protein phosphorylation in the healthy and failing heart
Activation of the α-adrenergic receptors stimulates the phospholipase C-dependent hydrolysis of membrane phosphatidylinositol, which in turn results in the generation of the second messengers, namely, inositol triphosphate (IP3) and DAG. IP3 induces Ca2+ release from the SR, and DAG is the endogenous activator of the conventional and novel PKC isoforms (Konopatskaya and Poole 2010; Parker and Murray-Rust 2004; Rybin and Steinberg 1994). Once activated, PKC has several target proteins within the cardiac muscle cell. For example, myofilament proteins troponin, cMyBP-C, and titin (Hidalgo et al. 2009; Kooij et al. 2010a), but also cytoskeletal protein desmin and protein phosphatase inhibitor-1 (I-1) have been implicated as PKC substrates in vitro. Interestingly, Molnar et al. (2009) showed a significant increase in PKCα binding to the cardiomyofilament contractile proteins following application of Ca2+ (Molnar et al. 2009), indicating a role for PKCα-mediated phosphorylation on myofilament contractile function. Braz et al. (2004) stated that PKCα is also localized in a weak sarcomeric pattern in unstimulated adult cardiac myocytes and that upon stimulation with phorbol myristate acetate, PKCα translocates to the Z-lines and the T-tubular network.
In transgenic mice over-expressing PKCα, Braz et al. (2004) observed signs of cardiac hypertrophy at the age of 8 months. PKCα directly phosphorylated I-1 and thereby altered the activity of protein phosphatase-1 (PP-1). Via this pathway, changes in PKCα activity might indirectly dephosphorylate phospholamban (PLB) and thereby alter SR Ca2+ loading and the calcium transient (Braz et al. 2004).
Two studies by our group showed positive lusitropic effects with the application of the PKC catalytic subunit and the active PKCα and -ε isoforms in cardiomyocytes from human explanted end-stage failing hearts. PKC-mediated phosphorylation of cTnI, cardiac troponin T (cTnT), and cMyBP-C was accompanied by a decrease in Ca2+-sensitivity of force without any alteration of the maximal force (Kooij et al. 2010a; van der Velden et al. 2006). These results indicated that PKC-mediated phosphorylation of the myofilament proteins may be beneficial for the contractile function of the cardiac myofilaments as the desensitizing effect of PKC may exert a lusitropic effect. However, it remains to be determined whether PKC is active and localized at the myofilaments in the end-stage failing heart.
PKC-mediated phosphorylation of myosin light chain-2
The myofilament protein myosin light chain-2 (MLC-2) can be phosphorylated by myosin light chain kinase (MLCK) (Dabrowska et al. 1977, 1978; Walsh et al. 1979), which results in an increased Ca2+-sensitivity in the cardiac muscle (Morano et al. 1985; Clement et al. 1992). In rat skinned cardiac cells, this increase in Ca2+-sensitivity by MLCK was found to be further enhanced when PKC was added together with MLCK (Clement et al. 1992). Noland et al. (1993) investigated the role of MLC-2 phosphorylation by PKC by substituting PKC phosphorylated MLC-2 back into cardiac myofibrils from the bovine heart. Competitive replacement experiments showed that phosphorylation of MLC-2 by MLCK and/or PKC increased the maximal Ca2+-stimulated MgATPase activity without altering Ca2+-sensitivity. Venema et al. (1993) analyzed site-specificity using two-dimensional tryptic phosphopeptide mapping and observed two phosphopeptides for both MLCK and PKC; these appeared to be the same two phosphopeptides for both kinases. Using mass spectrometry (MS), Ser15 in rat cardiac MLC-2 was identified as a phosphorylation site (Fig. 1); the authors did not observe the second site using MS. Therefore, it remains to be established if there is a second phosphorylation site present in cardiac MLC-2 (Yuan et al. 2008).”
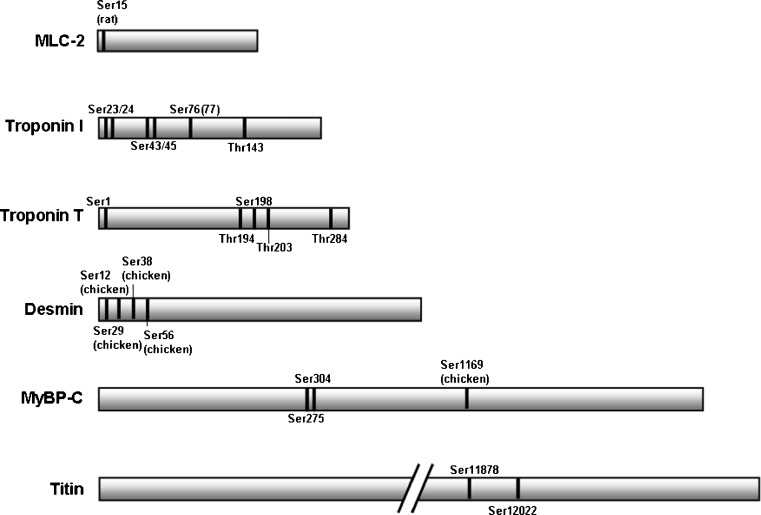
Fig. 1.
Schematic representation of protein kinase C (PKC) phosphorylation sites identified on the cardiac myofilament proteins. Phosphorylation sites of the human sequence are shown unless noted otherwise. MLC myosin light chain, MyBP-C myosin binding protein-C
PKC-mediated phosphorylation of troponin and changes therein during heart failure
Thin filament regulation
The cardiac troponin complex (cTn) is a thin filament regulatory protein complex that comprises three subunits: (1) cardiac TnI, the so-called inhibitory subunit, which inhibits actomyosin interactions at low levels of intracellular calcium; (2) cardiac troponin C (cTnC), which is the Ca2+ receptor subunit; (3) cTnT, which is the tropomyosin binding subunit. Cardiac TnI has a direct interaction with actin in the relaxed myofilament lattice, while cTnC is attached to the thin filament via interactions with both cTnT and cTnI (Solaro and Van Eyk 1996).
The phosphorylation status of cTnT and cTnI are prominent determinants of sarcomeric function, both in heath and disease. Walker et al. (2010) observed distinct changes in myofilament protein phophosphorylation, including troponin, in mice which underwent myocardial infarction. Two rat models that underwent pressure overload-induced left ventricular (LV) hypertrophy and myocardial infarction both showed depressed LV myofilament function. The authors showed that this was due, in part, to functionally important alterations in the cTn complex, which involve augmented phosphorylation levels as functional deficits that are partly restored to control values upon phosphatase treatment (Belin et al. 2007).
Analysis of the myofilament proteins from human heart samples in normal and pathological muscle has revealed unaltered cTnT phosphorylation in cardiac disease, while cTnI phosphorylation varies considerably and is significantly reduced in end-stage failing hearts compared to non-failing donor hearts (Marston et al. 2008; van der Velden et al. 2003). It has also been shown that both cTnI and cTnT are substrates for PKC in vitro, but the functional effects on contractility are less well defined compared to PKA-mediated phosphorylation of cTnI (Jideama et al. 1996). PKC is able to catalyze the incorporation of around 2 mol of phosphate/mol to cTnI and cTnT (Katoh et al. 1983).
Cardiac TnI is a target of multiple kinases, including PKA, PKG, PKD, and PKC, most of which have overlapping targets within the molecule (Belin et al. 2007; Cuello et al. 2007; Haworth et al. 2004; Kentish et al. 2001; Kooij et al. 2010a, b; Layland et al. 2002; Noland et al. 1989). Serines 23 and 24, which are located in the cardiac specific N-terminal domain (residues 1–30) of cTnI (absent in skeletal TnI) are the main targets of PKA (Kooij et al. 2010b; Solaro 2008). It has recently became evident that these sites can also be cross-phosphorylated by PKCδ, PKCα, PKCβ, PKG, and PKD (Table 1) (Haworth et al. 2004; Kobayashi et al. 2005; Layland et al. 2002, 2005; Swiderek et al. 1990; Sumandea et al. 2008). It has been generally accepted that phosphorylation of these sites is an important mechanism for increasing the rate of relaxation during β-adrenergic receptor stimulation in the heart. Also, cross-phosphorylation of Ser23/24 by PKC, PKG, or PKD, even though less efficient than that of PKA, has a similar effect on the sensitivity of the myofilaments to calcium, indicating a beneficial role of phosphorylation of these sites for the relaxation rate of the heart
Table 1.
Summary of the individual protein kinase C phosphorylation sites on all myofilament proteinsa
| Myofilament protein | PKC phospho-site | Primary or secondary PKC site | PKC isoform | Effect on the myofilament properties of the heart |
|---|---|---|---|---|
| MLC-2 | Ser15 | Secondary | Isoform unknown | - Phosphorylation of MLC-2 by PKC increased the maximal Ca2+-stimulated MgATPase activity without altering the Ca2+-sensitivity in cardiac rat whole myosin (Noland and Kuo 1993). |
| cTnI | Ser23/24 | Secondary | -PKCδ, PKCα, PKCβ, PKCδ | - PKCδ phosphorylation of bovine cTn complex decreased Ca2+-sensitivity of actomyosin S-1 MgATPase (Jideama et al. 1996). |
| -PKCβII, PKCε | - PKCδ-dependent phosphorylation decreased force development at submaximal Ca 2+ in skinned rat cardiomyocytes (Sumandea et al. 2008). | |||
| - Phospho mimicked Ser23/24 causes a decreased Ca2+-sensitivity (Lu et al. 2010). | ||||
| Ser43/45 | Primary | - PKCδ | - PKCδ and PKCα phosphorylation of bovine cTn complex reduced maximal activity of MgATPase (Jideama et al. 1996). | |
| - PKCα | - TG mice harboring unphosphorylatable sites showed systolic and diastolic lower intracellular Ca2+ and prolonged duration of Ca2+ transient (MacGowan et al. 2004). | |||
| - Papillary muscles from TG mice harboring unphosphorylatable alanines showed decreased maximal force upon α-adrenergic stimulation (Montgomery et al. 2002). | ||||
| - Phosphorylation mimicking reduces the maximal force of tension in skinned fibers from mice and in vitro motility assays (Burkart et al. 2003). | ||||
| - TG mice overexpressing PKCε and harboring non-phosphorylatable Ser43 and 45 sites showed less declination in LV function compared to the PKCε mice, indicating that PKCε phosphorylation of Ser43 and 45 plays a prominent role in depressing contractility in normal, healthy mice (Scruggs et al. 2006). | ||||
| - Skinned fibers from mice harboring alanines at sites 43 and 45 showed deceased maximal tension and increased MgATPase activity. PKC activation increased maximum tension and stiffness (Pyle et al. 2006). | ||||
| Ser76(77) | Unknown | Isoform unknown | - No data available on site specific function (Zabrouskov et al. 2008). | |
| Thr143 | Primary | -PKCβII | - PKCβII showed a phosphorylation preference for Thr144 and showed a decreased Ca2+ -sensitivity of force in skinned myocytes from TG mice (Wang et al. 2006). | |
| -PKCδ | - Phosphorylation mimicked Thr143 causes a depression of Hill coefficient in the presence of Ser23/24 phospho in skinned mice muscle fibers (Lu et al. 2010). | |||
| - cTnI Thr143 In rat skinned trabeculae modulates length-dependent activation (Tachampa et al. 2007). | ||||
| - Alanine substitution of Thr143 in reconstituted thin filaments showed involvement in NEM-S1-dependent activation of ATPase activity in the absence of Ca2+ (Kobayashi et al. 2009). | ||||
| cTnT | Ser1 | Primary | Isoform unknown | - No data available on site specific function (Sancho Solis et al. 2008) |
| Ser198 | Primary | Unknown | - No functional effects measured (Sumandea et al. 2003). | |
| Thr194 | Primary | Isoform unknown | - No functional effects measured (Sumandea et al. 2003). | |
| Thr203 | Primary | PKCα | - Phospho mimicking in mice resulted in decreased maximal tension, actomyosin Mg-ATPase activity, Ca2+-sensitivity and cooperativity (Sumandea et al. 2003) | |
| Thr284 | Primary | Unknown | - No functional effects measured (Sumandea et al. 2003) | |
| MyBP-C | Ser304 | Secondary | PKCε | - PKCε overexpressing mice developed dilated cardiomyopathy at 9–12 months of age (Xiao et al. 2007). |
| - TG mice with alanine substitution of phosphorylation sites displayed depressed cardiac contractility and altered sarcomeric structure (Sakthivel et al. 2005). | ||||
| Ser275 | Secondary | Isoform unknown | - TG mice with alanine substitution of phosphorylation sites displayed depressed cardiac contractility and altered sarcomeric structure (Sakthivel et al. 2005). | |
| Titin | Ser11878/ 12022 | Primary | PKCα | - PKCα phosphorylation increases passive force (Hidalgo et al. 2009) |
PKC, Protein kinase; MLC-2, myosin light chain-2; cTnl, cardiac troponin I; cTnT, cardiac troponin T; MyBP-C, myosin binding protein-C
The table lists whether the sites are primary or secondary substrates for protein kinase C (PKC)-mediated phosphorylation and the site specificity of the different PKC isoforms (as far as known). The function of the individual sites are described and their role in the propensity towards heart failure. The myofilament protein desmin has not been included in the table since, to the best of our knowledge; there is no site-specific information available for desmin
Effects of troponin I phosphorylation: transgenic animal studies
PKC is able to phosphorylate cTnI at Ser23/24, Ser43/45, Thr143, and Ser76 (or Thr77) (Fig.1, Table 1) (Kobayashi et al. 2005; Noland et al. 1989; Swiderek et al. 1990; Zabrouskov et al. 2008). Sakthivel et al. (2005) studied the effects of cTnI phosphorylation by PKA and PKC using transgenic mice models in which either all five phosphorylation sites on cTnI (Ser23/24, Ser43/45, and Thr143) were changed into aspartic acid to mimic complete phosphorylation, or only the PKA sites (Ser23/24). Their results showed that PKC-mediated phosphorylation of cTnI plays a primary role in reducing maximal Mg2+-ATPase activity, whereas the role of PKA phosphorylation is to mediate decreased Ca2+-sensitivity. The primary functional consequence of PKC-mediated cTnI phosphorylation in vivo is decreased contractility at baseline and increased relaxation during β-adrenergic stimulation (Sakthivel et al. 2005).
Transgenic mice in which two cTnI amino acids (Ser43/45) were mutated to unphosphorylatable alanines (A) (cTnI S43A/S45A) have been used to study the effects of PKC target sites in cTnI. PKC phosphorylation sites Ser43 and Ser45 on cTnI were found to regulate maximum tension. In one study, muscles from transgenic mice showed a 45% reduction in maximal force, while the fiber bundles of the transgenic mice in a different study showed a 13% decrease in maximum tension and a 20% increase in maximum MgATP activity compared to fibers from wild-type mice (Pyle et al. 2002; Montgomery et al. 2002). Burkart et al. (2003) studied phosphorylated cTnI at sites Ser43 and 45 using charge mutations in skinned fibers from mice and in vitro motility assays. Their results showed that charge change at these PKC sites inhibits the actin–myosin interaction by causing decreases in maximum tension, Ca2+-sensitivity, and thin filament sliding speed.
Residue Thr143 on cTnI is also known to be a major phosphorylation site for PKC, but the effects of cTnI phosphorylation at Thr143 still remain elusive. Thr143 is a cardiac-specific residue (proline in skeletal muscle) located in the inhibitory region of cTnI (residues 137–148 of the human cTnI sequence). In the absence of Ca2+, this region interacts with actin and thereby inhibits actomyosin ATPase activity, while upon Ca2+ binding to cTnC, the inhibitory region interacts with the N-terminal regulatory domain of cTnC, allowing actin to interact with myosin (Kobayashi and Solaro 2005; Kobayashi et al. 2009; McKay et al. 1999). Alanine substitution of Thr143 did not have any significant effect on the Ca2+-sensitivity of force, but there was the involvement of strong cross-bridge-dependent activation of the thin filaments in the absence of Ca2+ (Table 1) (Kobayashi et al. 2009). Also, a reduction in myofilament length-dependent activation has been observed in cardiac rat trabeculae expressing slow skeletal troponin (ssTn), which has a proline at position 122 versus a threonine at position 143 in cTnI (Table 1) (Tachampa et al. 2007). Pseudophosphorylation of Thr143 has been shown to desensitize the filaments to Ca2+ in an in vitro motility assay (Burkart et al. 2003), while phosphorylation of Thr143 by PKCβII in permeabilized cadiac myocytes from mouse in which the PKA sites Ser23/24 were replaced with alanines to avoid cross-phosphorylation showed an increase in the Ca2+-sensitivity of tension. Replacement of Thr143 with alanine abolished the response to PKC-βII treatment. These authors also showed that PKCβII phosphorylated the PKA sites Ser23/24 in wild-type myofibrils and demonstrated that such cross-phosphorylation resulted in a rightward shift of the pCa-tension curve (Table 1) (Wang et al. 2006). In a more recent study by Lu et al. (2010), pseudophosphorylation of Thr143 with glutamic acid (T143E) and of Ser23/24 with aspartic acid (S23D/S24D/T143E) caused no alterations in Ca2+-sensitivity upon exchange with cTn(T143E) compared to a decrease measured in the group exchanged with (S23D/S24D/T143E), suggesting that phosphorylation of Thr143 does not affect Ca2+-sensitivity. However, the latter study does demonstrate that cTnI(T143E) depresses the Hill coefficient (nH) in the presence of cTnI(S23D/S24D), which is a measure of the cooperative behavior of the cardiac thin filaments (Table 1) (Lu et al. 2010).
Effects of PKC-mediated phosphorylation of troponin T
Phosphorylation sites in cTnT are localized in the T2 domain at Thr 194, 203, 284, and Ser 198 (Fig. 1) (human sequence, isoform 3). Noland et al. (1989) showed that PKC phosphorylates Thr190 and Thr199 in bovine cTnT (Noland et al. 1989), and Swiderek et al. demonstrated the phosphorylation of Ser194 also occurs in bovine cTnT (Swiderek et al. 1990). The Ser1 of cTnT was also reported in earlier studies to be a phosphorylation site (Fig. 1) (Gusev et al. 1980). This site was ‘forgotten’ for a long time, but re-emerged in the mass spectrometry measurements on human heart troponin by Sancho Solis and co-workers who found that cTnT is almost completely constutively monophosphorylated. They subsequently reported Ser1 as the most likely candidate of PKC (Marston and Walker 2009; Sancho Solis et al. 2008).
The functional effects of PKC-mediated phosphorylation of cTnT are not as extensively studied as those of cTnI. The first studies suggested that PKC-mediated phosphorylation exerts a negative inotropic effect. Phosphorylation of cTnI and cTnT by PKC was found to cause a reversible inhibition of Ca2+-stimulated actomyosin MgATPase activity in the reconstituted contractile apparatus from bovine hearts, with little or no effect on Ca2+-sensitivity. However, under the latter conditions, both cTnI and cTnT were phosphorylated (Noland and Kuo 1991). An additional study indicated that PKC-mediated phosphorylation of cTnT lowered the apparent affinity for binding tropomyosin(Tm)-F-actin, which correlated with the inhibition of Ca2+-stimulated actomyosin MgATPase activity (Noland and Kuo 1992). A more direct study in which transgenic mice lacking PKC phosphorylation sites expressed approximately 50% fast skeletal TnT (fsTnT) provided evidence for a potential role of cTnT phosphorylation in depressing/reducing myofilament force (Montgomery et al. 2001).
Sumandea et al. (2003) studied the specific functional effects by charge mutating the cTnT sites (Thr197, Ser201, Thr206, Thr287, mouse sequence) into aspartic acid and replacing the recombinant cTn into detergent-treated mouse LV papillary muscle fibers. Phospho-mimicking of Thr206, but not the other sites, in the TG mice resulted in decreased maximal tension, actomyosin Mg-ATPase activity, Ca2+-sensitivity, and cooperativity (Table 1).
The cytoskeletal protein desmin as PKC substrate
Desmin is the major intermediate filament protein in cardiac muscle where it forms a three-dimensional scaffold which seems to extend across the entire diameter of the cardiac muscle cell. The intermediate filaments surround the Z-discs, which are associated with other organelles, and extend from the Z-disc to the plasma membrane (Capetanaki 2000). An analysis of specific desmin fragments indicated that desmin is a phosphoprotein (Evans 1988). In an in vitro study, Kitamura et al. (1989) identified four phosphorylation sites as substrates for PKC phosphorylation, namely, Ser12, Ser29, Ser38, and Ser56 (chicken sequence) (Fig. 1), which are all located in the head domain of desmin. A more recent study demonstrated that desmin is also phosphorylated by PKC in skinned myofibrils of hamster cells. These authors stated that desmin from failing hamster hearts contains more phosphorylated serines than that of the normal control hearts. It has also been shown that PKC-mediated phosphorylation of desmin can induce a disassembly of desmin filaments, which may be involved in myofibril disarray (Huang et al. 2002). This latter study indicates that PKC-mediated phosphorylation of desmin has a detrimental effect on myocardial structure. To the best of our knowledge, no PKC phosphorylation targets have been reported on human desmin.
Cardiac MyBP-C contains several PKC phosphorylation sites
In several animal and human studies, the total phosphorylation of cMyBP-C has been found to be decreased in hypertrophy and end-stage heart failure, suggesting a role of cMyBP-C in the modulation of cardiac function (El Armouche et al. 2007; Hamdani et al. 2008; Sadayappan et al. 2005). Lim et al. (1985) reported that cMyBP-C from bovine is a target for PKC phosphorylation. Ser265 (Ser275 human sequence), Ser300 (Ser304 human sequence), and Ser1169 are the major PKC-mediated phosphorylation sites found in chicken cMyBP-C (Fig. 1, Table 1). There is no equivalent of site Ser1169 in mouse and human, which suggests that this site is specific for chicken. Mohamed et al. (1998) demonstrated that site Ser1169 in chicken is phosphorylated by PKC to 2.0 mol phosphate/mol. Xiao et al. (2007) reported that Ser302 on mouse cMyBP-C is a PKCε phosphorylation site both in vivo and in vitro. A specific role for PKC-mediated phosphorylation of cMyBP-C on the contractility and during heart failure has yet to be determined. However, it has been suggested that cMyBP-C phosphorylation by PKC influences actomyosin Mg2+-ATPase activity, the kinetics of cross-bridge cycling, and the rate of relaxation (Barefield and Sadayappan 2010).
Effect of PKC-mediated titin phosphorylation
The giant protein titin has been found to be a phosphorylation target for several kinases, among which are PKA (Kruger and Linke 2006; Yamasaki et al. 2002) and PKG (Kruger et al. 2009). Both the PKA- and PKG-mediated phosphorylation of titin have been correlated with a reduction of the passive tension in human and rat myocardial tissue. Using in vitro phosphorylation assays and mass spectrometry, Hidalgo et al. (2009) recently identified that the PEVK element of titin functions as a PKCα substrate. The authors identified two highly conserved sites, i.e. Ser11878 and Ser12022, as PKCα phosphorylation sites (Fig. 1, Table 1). They also observed that PKCα increases passive tension in mouse and bovine tissue. This effect was found to be reversible by the dephosphorylation of titin by protein phosphatase 1. In contrast to the observed increase in passive force, our own study showed a slight but significant decrease in passive tension when single human cardiomyocytes from end-stage failing tissue were incubated with PKCα (Kooij et al. 2010a). However, using ProQ Diamond phosphorylation staining of titin, we did not find an increase in titin phosphorylation after incubation with PKCα, possibly because of the low sensitivity of the assay. In addition to these results, incubation of cardiomyocytes from end-stage failing tissue with the catalytic subunit of PKC also showed a slight decrease in passive tension (van der Velden et al. 2006). Taken together, these results suggest that PKC-mediated phosphorylation of titin most likely exerts a detrimental effect on passive stiffness of the heart muscle cells, but its role in the human heart remains to be determined.
Future perspectives
Protein kinase C is able to phosphorylate multiple myofilament proteins, with cTn, cMyBP-C, titin and also desmin having been reported as a target protein. The phosphorylation of individual sites on these proteins have been shown to have different function. Phosphorylation of cTnI at Ser43/45 has been implicated in the reduction of maximum tension, while phosphorylation of the PEVK region of titin has been implicated in an increase in passive tension. Thr143 phosphorylation by PKC has been implicated in alterations in Ca2+-sensitivity, but also to be involved in the cooperativity of the myofilaments by depression of the Hill coefficient. Overall, most biochemical and transgenic studies indicate a detrimental inotropic and/or lusitropic effect of PKC-mediated phosphorylation of the cardiac myofilament proteins on the contractility of the heart. However, our studies using human skinned cardiomyocytes incubated with PKC showed a reduction in the Ca2+-sensitivity, indicating a positive lusitropic effect for the failing heart. These results indicate that most PKC effects are detrimental, but that the effects of PKC-mediated cTn phosphorylation on the myofilament Ca2+-sensitivity of force is less straightforward and that more research is warranted.
Since the above-mentioned effects of PKC-mediated phosphorylation of myofilament proteins are able to greatly influence the contractile function of the heart, it is important to establish the function of PKC in the in vivo situation. An important question that must be answered is whether the myofilament proteins are actually targeted in the human failing heart, and if so, under which conditions. Studies determining the expression and translocation of the isoforms in the failing heart are therefore very important and must be studied in detail to gain more insights in the function of PKC in the human failing heart.
References
- Agnetti G, Kane LA, Guarnieri C, Caldarera CM, van Eyk JE. Proteomic technologies in the study of kinases: novel tools for the investigation of PKC in the heart. Pharmacol Res. 2007;55:511–522. doi: 10.1016/j.phrs.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Parker PJ, Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and it is activated by phorbol esters, epinephrine, and endothelin. Circ Res. 1993;72:757–767. doi: 10.1161/01.res.72.4.757. [DOI] [PubMed] [Google Scholar]
- Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003;278:11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y. Desmin cytoskeleton in healthy and failing heart. Heart Fail Rev. 2000;5:203–220. doi: 10.1023/A:1009853302447. [DOI] [PubMed] [Google Scholar]
- Clement O, Puceat M, Walsh MP, Vassort G. Protein kinase C enhances myosin light-chain kinase effects on force development and ATPase activity in rat single skinned cardiac cells. Biochem J. 1992;285(Pt 1):311–317. doi: 10.1042/bj2850311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S, Thomas GM. Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem J. 1992;288(Pt 1):1–14. doi: 10.1042/bj2880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello F, Bardswell SC, Haworth RS, Yin X, Lutz S, Wieland T, Mayr M, Kentish JC, Avkiran M. Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circ Res. 2007;100:864–873. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- Dabrowska R, Aromatorio D, Sherry JM, Hartshorne DJ. Composition of the myosin light chain kinase from chicken gizzard. Biochem Biophys Res Commun. 1977;78:1263–1272. doi: 10.1016/0006-291X(77)91429-2. [DOI] [PubMed] [Google Scholar]
- Dabrowska R, Sherry JM, Aromatorio DK, Hartshorne DJ. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978;17:253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- Marston SB, Tombe PP. Troponin phosphorylation and myofilament Ca(2+)-sensitivity in heart failure: Increased or decreased? J Mol Cell Cardiol. 2008;45(5):603–607. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- El Armouche A, Pohlmann L, Schlossarek S, Starbatty J, Yeh YH, Nattel S, Dobrev D, Eschenhagen T, Carrier L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J Mol Cell Cardiol. 2007;43:223–229. doi: 10.1016/j.yjmcc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Evans RM. The intermediate-filament proteins vimentin and desmin are phosphorylated in specific domains. Eur J Cell Biol. 1988;46:152–160. [PubMed] [Google Scholar]
- Gaponenko V, Abusamhadneh E, Abbott MB, Finley N, Gasmi-Seabrook G, Solaro RJ, Rance M, Rosevear PR. Effects of troponin I phosphorylation on conformational exchange in the regulatory domain of cardiac troponin C. J Biol Chem. 1999;274:16681–16684. doi: 10.1074/jbc.274.24.16681. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94:284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- Gusev NB, Dobrovolskii AB, Severin SE. Isolation and some properties of troponin T kinase from rabbit skeletal muscle. Biochem J. 1980;189:219–226. doi: 10.1042/bj1890219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, dos Remedios CG, Duncker DJ, Stienen GJM, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2008;77:649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, Avkiran M. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H. PKC phosphorylation of titin's PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105(631–8):17. doi: 10.1161/CIRCRESAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Li J, Foster D, Lemanski SL, Dube DK, Zhang C, Lemanski LF. Protein kinase C-mediated desmin phosphorylation is related to myofibril disarray in cardiomyopathic hamster heart. Exp Biol Med (Maywood) 2002;227:1039–1046. doi: 10.1177/153537020222701113. [DOI] [PubMed] [Google Scholar]
- Jideama NM, Noland TA, Jr, Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF. Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J Biol Chem. 1996;271:23277–23283. doi: 10.1074/jbc.271.38.23277. [DOI] [PubMed] [Google Scholar]
- Katoh N, Wise BC, Kuo JF. Phosphorylation of cardiac troponin inhibitory subunit (troponin I) and tropomyosin-binding subunit (troponin T) by cardiac phospholipid-sensitive Ca2+-dependent protein kinase. Biochem J. 1983;209:189–195. doi: 10.1042/bj2090189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Ando S, Shibata M, Tanabe K, Sato C, Inagaki M. Protein kinase C phosphorylation of desmin at four serine residues within the non-alpha-helical head domain. J Biol Chem. 1989;264:5674–5678. [PubMed] [Google Scholar]
- Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yang X, Walker LA, Van Breemen RB, Solaro RJ. A non-equilibrium isoelectric focusing method to determine states of phosphorylation of cardiac troponin I: identification of Ser-23 and Ser-24 as significant sites of phosphorylation by protein kinase C. J Mol Cell Cardiol. 2005;38:213–218. doi: 10.1016/j.yjmcc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Patrick SE, Kobayashi M. Ala scanning of the inhibitory region of cardiac troponin I. J Biol Chem. 2009;284:20052–20060. doi: 10.1074/jbc.M109.001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopatskaya O, Poole AW. Protein kinase Calpha: disease regulator and therapeutic target. Trends Pharmacol Sci. 2010;31:8–14. doi: 10.1016/j.tips.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij V, Boontje N, Zaremba R, Jaquet K, dos Remedios CG, Stienen GJM, van der Velden J. Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca2+ sensitivity in human myocardium. Basic Res Cardiol. 2010;105:289–300. doi: 10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij V, Saes M, Jaquet K, Zaremba R, Foster DB, Murphy AM, dos Remedios C, van der Velden J, Stienen GJM. Effect of troponin I Ser23/24 phosphorylation on Ca(2+)-sensitivity in human myocardium depends on the phosphorylation background. J Mol Cell Cardiol. 2010;48:954–963. doi: 10.1016/j.yjmcc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil. 2006;27:435–444. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- Lamberts RR, Hamdani N, Soekhoe TW, Boontje NM, Zaremba R, Walker LA, de Tombe PP, van der Velden J, Stienen GJM. Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J Physiol. 2007;582:695–709. doi: 10.1113/jphysiol.2007.134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Kushnir A, Marks AR. Cardiac ryanodine receptor function and regulation in heart disease. Ann N Y Acad Sci. 2004;1015:144–159. doi: 10.1196/annals.1302.012. [DOI] [PubMed] [Google Scholar]
- Lim MS, Sutherland C, Walsh MP. Phosphorylation of bovine cardiac C-protein by protein kinase C. Biochem Biophys Res Commun. 1985;132:1187–1195. doi: 10.1016/0006-291X(85)91932-1. [DOI] [PubMed] [Google Scholar]
- Lu QW, Hinken AC, Patrick SE, Solaro RJ, Kobayashi T. Phosphorylation of cardiac troponin I at protein kinase C site threonine-144 depresses cooperative activation of thin filaments. J Biol Chem. 2010;285(16):11810–11817. doi: 10.1074/jbc.M109.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGowan GA, Evans C, Hu TC, Debrah D, Mullet S, Chen HH, McTiernan CF, Stewart AF, Koretsky AP, Shroff SG. Troponin I protein kinase C phosphorylation sites and ventricular function. Cardiovasc Res. 2004;63:245–255. doi: 10.1016/j.cardiores.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Marston SB, Walker JW. Back to the future: new techniques show that forgotten phosphorylation sites are present in contractile proteins of the heart whilst intensively studied sites appear to be absent. J Muscle Res Cell Motil. 2009;30:93–95. doi: 10.1007/s10974-009-9184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay RT, Tripet BP, Pearlstone JR, Smillie LB, Sykes BD. Defining the region of troponin-I that binds to troponin-C. Biochemistry. 1999;38:5478–5489. doi: 10.1021/bi9829736. [DOI] [PubMed] [Google Scholar]
- Mohamed AS, Dignam JD, Schlender KK. Cardiac myosin-binding protein C (MyBP-C): identification of protein kinase A and protein kinase C phosphorylation sites. Arch Biochem Biophys. 1998;358:313–319. doi: 10.1006/abbi.1998.0857. [DOI] [PubMed] [Google Scholar]
- Molnar A, Borbely A, Czuriga D, Ivetta SM, Szilagyi S, Hertelendi Z, Pasztor ET, Balogh A, Galajda Z, Szerafin T, Jaquet K, Papp Z, Edes I, Toth A. Protein kinase C contributes to the maintenance of contractile force in human ventricular cardiomyocytes. J Biol Chem. 2009;284:1031–1039. doi: 10.1074/jbc.M807600200. [DOI] [PubMed] [Google Scholar]
- Montgomery DE, Chandra M, Huang Q, Jin J, Solaro RJ. Transgenic incorporation of skeletal TnT into cardiac myofilaments blunts PKC-mediated depression of force. Am J Physiol Heart Circ Physiol. 2001;280:H1011–H1018. doi: 10.1152/ajpheart.2001.280.3.H1011. [DOI] [PubMed] [Google Scholar]
- Montgomery DE, Wolska BM, Pyle WG, Roman BB, Dowell JC, Buttrick PM, Koretsky AP, Del Nido P, Solaro RJ. alpha-Adrenergic response and myofilament activity in mouse hearts lacking PKC phosphorylation sites on cardiac TnI. Am J Physiol Heart Circ Physiol. 2002;282:H2397–H2405. doi: 10.1152/ajpheart.00714.2001. [DOI] [PubMed] [Google Scholar]
- Morano I, Hofmann F, Zimmer M, Ruegg JC. The influence of P-light chain phosphorylation by myosin light chain kinase on the calcium sensitivity of chemically skinned heart fibres. FEBS Lett. 1985;189:221–224. doi: 10.1016/0014-5793(85)81027-9. [DOI] [PubMed] [Google Scholar]
- Noland TA, Jr, Kuo JF. Protein kinase C phosphorylation of cardiac troponin I or troponin T inhibits Ca2(+)-stimulated actomyosin MgATPase activity. J Biol Chem. 1991;266:4974–4978. [PubMed] [Google Scholar]
- Noland TA, Jr, Kuo JF. Protein kinase C phosphorylation of cardiac troponin T decreases Ca(2+)-dependent actomyosin MgATPase activity and troponin T binding to tropomyosin-F-actin complex. Biochem J. 1992;288(Pt 1):123–129. doi: 10.1042/bj2880123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland TA, Jr, Kuo JF. Phosphorylation of cardiac myosin light chain 2 by protein kinase C and myosin light chain kinase increases Ca(2+)-stimulated actomyosin MgATPase activity. Biochem Biophys Res Commun. 1993;193:254–260. doi: 10.1006/bbrc.1993.1617. [DOI] [PubMed] [Google Scholar]
- Noland TA, Jr, Raynor RL, Kuo JF. Identification of sites phosphorylated in bovine cardiac troponin I and troponin T by protein kinase C and comparative substrate activity of synthetic peptides containing the phosphorylation sites. J Biol Chem. 1989;264:20778–20785. [PubMed] [Google Scholar]
- Parker PJ. Protein kinase C phosphorylation: an introduction. Methods Mol Biol. 2003;233:159–162. doi: 10.1385/1-59259-397-6:159. [DOI] [PubMed] [Google Scholar]
- Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- Pyle WG, Sumandea MP, Solaro RJ, de Tombe PP. Troponin I serines 43/45 and regulation of cardiac myofilament function. Am J Physiol Heart Circ Physiol. 2002;283:H1215–H1224. doi: 10.1152/ajpheart.00128.2002. [DOI] [PubMed] [Google Scholar]
- Pyle WG, La Rotta G, de Tombe PP, Sumandea MP, Solaro RJ. Control of cardiac myofilament activation and PKC-betaII signaling through the actin capping protein, CapZ. J Mol Cell Cardiol. 2006;41:537–543. doi: 10.1016/j.yjmcc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Rouet-Benzineb P, Mohammadi K, Perennec J, Poyard M, Bouanani N, Crozatier B. Protein kinase C isoform expression in normal and failing rabbit hearts. Circ Res. 1996;79:153–161. doi: 10.1161/01.res.79.2.153. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994;74:299–309. doi: 10.1161/01.res.74.2.299. [DOI] [PubMed] [Google Scholar]
- Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97:1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthivel S, Finley NL, Rosevear PR, Lorenz JN, Gulick J, Kim S, VanBuren P, Martin LA, Robbins J. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem. 2005;280:703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- Sancho Solis R, Ge Y, Walker JW. Single amino acid sequence polymorphisms in rat cardiac troponin revealed by top-down tandem mass spectrometry. J Muscle Res Cell Motil. 2008;29:203–212. doi: 10.1007/s10974-009-9168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs SB, Walker LA, Lyu T, Geenen DL, Solaro RJ, Buttrick PM, Goldspink PH. Partial replacement of cardiac troponin I with a non-phosphorylatable mutant at serines 43/45 attenuates the contractile dysfunction associated with PKCepsilon phosphorylation. J Mol Cell Cardiol. 2006;40:465–473. doi: 10.1016/j.yjmcc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J Biol Chem. 2008;283:26829–26833. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro RJ, de Tombe PP. Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Cardiovasc Res. 2008;77:616–618. doi: 10.1093/cvr/cvn004. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Van Eyk J. Altered interactions among thin filament proteins modulate cardiac function. J Mol Cell Cardiol. 1996;28:217–230. doi: 10.1006/jmcc.1996.0021. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99:884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003;278:35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- Sumandea MP, Burkart EM, Kobayashi T, de Tombe PP, Solaro RJ. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann N Y Acad Sci. 2004;1015:39–52. doi: 10.1196/annals.1302.004. [DOI] [PubMed] [Google Scholar]
- Sumandea MP, Rybin VO, Hinken AC, Wang C, Kobayashi T, Harleton E, Sievert G, Balke CW, Feinmark SJ, Solaro RJ, Steinberg SF. Tyrosine phosphorylation modifies protein kinase C delta-dependent phosphorylation of cardiac troponin I. J Biol Chem. 2008;283:22680–22689. doi: 10.1074/jbc.M802396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiderek K, Jaquet K, Meyer HE, Schachtele C, Hofmann F, Heilmeyer LM., Jr Sites phosphorylated in bovine cardiac troponin T and I. Characterization by 31P-NMR spectroscopy and phosphorylation by protein kinases. Eur J Biochem. 1990;190:575–582. doi: 10.1111/j.1432-1033.1990.tb15612.x. [DOI] [PubMed] [Google Scholar]
- Tachampa K, Wang H, Farman GP, de Tombe PP. Cardiac troponin I threonine 144: role in myofilament length dependent activation. Circ Res. 2007;101:1081–1083. doi: 10.1161/CIRCRESAHA.107.165258. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJM. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003;57:37–47. doi: 10.1016/S0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbely A, Zaremba R, Bronzwaer JG, Papp Z, Jaquet K, Paulus WJ, Stienen GJM. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res. 2006;69:876–887. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJM, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- Venema RC, Raynor RL, Noland TA, Jr, Kuo JF. Role of protein kinase C in the phosphorylation of cardiac myosin light chain 2. Biochem J. 1993;294(Pt 2):401–406. doi: 10.1042/bj2940401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LA, Walker JS, Ambler K, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol. 2010;48(6):1180–1186. doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Vallet B, Autric F, Demaille JG. Purification and characterization of bovine cardiac calmodulin-dependent myosin light chain kinase. J Biol Chem. 1979;254:12136–12144. [PubMed] [Google Scholar]
- Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. PKC-betaII sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41:823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Xiao L, Zhao Q, Du Y, Yuan C, Solaro RJ, Buttrick PM. PKCepsilon increases phosphorylation of the cardiac myosin binding protein C at serine 302 both in vitro and in vivo. Biochemistry. 2007;46:7054–7061. doi: 10.1021/bi700467k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.RES.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- Yuan C, Sheng Q, Tang H, Li Y, Zeng R, Solaro RJ. Quantitative comparison of sarcomeric phosphoproteomes of neonatal and adult rat hearts. Am J Physiol Heart Circ Physiol. 2008;295:H647–H656. doi: 10.1152/ajpheart.00357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabrouskov V, Ge Y, Schwartz J, Walker JW. Unraveling molecular complexity of phosphorylated human cardiac troponin I by top down electron capture dissociation/electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2008;7:1838–1849. doi: 10.1074/mcp.M700524-MCP200. [DOI] [PubMed] [Google Scholar]