Abstract
Skeletal muscle is a highly organized tissue that has to be optimized for fast signalling events conveying electrical excitation to contractile response. The site of electro-chemico-mechanical coupling is the skeletal muscle triad where two membrane systems, the extracellular t-tubules and the intracellular sarcoplasmic reticulum, come into very close contact. Structure fits function here and the signalling proteins DHPR and RyR1 were the first to be discovered to bridge this gap in a conformational coupling arrangement. Since then, however, new proteins and more signalling cascades have been identified just in the last decade, adding more diversity and fine tuning to the regulation of excitation-contraction coupling (ECC) and control over Ca2+ store content. The concept of Ca2+ entry into working skeletal muscle has become attractive again with the experimental evidence summarized in this review. Store-operated Ca2+ entry (SOCE), excitation-coupled Ca2+ entry (ECCE), action-potential-activated Ca2+ current (APACC), and retrograde EC-coupling (ECC) are new concepts additional to the conventional orthograde ECC; they have provided fascinating new insights into muscle physiology. In this review, we discuss the discovery of these pathways, their potential roles, and the signalling proteins involved that show that the triad may become a crowded place in time.
Keywords: Skeletal muscle, T-system, Triad, DHPR, RyR, Ca2+ sparks, Retrograde coupling, Store-operated Ca2+ entry, Excitation-coupled Ca2+ entry, Action-potential-activated Ca2+ entry
Introduction
Skeletal muscle is a highly organized tissue that is optimized for fast contractile activation and relaxation in order to serve multiple mechanical demands, from single twitches to more physiological brief or extended bouts of tetani. The process linking membrane excitation to mechanical contraction is called excitation-contraction coupling (ECC), and it was a milestone to discover that ECC is conveyed by a fast conformational coupling of two key molecules forming a protein-protein link: the ryanodine receptor (RyR) in the sarcoplasmic reticulum membrane and the dihydropyridine receptor (DHPR) in the sarcolemmal t-tubule. For a long time it has been thought that, apart from the tubular ion channels responsible for action potential transmission, the DHPR was the only major protein present in the tubules for trans-tubular signalling. Exciting new experiments in the last couple of years, however, have revealed more and more neighbors the DHPR must share the narrow tubular space with. Most of these new proteins are indeed functional channels involved in signalling cascades apart from the EC-coupling process.
This review aims to give an overview of current concepts involved in trans-tubular signalling events that are associated with ECC, retrograde signalling, and the recently discovered Ca2+ entry pathways connected to store-depletion, excitation, and membrane depolarization even during single action potentials. The different pathways and the associated channel proteins are referenced to the key publications in the field. In view of all these different channels, we will discuss whether the triad will indeed get more crowded over the years. The current review does not aim to cover the equally exciting developments related to SR proteins regulating transtubular-SR signalling.
Elementary calcium release events
Excitation-contraction coupling (ECC) in skeletal and cardiac muscle is initiated by the interaction of two calcium channels: (1) the plasma membrane L-type calcium channel (LCC) or dihydropyridine receptor (DHPR) and (2) the intracellular ryanodine receptor Ca2+ release channel (RyR) located in the sarcoplasmic reticulum (SR) membrane. Elementary RyR channel activity and DHPR-RyR interaction in these cells is often studied with confocal Ca2+ fluorescence imaging, where elementary Ca2+ release events (ECRE) via RyR Ca2+ channel clusters, with or without control exerted by the DHPR, can be observed. Elementary calcium release events are localized, short events of SR Ca2+ release detectable by fluorescence microscopy. Ryanodine-receptor-mediated ECRE and in particular, calcium “sparks” were initially described and studied in cardiac myocytes, skeletal and smooth muscle cells (Cheng et al. 1993; Cheng and Lederer 2008). Today, ECRE comprise a large family of events with different spatiotemporal characteristics occurring in a variety of cell types expressing ryanodine- and 1,4,5-inositoltrisphosphate (IP3) receptors. Recently, the history and current state of “sparkology” have been reviewed in detail (Cheng and Lederer 2008).
Here, we want to briefly discuss future challenges related to elementary calcium release events in adult mammalian skeletal muscle. Spontaneous calcium release events in fully polarized resting fibers are extremely rare. Upon depolarization, amphibian skeletal muscle fibers show distinct and monomorphic Ca2+ sparks, whereas their mammalian counterparts show longer lasting, low amplitude events termed (lone) embers (Zhou et al. 2003). Under certain experimental conditions, however, mammalian skeletal muscle fibers also show clear sparks, embers, and several morphological combinations of these. In the first study to reveal these events (ECRE) in adult mammalian fibers, chemical and mechanical membrane skinning was used (Kirsch et al. 2001). The study revealed a wealth of morphologically different elementary Ca2+ release events occurring spontaneously in saponin-permeabilized adult mammalian skeletal muscle fibers. The most obvious difference in this preparation is the absence of DHPR-mediated voltage control over RyR activity. However, the same study showed that—in contrast to the common belief that in the presence of functional DHPR voltage sensors, ECRE and in particular Ca2+ sparks cannot occur—mechanically skinned fibers with resealed t-tubules could be activated by depolarization with high [Na+] solution, and then showed distinct Ca2+ sparks preceding whole cell Ca2+ transients (Kirsch et al. 2001). Other effects however, such as the loss of small diffusible RyR regulating molecules and cytosolic Ca2+ buffers, have not been taken into account or quantified yet.
Of the many open questions concerning ECRE regulation, one becomes especially salient in the context of skinned mammalian fibers. Which mechanisms initiate and terminate ECRE? Despite significant effort, there is no conclusive answer to this question. In permeabilized fibers, a continuum of sparks, embers, sparks followed by embers, embers terminating in a spark, and polymorphic repetitive events can be observed, challenging the question of a unique mechanism of Ca2+ release control in these preparations. The crucial question of which trans-SR-membrane currents counterbalance Ca2+ release via arrays of RyR channels during individual ECRE has recently been treated computationally (Gillespie and Fill 2008). Experimental data, however, are sparse.
Another exciting line of research opened recently when the effects of mechanical stress on healthy and dystrophic skeletal muscle fibers were related to the properties of global and elementary Ca2+ release. Initially, it was reported that Ca2+ release in muscle fibers from mdx-mice lacking the large intracellular protein dystrophin are highly sensitive to the combination of hypercalcium extracellular medium and mechanical stress as induced by osmotic challenges (Wang et al. 2005). In a subsequent study (Teichmann et al. 2008), it could be shown that also under physiological concentrations of extracellular Ca2+, osmotic challenge elicits ECRE and travelling waves of Ca2+ release in dystrophic muscle and that RyR1 channels in these cells are activated via mechanosensitive cation channels (MsC). Moreover, in the same study it was shown that Ca2+ dependent and independent pathways contribute to this effect (Teichmann et al. 2008).
Taken together, these results lead to an even more intricate picture of calcium currents and signalling pathways in the skeletal muscle triad. Integrating the roles of DHP receptors, SR proteins [e.g., calsequestrin, triadin, junctin (Beard et al. 2009)], mechanosensitive channels and cytoskeletal proteins themselves, and finally the more recently discovered mechanisms of Ca2+ entry reviewed in this article is a major challenge on the way towards a comprehensive model of SR Ca2+ release control.
Retrograde RyR-DHPR coupling
Excitation-contraction coupling (ECC) in skeletal and cardiac muscle is initiated by DHPR-RyR interaction; the molecular architecture and the mechanism of channel interaction, however, show large differences between both cell types (Melzer et al. 1995). Functionally, skeletal and cardiac L-type channels act as voltage sensors and are activated by membrane depolarization, usually during an action potential when channels undergo a fast conformational change within milliseconds. Activation of the L-type Ca2+ current in skeletal muscle, however, is a slow process (∼50–100 ms) compared with the duration of the skeletal muscle action potential (∼2–5 ms) and therefore, under physiological conditions, no significant Ca2+ current is mediated by this channel. Experimentally, this is reflected by the fact that skeletal muscle fibers maintain Ca2+-dependent contractility for sustained periods when repetitively depolarized in a Ca2+-free extracellular medium. In contrast, during the cardiac action potential, which lasts for several hundred milliseconds, a significant number of Ca2+ ions enter the cytosol and activate the type 2 ryanodine receptor (RyR2) in a process known as calcium-induced calcium release (CICR) (Melzer et al. 1995).
RyR activation by the skeletal muscle DHPR is a much faster process mediated by conformational coupling of both molecules (Melzer et al. 1995). Although the exact molecular nature of this protein-protein interaction is not yet known completely, several amino acid segments on both channel proteins have been found to regulate this process. It is clear, however, that the II-III loop of the DHPR α1S subunit is essential for ECC (Bannister 2007). Further RyR interaction sites are found within the cytoplasmic DHPR β1a subunit, the α1S III-IV loop, and the α1S C-terminus. Interestingly, the experimental evidence for the long-held view of a direct physical link between the DHPR and the RyR only is not compelling. For a thorough discussion of this topic and the potentially crucial, hitherto neglected role of the β1a subunit of the DHPR, possibly acting as the missing “dancing partner” between both Ca2+ channels, the reader is referred to the review of A. Dulhunty and co-workers (2009) in this Journal.
As a convention, the term “orthograde coupling” refers to RyR activation by conformational coupling to the DHPR, and “retrograde coupling” refers to altered L-type channel gating in the presence of RyR. Such a novel, retrograde coupling by which the RyR alters the gating kinetics of the DHPR was first reported by Ma and colleagues (Ma et al. 1991). They found faster activation and inactivation kinetics of DHPRs incorporated in lipid bilayers when purified RyR1 was added to the cis-chamber. However, the term “retrograde signaling” was coined several years later when DHPR-RyR interactions were investigated by electrophysiological characterization of dyspedic skeletal muscle myotubes (Nakai et al. 1996). Dyspedic myotubes have a homozygous mutation of the RyR1 gene and do not express functional RyR Ca2+ release channels. Consequently, EC-coupling cannot be observed in this genotype. Interestingly, also the L-type Ca2+ current density (ICa) in these cells was ∼30-fold reduced, and both ECC and ICa were partially restored by injection of cells with RyR1-cDNA (Nakai et al. 1996).
Two natural questions arise from the aforementioned findings: (1) How is the retrograde signal mediated, e.g., only by direct conformational coupling, and/or other factors? (2) Which segments of the DHPR and RyR are involved in the process, i.e., are they the same segments that mediate the orthograde signal?
Regarding the first question, mainly two mechanisms have been considered. First, a direct protein-protein interaction between DHP receptors and RyR and second, DHPR activation by Ca2+ ions released from the SR via the RyR Ca2+ channel. The first model is suggested by the early lipid bilayer experiments (Ma et al. 1991) where soluble cytosolic factors could be excluded. Further experiments using RyR1/RyR2 chimeras showed that the RyR1 aa-residues 2659-3720 (chimera R9) and 1635-2636 (chimera R10) mediate both ortho- and retrograde coupling. The same experiments basically ruled out the second model of interaction, i.e., that retrograde coupling is connected to RyR1 Ca2+ release, as R9 restores retrograde coupling but not ECC (Nakai et al. 1998).
The conformational coupling model however, was challenged by recent findings showing that extrajunctional RyR3 mediates a retrograde signal to the DHPR in dyspedic myotubes without restoring tetrade formation, i.e., the skeletal muscle-specific spatial alternating arrangement of DHP and ryanodine receptors, where each other RyR channel faces a group of four DHP receptors and where DHPR-RyR coupling takes place (Sheridan et al. 2006). This result indicates a mechanism different from a direct physical link between both molecules. Other mechanisms of interaction discussed involve covalent modification of the DHPR, reactive oxygen species, and nitrosylation (Durham et al. 2008; Andronache et al. 2009). Thus, indirect models of interaction will have to be tested, possibly involving other molecular “players” in the crowded field of the triad junction and especially in the macromolecular complex of the tetrade.
The second question regarding which segments of DHPR and RyR1 contribute to the retrograde signal has also been addressed using molecular chimeras. After a series of reports elucidating the critical role of the DHPR II-III loop in EC-coupling, a short 46 amino acid sequence (s53, Leu720-Leu765) of the skeletal muscle DHPR α1S subunit was shown to restore both orthograde and retrograde DHPR-RyR1 coupling (Grabner et al. 1999). Within this sequence, a minimum 15 amino acid segment (Asp734-Asp748) was found to mediate bidirectional coupling (Kugler et al. 2004). Furthermore, four of those amino acids were identified to mediate orthograde EC-coupling (Ala739, Phe741, Pro742, Asp744), two of which (Ala739, Phe741) were shown also to be crucial for retrograde signaling. On the RyR, an interaction motif within the R10 chimera, the sR16 region (RyR1 aa 1837-2168), was shown to contribute to bidirectional coupling. However, this region is not essential as substitution of an RyR1 segment containing sR16 for the corresponding cardiac RyR2 analogue showed full bidirectional coupling (Proenza et al. 2002). In the recent study of Sheridan and colleagues, RyR3 segments in RyR1/RyR3 chimeras were unexpectedly found to support retrograde signaling whereas a large N-terminal RyR1 segment (aa 1-1681) was only involved in orthograde signaling.
An important pathophysiological implication of altered bidirectional coupling was reported recently in the context of malignant hyperthermia using RyR1Y522S knock-in mice (Andronache et al. 2009). Animals with this mutation suffer from potentially lethal episodes of hyperthermia triggered by elevated ambient temperatures. The pathophysiological correlate of this disorder is an uncontrolled, massive Ca2+ release from the SR. In adult muscle fibers with the RyR1Y522S mutation, the voltage-dependent activation and inactivation curves of the DHPR are left-shifted, i.e., towards more negative potentials. This marked left-shift of voltage-dependent DHPR-inactivation induced by the retrograde RyR1Y522S signal controls the extent of window Ca2+ release and can be interpreted as a compensatory mechanism that limits SR Ca2+ release.
Store-operated Ca2+ entry (SOCE) in skeletal muscle
Fifteen years after suggesting a model for store-dependent Ca2+ entry that involved IP3-signalling in nonexcitable exocrine gland cells (Putney 1986), store-operated Ca2+ entry (SOCE), previously termed Ca2+-release-activated Ca2+ current (CRAC) or capacitive Ca2+ current was demonstrated in adult rat skeletal muscle for the first time by Kurebayashi and Ogawa (2001). Before, SOCE had been identified in T-lymphocytes (Zweifach and Lewis 1995) and endothelial tissue (Holder and Blatter 1997). In their original study, Kurebayashi and Ogawa used intact thin mouse EDL fiber bundles that they subjected to short (30 s) high K+ pulses in Ca2+-free solution and recorded force transients and fura-2 Mn2+ influx. After 7–10 successive K+ contractures, a steady-state partial depletion with force transients down to ∼15% of the initial amplitude was reached that could be further lowered by severe SR depletion upon addition of 10 µM CPA to block SERCA1 so that force was diminished by the 15th or so high K+ contracture. When extracellular Ca2+ was raised to 2 mM, Mn2+ influx increased tremendously. When the SERCA blockers were washed out, Mn2+ influx became smaller and force transients increased during successive K+ contractures as the store refilled with extracellular Ca2+ (Kurebayashi and Ogawa 2001). Subsequent attempts to identify the SOCE pathways showed that Ca2+ entry was blocked by Ni2+ and 2-APB (Prakriya and Lewis 2001) but was insensitive to nifedipine (Kurebayashi and Ogawa 2001) or verapamil (Pan et al. 2002), and operated at negative membrane potentials with large driving force, as Ca2+ entry stopped at depolarized membrane potentials (Kurebayashi and Ogawa 2001).
Studies in dysgenic myotubes that lack α1sDHPR showed that SOCE worked normally and that Ca2+ entry did not occur via DHPRs at resting membrane potentials where L-type Ca2+ conductance would be virtually zero in wild-type fibers also (Cherednichenko et al. 2004). Likewise, RyR1 is not essentially required for SOCE, as SOCE was still present in dyspedic myotubes (Cherednichenko et al. 2004), although some reduction in Mn2+ quench rates in dyspedic myotubes (Lyfenko and Dirksen 2008) and in myotubes from mice lacking RyR1/RyR3 or the synaptophysin-family-related protein mitsugumin-29 in the triad junction suggests a modulation of SOCE by the release channels (Pan et al. 2002; Zhao et al. 2006). Other myotube studies have established a link between mitochondrial depolarization and reduced SOCE (Vandebrouck et al. 2006) or sarcolemmal TRPC1 channels anchored to the dystrophin-associated glycoprotein complex via α1-syntrophin with compromised SOCE in dystrophic myotubes where this molecular lattice would be disrupted (Vandebrouck et al. 2007). Another early study found a store-dependent capacitive Ca2+ current in cultured myotubes that was mediated by Ca2+-specific leak channels and, interestingly, inhibited by dihydropyridines (Hopf et al. 1996).
When interpreting the possible physiological role of SOCE in the different studies presented, one has to consider both preparation and technical impacts on SOCE. Myotubes can be easily used for genetic modifications in knock-down and silencing studies for tubular signalling proteins (Lyfenko and Dirksen 2008), but myotubes do not necessarily compare to adult fibers due to maturation plasticity (Merrick et al. 2009). For example, sarcolemmal mechanosensitive channel open probabilities decline substantially from myotubes to adult fibers (Franco-Obregon and Lansman 1994), and dedifferentiating cultured adult fibers show spontaneous elementary calcium-release events that can be blocked by DHPR antagonists (Brown et al. 2007). The latter observation was associated with a loss of t-tubular structure and has been interpreted as a loss of DHPR-mediated control of RyR channels (Brown et al. 2007).
Another concern in most electrophysiological cell-attached patch clamp studies connecting SOCE to plasma membrane Ca2+ influx is the use of >100 mM Ca2+ concentrations in the pipettes that vastly increase driving force both in myotubes (Hopf et al. 1996) and adult fibers. In this regard, Allard et al. (2006) challenged the physiological role of store-operated membrane conductance in adult skeletal muscle by showing no increase in whole cell conductance or single channel activity in single fibers severely depleted by prolonged depolarizing pulses under voltage-clamp conditions, either in the presence or in the absence of CPA or in the presence of 25 mM caffeine (Allard et al. 2006).
In a subsequent study not related to SOCE, the same author(s) recently showed that membrane Ca2+ flux determined by Mn2+ quenching in adult fibers crucially depends on the membrane potential and the Mn2+ concentrations used (Berbey and Allard 2009). This has to be kept in mind, as most studies on SOCE detected by Mn2+ quench did not keep divalent ion concentrations constant (Hopf et al. 1996; Boittin et al. 2006; Zhao et al. 2006). A further artificial condition in the quest for the physiological relevance of SOCE is introduced by severe SR depletion using high-dose caffeine or SERCA pump blockers to chronically deplete the store in Ca2+-free external solution with subsequent recordings of force, intracellular Ca2+, or Ca2+ entry (Zhao et al. 2006; Boittin et al. 2006; Kurebayashi and Ogawa 2001; Cherednichenko et al. 2004). Under these conditions, SOCE develops with a time delay of many seconds and is maintained during ongoing SERCA blockade and shuts off as the store refills (Kurebayashi and Ogawa 2001). However, the SR Ca2+ thresholds for SOCE have not been defined yet.
It has been suggested that SOCE might be a mechanism to replenish the SR during severe store depletion at strenuous exercise (Pan et al. 2002). Fast-twitch fibers are known to operate with a ∼30% partially filled SR only, whereas slow-twitch fibers are almost completely filled under resting conditions (Fryer and Stephenson 1996). Whether SR depletion even in exercised fast-twitch muscle would be severe enough to trigger SOCE could not be answered in intact muscle, especially in view of mechanisms that counteract fatigue (Allen et al. 2008) and given the fact that SOCE operates mostly at negative membrane potentials (Stiber et al. 2008; Kurebayashi and Ogawa 2001).
Studies in mechanically skinned fibers, however, have substantially increased our understanding of SOCE mechanisms. Launikonis et al. (2003) found a coupling between tubular SOCs and heparin-sensitive IP3 receptor channels in amphibian skeletal muscle using confocal Ca2+ imaging of mechanically skinned fibers. SOCE in nonexcitable cells develops in tens of seconds after store depletion (Bennett et al. 1998) and also takes similar times (∼100 ms) in chronically store-depleted intact adult muscle fibers (Gonzalez Narvaez and Castillo 2007) or <1 min following halothane-induced Ca2+ waves in mechanically skinned adult rat muscle fibers (Duke and Steele 2006). Myotubes even showed SOCE dynamics in the ∼10 min range (Zhao et al. 2006). Launikonis and Rios (2007), however, speculated that SOCE in adult skeletal muscle should be much faster given the highly organized structure of the tubules and the SR, being determined for fast signaling. In order to resolve fast SOCE during more physiological release stimuli as compared to chronic SR depletion, the authors simultaneously monitored tubular (trapped mag-indo-1) and cytoplasmic Ca2+ (rhod-2) dynamics and found that SOCE activation was locally controlled within 1 s following low Mg2+-induced SR Ca2+ release. T-system Ca2+ ([Ca2+]t) dropped from a peak value of ∼1 mM to ∼0.2 mM within 4 s during global release as visualized with ratiometric SEER (shifted-excitation and emission ratioing of fluorescence) (Launikonis and Rios 2007).
Suggested models of SOCE included association of SR Ca2+ sensors to tubular TRP channels, IP3-receptors and another CRAC or SOC channel, the identity of which was obscure for a long time. Stimulating help came from the nonexcitable tissue area, where the molecular players for SOCE were identified as the ER Ca2+ sensor stromal interaction molecule 1 (Stim1; Roos et al. 2005; Wu et al. 2006; Liou et al. 2005) and the Ca2+ selective CRAC channel Orai1 (Feske et al. 2006). Orai1 was identified as a key regulator of the CRAC channel complex that was mutated in compromised T cells from patients with severe combined immune deficiency (SCID). Its gene locus is located on chromosome 12q24 and it consists of four transmembrane segments (M1–M4) with both N- and C-terminuses being cytoplasmic (Feske et al. 2006). Three human homologues exist, Orai1–3, named after the keepers of the gates of heaven in Greek mythology (Feske et al. 2006). The CRAC channel itself is a tetramer composed of four Orai1 subunits (Mignen et al. 2008). Stim1 is one of two human homologue ER-Ca2+ sensors that contains one transmembrane N-luminal and C-cytosolic domain and is anchored in the ER membrane. It binds luminal Ca2+ at its EF hand with an affinity of Kd ∼400 µM. When Ca2+ dissociates from Stim1 during SR Ca2+ depletion, a conformational unfolding of the EF and the sterile-α-motif permits aggregation of Stim1 molecules into clusters (Stathopulos et al. 2008). In Jurkat cells, ER Ca2+ depletion was associated with local accumulations of Stim1 clusters as “puncta” near the plasma membrane within tens of seconds that coincided with the development of CRAC currents (Wu et al. 2006). This suggested a slow signalling event during which ER Ca2+ depletion leads to collection and relocation of Stim1 molecules from regions distant from the plasma membrane towards the membrane to interact with the CRAC channels. During myoblast-to-myotube differentiation, an increased expression and redistribution of Stim1 to the cell periphery was observed (Stiber et al. 2008). Myotubes lacking Stim1 do not show SOCE, and muscles from Stim1 knockout mice fatigue more quickly (Stiber et al. 2008; Lyfenko and Dirksen 2008). Here as well, Orai1 and Stim1 are the crucial molecular players of SOCE (Lyfenko and Dirksen 2008). Both proteins are highly expressed in the triads of adult skeletal muscle (Stiber et al. 2008).
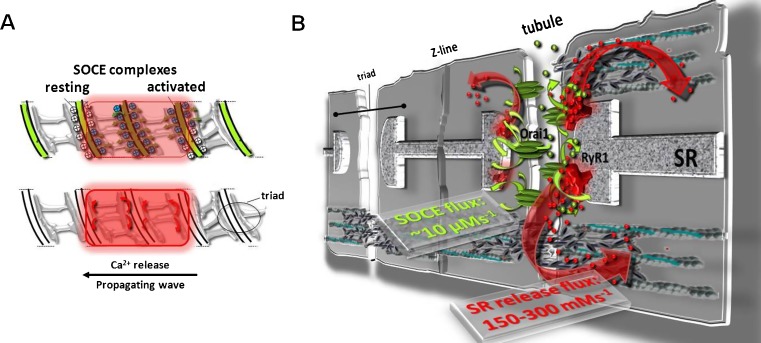
In a mechanistic model of SOCE, Stim1 molecules either have to be relocated from the SR membrane closer to the t-tubule membrane to realize SOCE within less than 1 s (Launikonis and Rios 2007) or they are already pre-arranged in a ready-to-go array suggesting conformational coupling with even ultra-fast SOCE. Recently presented experimental evidence using mechanically skinned fibers and simultaneous t-tubular and myoplasmic Ca2+ imaging shows low-Mg2+ induced “wave-like” SR Ca2+ releases. Those waves underlie local control of RyR1 with short and partial SR depletions that mimic release patterns during physiological activity, and ultra-fast SOCE activation and deactivation dynamics that are consistent with conformational coupling were detected (Launikonis et al. 2009a, b). SOCE activated within ∼25 ms of SR Ca2+ release. As with EC-coupling, “structure fits function,” with tubular and SR membrane proteins being set in place for conformational and ultra-fast coupling (Fig. 1).
Fig. 1a, b.
Conformational coupling of SOCE complexes in adult skeletal muscle. a Model of locally controlled EC-coupling during a low Mg2+-induced Ca2+ wave propagating along the fiber axis in a mechanically skinned fiber with Fluo-5N trapped in the t-system and rhod-2 in the cytoplasm. SOCE can be detected with a “coupling delay” of ∼25 ms from local SR release (according to data from Launikonis et al. 2009a, b). b Proposed model of Orai1 channels bridging the “Ca2+ supply gap” that may be caused by Ca2+ during the global release not being immediately available for SR refilling due to buffering by cytoplasmic buffers. SOCE may provide a shortcut for Ca2+ being directly transferred from the tubules through the triad junction into the SR and maintains SR filling during sustained physiological activity
What then is the physiological relevance of a conformationally coupled SOCE-complex to the rapid activation of Ca2+ entry? As calculated by Launikonis and Rios (2007), the SR release flux into the cleft is massive (up to 300 mM/s), however, cytoplasmic Ca2+ is only raised to 10–20 µM during a twitch (Hollingworth et al. 1996) due to the buffering capacity of troponin, parvalbumin, and other Ca2+ binding proteins. Ca2+ is sequentially transferred between buffers before it can be pumped back into the SR for subsequent releases. The SOCE flux into the triad junction is about four orders of magnitude smaller (Fig. 1), however, it can provide a direct pathway for extracellular Ca2+ to rush into the organelle where it is needed during sustained activity. Ca2+ entering the cleft can be directly pumped into the SR via SERCA while the cytoplasmic Ca2+ is still “in the queue.”
Although there is no doubt anymore about the SOCE complex being conveyed through a concerted action of Stim1 and Orai1, the threshold levels for its activation and deactivation remain poorly defined. Future research directions would include SR Ca2+ imaging in conjunction with SOCE, as some dyes can be used for quantitative SR imaging of Ca2+ depletion (MacQuaide et al. 2009; Kabbara and Allen 2001).
Excitation-coupled Ca2+ entry (ECCE) in skeletal muscle
As stated above, SOCE only works at negative membrane potentials that would translate to the repolarizing phase in between action potentials. Another Ca2+ entry pathway that operates during depolarizations was identified in myotubes and is called excitation-coupled Ca2+ entry or ECCE (Cherednichenko et al. 2004). After pulses of K+-depolarizations or in response to electrical field stimulation (most pronounced above 10 Hz), initial Mn2+ quench rates increased up to 100-fold compared to resting conditions (Cherednichenko et al. 2004). ECCE required both the RyR1 and the DHPR because ECCE was absent in RyR1-negative dyspedic or α1s-DHPR-negative dysgenic myotubes (Cherednichenko et al. 2004). A distinct Ca2+ entry protein was originally suggested because expression of a nonconducting SkEIIIK-α1s pore in dysgenic myotubes (Dirksen and Beam 1999) seemed to resuscitate ECCE. ECCE was also unrelated to SOCE, as neither store depletion, Orai1 knockout, nor Stim1 silencing affected Ca2+ entry during K+-depolarizations (Lyfenko and Dirksen 2008). Also, TRPC3 was not a key element for ECCE (Lee et al. 2006). In myotubes, ECCE was two times faster compared to SOCE (Lyfenko and Dirksen 2008). Subsequently, ECCE was also identified in fully differentiated adult mouse muscle (FDB) fibers and found to be blocked by dantrolene with an IC50 of ∼4.5 µM in myotubes and a similar potency in adult fibers when they were pretreated with 250 µM ryanodine, but a lower potency when dantrolene (10 µM) was given without pretreatment (Cherednichenko et al. 2008). Dantrolene did not affect SOCE in this assay.
In the search for the molecular entity of the ECCE channel, Bannister et al. (2009) revisited the problem of the L-type channel itself conducting ECCE in myotubes and found that although ECCE shared some pharmacological overlap drug sensitivity with SOCE, there was a larger overlap with L-type Ca2+ channels, i.e., blocking of Ca2+ entry by 2-APB, SKF-96356, nifedipine (>10 µM) and Gd3+ and La3+ during depolarizations (Bannister et al. 2009). In contrast to previous studies (Cherednichenko et al. 2004), ECCE was not detectable in dysgenic α1S-DHPR myotubes expressing SkEIIIK. The authors found that the DHPR conducts Mn2+ and they based most of their findings on direct electrophysiological recordings of Ca2+ currents through the DHPR during step pulse depolarizations. The authors also gave several more reasons to explain these controversial results that are summarized in their work (Bannister et al. 2009) and in Dirksen (2009). On the other hand, there is recent experimental evidence that a channel or transporter distinct from the DHPR could mediate the ECCE current in addition to the DHPR, as the activation curves for Mn2+ influx were shifted towards more negative potentials compared to the respective curves for L-type currents (Berbey and Allard 2009). In summary, the question about the molecular identity of the ECCE pathway remains unanswered.
Action-potential-activated Ca2+ current (APACC) in skeletal muscle
During low frequency stimulation of muscle over extended periods of time, there is a documented net uptake of Ca2+ from the extracellular medium (Gissel and Clausen 1999). However, the technique of measuring radioactive 45Ca uptake over a 4-h period did not allow for resolution of the mechanisms of Ca2+ entry during brief tetany or even single action potentials. Even if this effect could be 100% accounted for by a combination of SOCE and ECCE, experimental evidence for such a synergism during single twitches is not available. Very recently, Launikonis et al. (2009a, b) presented evidence for Ca2+ entry during single action potentials in mechanically skinned and polarized rat muscle fibers during field-stimulation and confocal SEER imaging of mag-indo-1 trapped in the t-system and rhod-2 in the cytoplasm. Depending on the initial t-system Ca2+ concentration [Ca2+]t, an inward (for [Ca2+]t >0.15 mM) or an outward (for [Ca2+]t <0.15 mM) flux was seen following a single action potential. The peak influx was roughly linear with initial [Ca2+]t: about 7 mM s-1 at ∼1 mM [Ca2+]t and approx. 20 mM s-1 at 2 mM concentrations; [Ca2+]t was calculated to decrease by 0.25–0.5 mM during an action potential (Launikonis et al. 2009a, b). During brief trains of action potentials (APs), an additive Ca2+ entry was observed but only if APs were sufficiently spaced apart, i.e., trains of APs applied at 10 Hz were accompanied by Ca2+ entry during the first three or so APs, after which [Ca2+]t became stationary. In contrast, at 3 Hz stimulation, every successive AP was followed by a further decrease in [Ca2+]t. These results suggested that this ultra-fast Ca2+ entry was activated at the peak of the action potential, most likely through a voltage-gated channel, with rapid activation kinetics, but a recovery from inactivation kinetics that was much slower (∼100–200 ms).
Launikonis et al. (2009a, b) termed this Ca2+ entry action-potential-activated Ca2+ current (APACC) and discussed potential sources for its molecular entity. As APACC was clearly activated by voltage, it is distinct from SOCE. Although an unspecific TRPC blocker, 2-APB and SKF-96356, affected APACC, TRP channels seem unlikely to underlie APACC as even the voltage-sensitive TRPC family members are activated on a much slower time scale (Shimizu et al. 2009). As in ECCE, the L-type channel could be a prime candidate; however, with its slow activation kinetics it is generally believed not to be opened by brief action potentials. On the other hand, ECCE during single APs has never been confirmed, so at this stage, it is not clear whether the ECCE signaling complex is also responsible for APACC.
Whatever channel identity, the physiological relevance of APACC was suggested to prime ryanodine receptors by directing an early small Ca2+ flux to the junctional cleft that could help reduce the Mg2+ inhibition on the activation sites, as well as to the physical DHPR II-III loop RyR1 coupling (Laver et al. 2004).
One of the reasons why APACC could not be detected earlier in electrophysiological studies may be the use of defined ion composition conditions being used to separate ionic currents, e.g., use of Cs+, TEA+, Ba2+, under voltage clamp conditions. Although such ion compositions may be regarded as “less physiological” compared to normal saline, they are necessary to record L-type Ca2+ currents out of the otherwise much larger superposing Na+ and K+ conductances. Also, although potential conditions are clearly controlled during conventional step-pulse depolarization used in voltage-clamp, the driving force for depolarizing potential steps is reduced compared to action potentials where depolarization and repolarization phases are more graded. This was recently calculated for action potential time courses and step pulses of the same duration (Launikonis et al. 2009a, b). In particular, the driving force for Ca2+ remains low for the duration of the depolarizing potential step although channels might be fully activated at that potential (depending on the steady-state activation curves) whereas during the gradual repolarization phase of an action potential, driving force increases while channels may not be fully activated. It can be speculated that it might not really be necessary to fully activate the L-type channel, as more Ca2+ could enter in fact during repolarization of the AP through partially opened channels, given a larger driving force (Launikonis et al. 2009a, b; Zhou and Bers 2000). Future studies might use action potential waveforms as input for voltage-clamp experiments to see whether APACC could also be confirmed by conventional electrophysiology.
Tubular proteins—socializing for signaling
From the previous sections, it can be seen that the triads and in particular the t-tubules in skeletal muscle are an attractive place to gather for various signaling proteins. Our image of the skeletal triad has been one of two tightly coupled proteins dwelling in relative isolation. Now, the picture is filling in and the reduced triadic space even seems to become crowded.
This tight morphological arrangement of the triad junction might be a structural requirement that predetermines skeletal muscle for ultra-fast activation cascades.
Apart from DHPR, TRPC, Orai1, APACC, Na+, K+, and Cl- channels are at least present in the tubules. With SOCE and ECCE, there is a machinery available to directly replenish SR Ca2+ during bouts of exercise covering the whole activity range, i.e., SOCE operating in the repolarizing phase of excitation when stores are being depleted and ECCE in the depolarizing phase of excitation unrelated to store content. APACC seems to aid EC-coupling at the peak of action potentials. The still unknown identity of the ECCE-mediating molecule increases the “crowding pressure” in the tubules. If the DHPR is identical to the APACC, our picture would change again. Future research will show whether one and the same molecule serves different functions here, or whether there will be even more channel proteins to be discovered.
The role of computational models
The previous sections give an impression of the recent flow of experimental data expanding our view of the skeletal muscle triad. To integrate these data into a quantifiable system of Ca2+ fluxes across t-tubular and SR membranes in the presence of a number of stochastically operating ion channels with different kinetics, revised and well-defined biophysical models of the triad will have to be formulated and experimentally tested. Due to the restricted spatial domains concerned and the relatively small Ca2+ fluxes involved in the more recently discovered processes, stochastic chemical models will probably replace deterministic, large-scale models of calcium buffering and diffusion. Fast, exact sampling procedures that integrate channel gating, buffering, diffusion, and feedback regulation by single Ca2+-responsive molecules (e.g., calmodulin) in a single multivariate process are already at hand (von Wegner and Fink 2009).
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Allard B, Couchoux H, Pouvreau S, Jacquemond V. Sarcoplasmic reticulum Ca2+ release and depletion fail to affect sarcolemmal ion channel activity in mouse skeletal muscle. J Physiol. 2006;575(1):68–81. doi: 10.1113/jphysiol.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronache Z, Hamilton SL, Dirksen RT, Melzer W. A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice. Proc Natl Acad Sci USA. 2009;106:4531–4536. doi: 10.1073/pnas.0812661106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister RA. Bridging the myoplasmic gap: recent developments in skeletal muscle excitation-contraction coupling. J Muscle Res Cell Motil. 2007;28:275–283. doi: 10.1007/s10974-007-9118-5. [DOI] [PubMed] [Google Scholar]
- Bannister RA, Pessah IN, Beam KG. The skeletal L-type Ca2+ current is a major contributor to excitation-coupled Ca2+ entry. J Gen Physiol. 2009;133(1):79–91. doi: 10.1085/jgp.200810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard NA, Wei L, Dulhunty A (2009) Ca2+ signaling in striated muscle: the elusive roles of triadin, junctin, and calsequestrin. Eur Biophys J. doi:10.1007/s00249-009-0449-6 [DOI] [PubMed]
- Bennett DL, Bootman MD, Berridge MJ, Cheek TR. Ca2+ entry in PC12 cells initiated by ryanodine receptors or inositol 1,4,5,-trisphosphate receptors. Biochem J. 1998;329:349–357. doi: 10.1042/bj3290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbey C, Allard B. Electrically silent divalent cation entries in resting and active voltage-controlled muscle fibers. Biophys J. 2009;96:2648–2657. doi: 10.1016/j.bpj.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boittin FX, Petermann O, Hirn C, Mittaud P, Dorchies OM, Roulet E, Ruegg U. Ca2+ independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J Cell Sci. 2006;119:3733–3742. doi: 10.1242/jcs.03184. [DOI] [PubMed] [Google Scholar]
- Brown LD, Rodney GG, Hernandez-Ochoa E, Ward CW, Schneider MF. Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers. Am J Physiol Cell Physiol. 2007;292:1156–1166. doi: 10.1152/ajpcell.00397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Confromational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci USA. 2004;101(44):15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Ward CW, Feng W, Cabrales E, Michaelson L, Samso M, Lopez JR, Allen PD, Pessah IN. Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol Pharmacol. 2008;73:1203–1212. doi: 10.1124/mol.107.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen RT. Checking your SOCCS and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol. 2009;587(13):3139–3147. doi: 10.1113/jphysiol.2009.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen RT, Beam KG. Role of calcium permeation in dihydropyridine receptor function. Insights into channel gating and excitation-contraction coupling. J Gen Physiol. 1999;114:393–403. doi: 10.1085/jgp.114.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AM, Steele S. Store-operated Ca2+ entry following halothane-induced Ca2+ waves in mechanically skinned rat muscle fibres. Proc Physiol Soc. 2006;3:140P. [Google Scholar]
- Dulhunty A, Karunasekara Y, Casarotto M (2009) Skeletal excitation-contraction coupling: who exactly are the dancing partners? Biophys Rev (in press) [DOI] [PubMed]
- Durham WJ, Aracena-Parks P, Long C, Ross AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker M, Shirokova N, Protasi F, Dirksen R, Hamilton SL. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knock-in mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Lansman JB., Jr Mechanosensitive ion channels in skeletal muscle from nornal and dystrophic mice. J Physiol. 1994;481(2):299–309. doi: 10.1113/jphysiol.1994.sp020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D, Fill M. Intracellular calcium release channels mediate their own countercurrent: the ryanodine receptor case study. Biophys J. 2008;95:3706–3714. doi: 10.1529/biophysj.108.131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissel H, Clausen T. Excitation-induced Ca2+ uptake in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 1999;276:R331–R339. doi: 10.1152/ajpregu.1999.276.2.R331. [DOI] [PubMed] [Google Scholar]
- Gonzalez Narvaes AA, Castillo A. Ca2+ store determines gating of store operated calcium entry in mammalian skeletal muscle. J Muscle Res Cell Motil. 2007;28:105–113. doi: 10.1007/s10974-007-9105-x. [DOI] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Suda N, Beam KG. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the bi-directional coupling with the ryanodine receptor. J Biol Chem. 1999;274:21913–21919. doi: 10.1074/jbc.274.31.21913. [DOI] [PubMed] [Google Scholar]
- Holder JR, Blatter LA. Capacitive calcium entry is inhibited in vascular endothelial cells by disruption of cytoskeletal microfilaments. FEBS Lett. 1997;403(2):191–196. doi: 10.1016/S0014-5793(97)00051-3. [DOI] [PubMed] [Google Scholar]
- Hollingworth S, Zhao M, Baylor SM. The amplitude and time course of the myoplasmic free [Ca2+] transient in fast-twitch fibres of the mouse. J Gen Physiol. 1996;108:455–469. doi: 10.1085/jgp.108.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Reddy P, Hong J, Steinhardt RA. A capacitive calcium current in cultured skeletal muscle cells is mediated by the calcium-specific leak channel and inhibited by dihydropyridine compounds. J Biol Chem. 1996;271(37):22358–22367. doi: 10.1074/jbc.271.37.22358. [DOI] [PubMed] [Google Scholar]
- Kabbara A, Allen DG. The use of the indicator fluo-5 N to measure sarcoplasmic reticulum calcium in single muscle fibres of the toad. J Physiol. 2001;537:379–389. doi: 10.1111/j.1469-7793.2001.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch WG, Uttenweiler D, Fink RHA. Spark- and ember-like elementary Ca2+ release events in skinned fibres of adult mammalian skeletal muscle. J Physiol. 2001;534(1):87–97. doi: 10.1111/j.1469-7793.2001.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler G, Weiss RG, Flucher BE, Grabner M. Structural requirements of α1S II-III loop for skeletal-type excitation-contraction coupling. J Biol Chem. 2004;279:4721–4728. doi: 10.1074/jbc.M307538200. [DOI] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc Natl Acad Sci USA. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Rios E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583(1):81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Edwards JN, v Wegner F, Friedrich O. Store-operated Ca2+ entry in skeletal muscle can be activated and deactivated within milliseconds of Ca2+ release and store refilling. Biophys J. 2009;96(3):280a. doi: 10.1016/j.bpj.2008.12.1384. [DOI] [Google Scholar]
- Launikonis BS, Stephenson DG, Friedrich O. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J Physiol. 2009;587(10):2299–2312. doi: 10.1113/jphysiol.2009.168682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, O’Neill ER, Lamb GD. Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J Gen Physiol. 2004;124:741–758. doi: 10.1085/jgp.200409092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, Cherednichenko G, Pessah IN, Allen PD. Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins. J Biol Chem. 2006;281(15):10042–10048. doi: 10.1074/jbc.M600981200. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrel JE, Jr, Meyer T. Stim is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on Stim1 and Orai1. J Physiol. 2008;586(20):4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Hosey MM, Ríos E. Skeletal muscle dihydropyridine and ryanodine receptor proteins interact in bilayers. Biophys J. 1991;59:201a. [Google Scholar]
- MacQuaide N, Dempster J, Smith GL. Assessment of sarcoplasmic reticulum Ca2+ depletion during spontaneous Ca2+ waves in isolated permeabilized rabbit ventricular cardiomyocytes. Biophys J. 2009;96(7):2744–2754. doi: 10.1016/j.bpj.2008.12.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of calcium ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Merrick D, Stadler LK, Larner D, Smith J. Muscular dystrophy begins early in embryonic development deriving from stem cell loss and disrupted skeletal muscle formation. Dis Model Mechanism. 2009;2:374–388. doi: 10.1242/dmm.001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586:419–425. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Nakai J, Sekiguchi N, Rando TA, Allen PD, Beam KG. Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J Biol Chem. 1998;273:13403–13406. doi: 10.1074/jbc.273.22.13403. [DOI] [PubMed] [Google Scholar]
- Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima CH, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4(5):379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proenza C, O'Brien J, Nakai J, Mukherjee S, Allen PD, Beam KG. Identification of a region of RyR1 that participates in allosteric coupling with the α1S (CaV1.1) II-III loop. J Biol Chem. 2002;277:6530–6535. doi: 10.1074/jbc.M106471200. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated Ca2+ entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Calahan MD, Velicelebi G, Stauderman KA. Stim1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan DC, Takekura H, Franzini-Armstrong C, Beam KG, Allen PD, Perez CF. Bidirectional signaling between calcium channels of skeletal muscle requires multiple direct and indirect interactions. Proc Natl Acad Sci USA. 2006;103:19760–19765. doi: 10.1073/pnas.0609473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Janssens A, Voets T, Nilius B. Regulation of the murine TRPC3 channel by voltage, pH and changes in cell volume. Pflugers Arch. 2009;457:795–807. doi: 10.1007/s00424-008-0558-6. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into Stim1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. Stim1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10(6):688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M, von Wegner F, Fink RH, Chamberlain JS, Launikonis BS, Martinac B, Friedrich O. Inhibitory control over Ca2+ sparks via mechanosensitive channels is disrupted in dystrophin deficient muscle but restored my mini-dystrophin expression. PLoS One. 2008;3(11):e3644. doi: 10.1371/journal.pone.0003644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Ducret T, Basset O, Sebille S, Raymond G, Ruegg U, Gailly P, Cognard C, Constantin B. Regulation of the store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. FASEB J. 2006;20(1):136–138. doi: 10.1096/fj.04-3633fje. [DOI] [PubMed] [Google Scholar]
- Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Regulation of capacitive calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J. 2007;21(2):608–617. doi: 10.1096/fj.06-6683com. [DOI] [PubMed] [Google Scholar]
- von Wegner F, Fink RH (2009) Stochastic simulation of calcium microdomains in the vicinity of an L-type calcium channel. Eur Biophys J. doi:10.1007/s00249-009-0504-3 [DOI] [PubMed]
- Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol. 2005;7(5):525–530. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- Wu MM, Buchanan JA, Luik RM, Lewis RS. Ca2+ store depletion causes Stim1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174(6):803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Weisleder N, Han X, Pan Z, Parness J, Brotto M, Ma J. Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J Biol Chem. 2006;281:33477–33486. doi: 10.1074/jbc.M602306200. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Bers DM. Ca2+ influx via the L-type Ca2+ channel during tail current and above current reversal potential in ferret ventricular myocytes. J Physiol. 2000;523(1):57–66. doi: 10.1111/j.1469-7793.2000.t01-2-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Brum G, Gonzalez A, Launikonis BS, Stern MD, Rios E. Ca2+ sparks and embers of mammalian muscle. Properties of the sources. J Gen Physiol. 2003;122:95–114. doi: 10.1085/jgp.200308796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995;105(2):209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]