Abstract
Plasma modification and plasma polymer deposition are valuable technologies for the preparation of surfaces for the covalent binding of biomolecules for applications such as biosensors, medical prostheses, and diagnostic devices as well as surfaces for enzyme-mediated reactions. Covalency is conveniently tested by the ability of the surface to retain the attached molecules after vigorous washing with sodium dodecyl sulphate (SDS). Covalency is indicated if the fraction of protein retained lies above the curve characteristic of physisorption. Confidence in covalency is strengthened when the washing protocol is aggressive enough to remove all adsorbed protein from a control significantly more hydrophobic than the test surface. The use of linker chemistry to space the molecules from the surface is in some cases beneficial. However, the use of linker chemistry is not necessary to retain molecular function for long periods when the polymer surface is modified by energetic bombardment. The energetic bombardment retains hydrophilicity of the surface by crosslinking the subsurface, and this appears to facilitate retention of protein function. Energetic bombardment also increases the functional life of molecules immobilized and then freeze dried on plasma-modified surfaces. Analysis of the surfaces shows that the covalent binding mechanism is related to the presence of free radicals on the surface and in the subsurface regions. The unpaired electrons associated with the radicals appear to be mobile within the modified region and can diffuse to the surface to take part in binding interactions. Proactive implantable devices can make use of these principles of covalent attachment by seeding the surface of an implant with a biomolecule that elicits the desired interaction with cells and prevents undesirable responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-010-0028-1) contains supplementary material, which is available to authorized users.
Keywords: Plasma modification, Plasma polymer, Covalent immobilisation, Energetic bombardment
Introduction
Many applications in medicine, environmental sensing, food technology, and chemical processing utilize the biorecognition capability of biomolecules. For example, many sensing applications rely on the detection of a specific binding event between an immobilized sensor molecule and its binding partner. Analysis of the binding of a patient’s serum proteins to an array of antibodies can reveal expression patterns that are diagnostic of disease (Hartmann et al. 2009). Sensing of environmental toxins is now possible with sensors based on biomolecular interactions (Frisk et al 2009). Detecting stressors that unfold target proteins which then bind to immobilized molecular chaperones has been proposed (George et al. 2008). Enzymes are commonly used to catalyze chemical reactions in food and chemical processing. Surface immobilization of an enzyme has advantages in cases where the function of the enzyme is compromised by excessive concentrations of the reaction products (Trevan et al. 1987) and where a continuous flow process rather than a batch process can be an advantage. Affinity chromatography relies on immobilizing an enzyme on the surface of a particulate medium (Azarkan et al. 2007). In the concept of proactive implantable devices, surfaces are seeded with a layer of biomolecules that elicit desired cellular responses at the location of an implant through their specific interactions with cell membrane proteins. The utilization of ceramics, metals, polymers (Vallet-Regí et al. 2008; Sugita et al. 2009; Park et al. 2009; Bax et al. 2009), and polymer-coated metals (Yin et al. 2009a; f) functionalized with a variety of proteins for such applications has been described.
The biorecognition process is affected to varying degrees by the conformation state of the molecule. In some cases, the presence of a specific sequence of amino acids is sufficient for the recognition process to take place, and therefore the recognition is not very sensitive to conformation. In cases where the binding site consists of amino acids which are proximate by virtue of the native folded conformation, the native molecular conformation must be maintained to preserve the bioactivity. Conformation can be affected when a molecule is attached to a surface. Since the surface does not typically replicate the aqueous environment of the protein, the energetics of the competing conformation states will, in general, be changed. The change is severe in cases where the surface is very hydrophobic, since hydrophobic regions of the molecule, normally not exposed in aqueous solution, are encouraged to present to the surface.
This review is primarily concerned with strategies for covalent immobilization on surfaces treated with a plasma (ionised gas) process. The reader is referred elsewhere for coverage of other methods of immobilization (see, for example, Woodward 1985a, b). The advantage of a covalent link is that the molecule is tethered at a site on its surface rather than in contact over a significant part of its surface as in the case of physisorption. Provided that there are not too many tethering sites, the molecule is generally more remote from the binding surface and its energetics are less affected than in a pure physical adsorption process. A potential problem for covalent binding is the presence of more than one covalent bond that may excessively constrain the molecule or at least increase the probability of involving the active site for biorecognition in the interaction with the surface. Additionally, the proximity of the surface may impede the interaction between the bound molecule and other molecules in solution. For example, if the immobilized molecule is an enzyme, its ability to interact with large molecules especially may be impeded. For this reason, the use of linker or spacer chemistry is often recommended for active enzyme immobilization to allow the tethered molecule to be located further from the tethering surface. Linker chemistry has been used with plasma-activated surfaces and, in some cases, increasing the length of the spacer molecule was beneficial for enzyme activity (Ganapathy et al. 2000). However, increasing linker length does not always result in an increase in activity since a longer linker may allow the linker to adopt a conformation that interferes with the function of the protein (Ganapathy et al. 2001). The benefits of a linker are therefore not universal and, in some cases, binding to the surface without a linker is shown to give a higher density of active molecules (Itoyama et al. 2008).
We will not review in depth the treatments that employ the use of specific linker molecules. The advantages of a so-called linker-free approach are that a sequence of, often complex, wet chemical steps are not required to achieve protein binding. The simple immersion of the surface in a solution which contains the biomolecules to be immobilized is all that is required. Consequently, we will focus in this review on linker-free methods.
Plasma methods have been proven to be useful in the generation of reactive sites for the grafting of one polymer onto the surface of another to modify its surface chemistry. Plasma treatment has been successful, for example, in modifying the hydrophobic surface of polydimethylsiloxane (PDMS) to make it hydrophilic and suitable for conventional derivations for biomaterials (Karkhaneh et al. 2007).
Plasma processes for creating the binding surfaces can be used on complex shaped objects such as medical prostheses and are easily adapted to patterning for use on sensing arrays. In the first section of the review, we describe the key aspects of the plasma processes used to create linker-free protein binding surfaces. The second section focuses on the characterization and properties of these surfaces, while in the last section a model for the interaction mechanisms between the proteins and the surfaces is reviewed. A summary of the techniques used for characterizing plasma-treated surfaces and for testing their ability to covalently attach bioactive protein layers is given in the supplementary material.
Plasma processes for creation of covalent binding surfaces
Reports of the strong binding of proteins to polymers after treatment in a plasma are relatively recent, first appearing in the 1990s. There are two types of processes that use plasmas for producing surfaces capable of covalently coupling protein molecules without the use of intermediate linker molecules. The first is a modification process for polymeric surfaces while the second is the deposition of a polymer-like material from a plasma that can be deployed on any underlying material. The reader is referred to the reviews of Biederman for details of the deposition approach (Biederman 2004; Biederman and Osada 1992).
Plasma modification of polymers for covalent protein immobilization
A plasma, especially a DC or RF excited glow discharge plasma, is a convenient source of radiation, ions, atoms, and excited or ionized chemical groups that can be used to modify the surface of a polymer without the growth of a layer from the plasma. Etching of polymeric materials by plasmas has been described by d’Agostino (1990) and the plasma modification of polymeric materials has been described in Inagaki (1996) and Kondyurin and Bilek (2008). Since polymers are insulators, plasma treatment processes need to take account of surface charging phenomena. When a polymer material is placed in a glow discharge, it is bombarded by ions, electrons, and other species from the plasma. There is a tendency for the surface to charge negatively because of the greater mobility of electrons. The negative charge is limited by ion bombardment from the plasma. The energy of the bombardment by ions is difficult to measure but it is relatively low and is determined by the small voltage that appears across a sheath that forms in the plasma in response to the negatively charged surface. A sheath is a region of net charge that forms to shield the plasma from the charged surface. It is analogous to a double layer in solution.
The bombardment by ions and atoms from the plasma can remove as well as modify the polymer, in a process known as sputter etching. If a species such as a fluorocarbon is introduced into the plasma, it produces groups which react with surface and increase etching by the formation of volatile reaction products.
Sodium dodecyl sulphate (SDS) is a detergent capable of disrupting physical interactions between a protein and a surface. Bohnert and coworkers (Bohnert et al. 1990) exposed PET surfaces to argon plasma and observed a decrease in the ability of the treated PET surface to bind albumin when incubated in blood plasma. However, the adsorbed albumin was found to have an increased resistance to elution by SDS, indicating a different kind of attachment. The authors noted that the elution percentage of albumin was reduced substantially, but the significance of the result may not have been realised. This is, to our knowledge, the first report of SDS-resistant binding to a plasma-modified polymer in the literature.
An important advance in the treatment of polymers for immobilisation of biomolecules was the use of energetic ion bombardment (Bilek et al. 2006; Kondyurin and Maitz 2005). Additional energy can be provided to the bombarding ions when a negative DC voltage is applied to a polymer covered electrode. The process in which an object is placed in a plasma and biased with negative pulses is sometimes referred to as plasma immersion ion implantation (PIII) and is most commonly used to treat conductive objects. For a review of the application of PIII to polymers, see Bilek et al. (2007). The applied voltage, typically in the range 1–20 kV, creates a much larger sheath than normally present. All ions crossing the sheath boundary are accelerated by the electric field in the sheath and impact on the polymer surface with an energy equivalent to the voltage across the sheath less any energy lost in collisions which occurred while the ion was passing through the sheath (Bilek et al. 2007). The pulses must be kept short enough and at a low enough duty cycle to avoid overheating the polymer with the implanted ion energy. For insulating surfaces such as most polymers, however, while there is initial bombardment by positively charged ions with the full energy corresponding to the applied voltage, owing to the build up of charge, the sheath collapses and the bombardment ceases (Oates et al. 2003). The charging problem is most serious for thick polymer objects. The use of pulsed DC or RF voltage on the electrode can help sustain the modification process. The use of a mesh covering the object to which the bias pulses are applied may also be beneficial (Fu et al. 2003). The use of PIII with polymers is relatively new, but has been used successfully to modify the mechanical properties of a range of polymers (Kondyurin and Bilek 2008). Depending on the nature of the bombarding species, various rates of removal of the polymer surface occur, with the possible formation of surface roughness. If the species are inert gas ions, physical etching processes occur in which the incident energy removes constituents of the polymer by sputtering.
The benefits of energetic modification by the PIII process are several. First, it is confirmed that the covalent binding capacity conferred remains for much longer periods when the modification is energetic rather than consisting only of a simple exposure to a plasma. It has been shown that when the polymer samples are stored dry in a desiccator, the PIII-modified samples retain their covalent binding capability for longer than control surfaces exposed to plasma without PIII (Nosworthy et al. 2007).
Second, the function of attached biomolecules is retained for longer on surfaces that have received treatment with energetic ions as compared to those receiving plasma treatment without significant ion impacts (Ho et al. 2007; Nosworthy et al. 2007). At least in part this is likely to be related to the slower hydrophobic recovery of the ion-treated samples. Low energy plasma treatments typically lower the contact angle with water in a nonpermanent way (Nosworthy et al. 2007; Kondyurin et al. 2009b) while the energetic ion treatment crosslinks the subsurface and this limits the ability for molecules to rotate and diffuse to replace the modified surface (see "Longevity of protein function").
Plasma deposited polymeric materials for covalent protein Immobilisation
When a carbon-containing gas is injected into a plasma formed in a background gas, deposition of a coating consisting of components from the gas often occurs. The plasmas usually used for this purpose are also referred to as glow discharge plasmas. Suitable plasmas may be excited by application of a DC voltage, a pulsed DC voltage or a radiofrequency (RF) voltage to an electrode inside a vacuum chamber. Alternatively, RF or microwave power may be inductively or capacitively coupled into the chamber by means of an externally mounted electrode. The coatings are often referred to as plasma polymers and their molecular structure reflects to varying degrees the molecular structure of the precursor molecules. The plasma conditions of plasma density, electron temperature, and background gas type affect the properties of the coating. In addition, the presence of a bias voltage on the deposition surface strongly affects the structure by affecting the energy of the bombarding species. The temperature of the growth surface is also an important variable as it affects the mobility of the molecular groups on the surface and phenomena such as structural rearrangement and the exclusion of volatile components. Such a bias voltage can be applied by a separate DC or RF power supply, or it can be arranged by placing the growth surface on an actively powered electrode. Observations of the strong binding of proteins to plasma-created surfaces has been reported from the 1990s (Bohnert et al. 1990; Kiaei et al. 1992). The ability of surfaces coated with plasma deposited fluorocarbon polymer-like materials to retain albumen and fibrinogen after treatment with sodium dodecyl sulphate has been reported (Bohnert et al. 1990). It was observed that the coated surfaces retained the protein much better than untreated PTFE or PET surfaces.
It has been shown that there are clear benefits to plasma polymerization with simultaneous energetic bombardment of the surface. In a study of the performance of surfaces deposited from n-hexane with bias applied to the growth surface, it was found that the polymer surfaces deposited with bias voltages of around 200 V showed better retention of protein function than surfaces deposited without bias (Kondyurin et al. 2008a). Acetylene films deposited with bias showed better retention of protein binding capacity after shelf storage than unbiased controls (Yin et al 2009b). This mirrors the advantages of PIII in the plasma modification of polymers described above.
Adhesion of plasma-deposited layers is potentially a problem for many applications, especially those in which the surface is implanted into the body. A plasma polymer may appear to adhere well, but after immersion in an aqueous environment, adhesion failure may appear, especially after long-term immersion. The underlying surface onto which the plasma polymer is applied can be of many kinds, including metallic, ceramic, or polymer. Sputter etching to remove contaminants and to prepare the surface chemically may be used, but for the best results an interface mixing approach is applied. This approach is well illustrated by the deposition of an acetylene-derived plasma polymer onto a stainless steel surface (Yin et al. 2009c). First, the stainless steel surface is sputter cleaned in argon and the deposition begins with pure stainless steel. The deposition then proceeds to incorporate more of the plasma polymer into the coating and the coating finishes in pure plasma polymer. The pure plasma polymer deposition is then maintained long enough to achieve the desired thickness of the protein binding interlayer.
Surface structure of plasma prepared surfaces
There are a number of reports (Kondyurin et al. 2006, 2008b, c, 2009a, b; Gan et al. 2006; Ho et al. 2007) on the surface chemistries obtained using the methods described in the previous section. Although the chemistries of the polymer being ion implanted and the organic precursor used to deposit the plasma polymer affect the resulting surface chemistry, there are a number of features which are common to all plasma-prepared surfaces. PIII-modified polymers that have been studied include polyethylene, polystyrene, nylon, PET, and PTFE. Precursors used in the energetic ion-assisted plasma polymerization process include n-hexane and acetylene.
The presence of free radicals
Energetic ion impacts break bonds in polymers and create groups containing unpaired electrons at the surface and also in the implanted region below the surface. The free radicals exist to a depth determined by the penetration depth of the bombarding species.
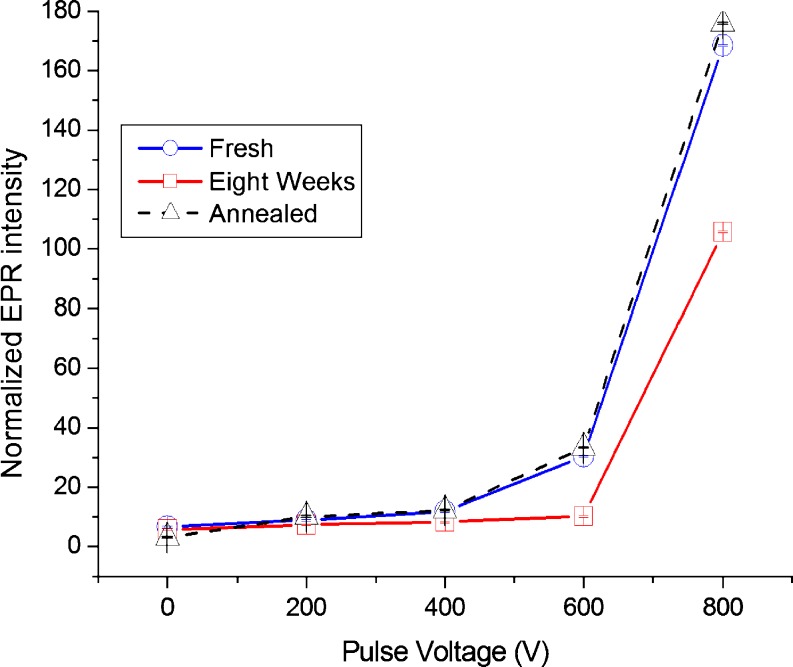
Plasma polymers typically incorporate a significant density of free radicals due to the dissociation of the precursor gas in the plasma prior to arrival at the deposition surface. Depositions using energetic ion bombardment show a dependence of the free radical density as measured by electron spin resonance (ESR) on the pulse bias voltage, an example of which is shown in Fig. 1. This dependence indicates that additional free radicals are generated by the energetic ion fluence caused by the substrate bias. New chemical bonds also form between proximate free radicals, resulting in a highly crosslinked material beneath the surface. This is observed in infrared spectra by the appearance of absorption features in the region 1,600–1,650 cm−1 associated with C=C.
Fig. 1.
ESR signal density as a function of pulse bias voltage applied to the substrate during plasma polymerisation from a mixture of acetylene, argon, and nitrogen. The film thicknesses were approximately 150 nm. A strong increase in the number of spins occurs for bias over 500 V. The density of free radicals remaining decreases with time in laboratory ambient (2 days to 8 weeks shown as the blue cicles and red squares, respectively). Annealing in vacuum for 30 min at 370°C was shown to recover the spins (black triangles). Data from Yin et al. 2009c
The unpaired electrons detected in ESR lie in chemical groups that could be of many types. The unpaired electrons do not only lie on the surface, but have a distribution with depth in the material. The concentration at each depth and the total number present depend on the energetics of the plasma process. For example, when a polymer is being modified by a plasma, the depth of the modification depends on the energy and type of the bombarding species. Argon creates a higher intensity of energy delivery to a shallower depth than hydrogen. An estimate of the modification depth can be obtained by simulating ion trajectories using the SRIM code (Ziegler and Biersack 1985). Table 1 shows a comparison of the depth achieved in PET by ions of various species at two energies (Bilek et al. 2007).
Table 1.
Projected range (in Å) of various 2.5–20 keV ions implanted into PET as simulated by the Monte-Carlo simulation code SRIM 2003.20 (from Bilek et al. 2007)
| Ion | Mass (amu) | 2.5 keV | 5.0 keV | 7.5 keV | 10.0 keV | 12.5 keV | 15.0 keV | 17.5 keV | 20.0 keV |
|---|---|---|---|---|---|---|---|---|---|
| H | 1.008 | 646 | 1,134 | 1,567 | 1,960 | 2,323 | 2,644 | 2,952 | 3,254 |
| He | 4.003 | 369 | 717 | 1,048 | 1,369 | 1,681 | 1,957 | 2,243 | 2,507 |
| O | 15.995 | 105 | 183 | 261 | 336 | 414 | 490 | 566 | 641 |
| C | 12.000 | 126 | 230 | 333 | 432 | 534 | 635 | 735 | 831 |
| N | 14.003 | 113 | 201 | 287 | 370 | 457 | 542 | 627 | 710 |
| Ar | 39.962 | 80 | 122 | 161 | 197 | 233 | 268 | 302 | 335 |
Good covalent immobilization on plasma-polymerized surfaces is observed when the layer is deposited from a mixture of argon and acetylene. These properties are retained when nitrogen is added while the covalent binding capacity is reduced with oxygen or hydrogen additions, even though the ESR signal is strong in both cases (Yin et al 2009e). This loss of binding capacity could be explained by a reduction in the mobility of unpaired electrons when hydrogen or oxygen are present in the structure. Alternatively, the presence of oxygen or hydrogen may give rise to different types of radical groups, some of which are unable to react with protein side chains.
Oxidation of the surface and crosslinking of the subsurface
A common observation is that argon treatment of a polymer causes oxygen and, to a lesser extent, nitrogen incorporation into the polymer. After examination of the C1s XPS peak, Alvarez-Blanco et al. (2001) attributed this to the creation of active radicals in the polymer by bombardment from the Ar plasma that react with atmospheric oxygen and nitrogen to form C-O(286.5 eV), C=O(288 eV), and C-N (285.8 eV) groups. These authors also report reactions of Ar plasma-treated polyethylene with other stable gas phase molecules in the absence of plasma. An example is the formation of amine groups by exposure to 1,3 Diamino propane. For PET and PP surfaces, Ganapathy et al (1998) reported that argon plasma treatment produced the C=O(287.8 eV) peak as well as a peak corresponding to greater oxygenation, O-C=O at 288.7 eV. Oxygen plasma treatment gave higher relative intensities of both peaks than argon treatment. Hydrogen ion implantation achieved similar oxygenation levels in PET (Tóth et al. 2006).
The effect of exposure to plasma on PET using PIII is similar but the O/C ratio is reduced, compared to plasma treatment without PIII (Kereszturi et al. 2008). This reduction in O/C ratio is consistent with the increasing carbonization of the polymer that accompanies energetic bombardment. Increasing carbonization has been observed in the Kr ion implantation of PPO as a function of fluence (Wasserman et al. 1985).
XPS examination of plasma-deposited polymers on a polymer surface shows that the underlying polymer is more or less completely covered by the deposited material, ensuring that the chemical status of the surface is determined by the deposited material and not by the underlying substrate (Kiaei et al. 1992). The chemistry of the deposited layer is determined by the composition of the precursors in the plasma as well as by the degree of ion bombardment, determined by the applied bias.
Infrared spectroscopy confirms that, on exposure to atmosphere, some of the free radicals created by the plasma process react with oxygen, forming a variety of oxygen-containing species (Kondyurin et al. 2006, 2008b, c, 2009a, b; Gan et al. 2006; Ho et al. 2007). Most common are groups containing C=O and C–O. These chemical bonds and the remaining free radicals are polar and their presence on the surface imparts a hydrophilic character as observed by a decrease of the water contact angle compared to an untreated polymer surface. Over time, the concentration of the oxygen containing groups gradually further increases (Kondyurin et al. 2009b).
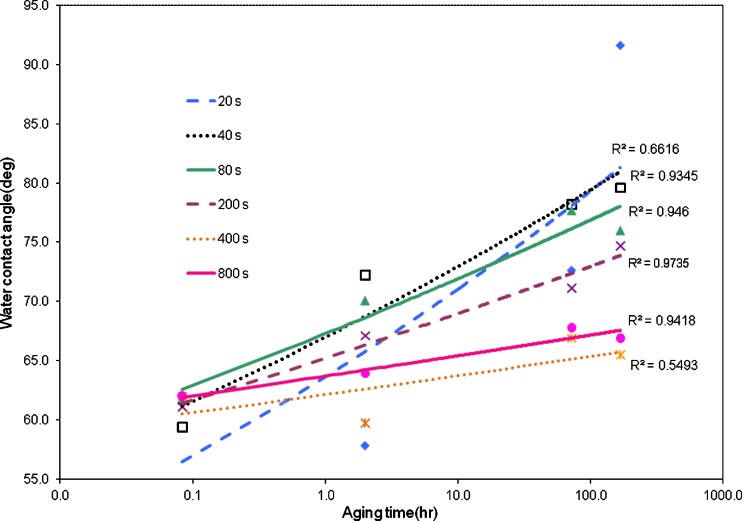
The highly crosslinked carbon network created under the immediate surface by ion collisions beneath the surface stabilizes the modification by arresting processes such as polar group rotation and polymer chain diffusion, both of which lead to hydrophobic recovery. This effect is demonstrated in Fig. 2, which shows the contact angle of PET as a function of time post-PIII treatment for a range of fluences of the energetic ions. For high fluences the final contact angle remains below that of an untreated surface.
Fig. 2.
The effect of ion implantation on degradation processes which contribute to hydrophobic recovery after plasma treatment. The graph shows variations in water contact angle of plasma immersion ion implantation (PIII)-treated PET as a function of time after removal from the treatment chamber. The treatment was carried out in a 2-mTorr nitrogen gas discharge maintained by 100 W rf power. PIII was administered using 20-μs pulses of 20 kV at a frequency of 50 Hz. The fluence of ions scales linearly with the treatment time with 400 s corresponding to a fluence of 1 × 1016 ions/cm2. Hydrophobic recovery characteristic curves are shown for fluencies corresponding to treatments of 20 s (diamonds), 40 s (squares), 80 s (triangles), 200 s (crosses), 400 s (stars) and 800 s (circles). Both the rate of hydrophobic recovery and the final contact angle are reduced by increasing fluence. Data provided by K. Mizuno
There is likely to be an optimum fluence of energetic ions that maximizes the ability of the surface to bind protein molecules covalently. At very high fluences, the ion bombardment process causes extensive carbonization and a consequent overlap of the electron spin wavefunctions, allowing them to move through the structure easily, so that many of the unpaired electrons can combine and be lost as active sites. The overlap of the wavefunctions is observed as a line narrowing of the ESR resonance and the increased mobility of the electrons is accompanied by a marked increase in electrical conductivity. The marked increase in conductivity has been observed for ion beam-treated PAN and PPO (Wasserman et al. 1985). At high fluences of energetic bombardment, most carbon-containing polymers are expected to produce similar structures that contain a highly crosslinked network. Under heavy bombardment, carbon becomes the dominant element and the structures evolve towards noncrystalline carbon with a dominance of graphite-like pi-conjugated bonding. The exceptions are polymers that contain, in addition to carbon, a high content of elements such as Si and O. These polymers can, at high fluences of ion bombardment, form a residual refractory structure in which carbon is not dominant and they may in fact contain very little carbon after ion modification.
Characteristics of the bound protein layer
There are a number of investigations (Gan et al. 2007; MacDonald et al. 2008; Yin et al. 2009c, d, f; Nosworthy et al. 2007; Ho et al. 2007; Gan et al. 2008; Kondyurin et al. 2008b, c; Bax et al. 2009, 2010) of protein layers attached to the surfaces produced by the energetic bombardment methods described in "P and Plasma deposited polymeric materials for covalent protein immobilization". Although the specific properties of the protein being attached affect the binding to the surface, there are a number of features which are common to all proteins studied so far. Protein attachment has been investigated using the enzymes horseradish peroxidise (HRP), catalase, and soybean peroxidise (SBP), and the extracellular matrix proteins, tropoelastin and collagen.
Attachment of dense protein monolayers
Protein attachment is achieved by incubation of the surfaces in a solution of protein in a buffer, such as phosphate buffer or phosphate-buffered saline (PBS). The protein layers are found to attach after incubation for times of the order of 1 h. Measurements using atomic force microscopy (AFM) and ellipsometry or surface plasmon resonance (SPR) have revealed that densely packed monolayers of HRP and SBP (Gan et al. 2007; MacDonald et al. 2008) are attached to ion-implanted polyethylene and polystryrene while comparatively sparse attachment is observed on untreated controls. A quartz crystal microbalance was used to observe the kinetics of attachment onto acetylene plasma-polymerized layers. The masses of catalase and HRP bound in an SDS-resistant manner corresponded to a dense monolayer coverage (Yin et al. 2009d).
Longevity of protein function
The catalytic function of the bound enzyme layers has been studied using colorimetric activity assays (Ho et al. 2007; Nosworthy et al. 2007; Gan et al. 2008; MacDonald et al. 2008) and, for extracellular matrix proteins, using cell spreading assays (Bax et al. 2009, 2010). In this section, we examine the retention of activity of surface attached biomolecules reported in the literature. A trend to better retention of activity was observed on the more hydrophilic surfaces as compared with hydrophobic ones. Modified or deposited surfaces subjected to energetic ion bombardment show better retention of activity of the attached protein than surfaces not exposed to ion bombardment. The highly crosslinked subsurface layer created by energetic bombardment may play a role in slowing down the hydrophobic recovery by reducing molecular diffusion/rotation as discussed in "Oxidation of the surface and crosslinking of the subsurface". Such a retention of hydrophilic character correlates with the increase in the shelf life of the surfaces when stored prior to protein immobilization and with the preservation of the protein function after immobilization (Ho et al 2007; Nosworthy et al 2007).
The activity of enzymes immobilised on polymers treated with ions from a plasma and stored in fresh buffer solution was observed to be retained over longer periods than that of enzymes attached to control surfaces (Nosworthy et al. 2007; Ho et al. 2007; Gan et al. 2008; Kondyurin et al. 2008c). The control surfaces were the untreated material and the same surface treated only by a plasma discharge in the absence of bias induced energetic impacts. For deposited plasma polymers, the retention of biological activity for bound protein increased with increasing hydrophilicity of the deposited film (Chen et al. 1993; Bax et al. 2009). Comparison of biased and unbiased depositions showed that those layers deposited with energetic ion impacts (i.e. bias deposition) performed better with respect to bioactivity retention (Kondyurin et al. 2008b).
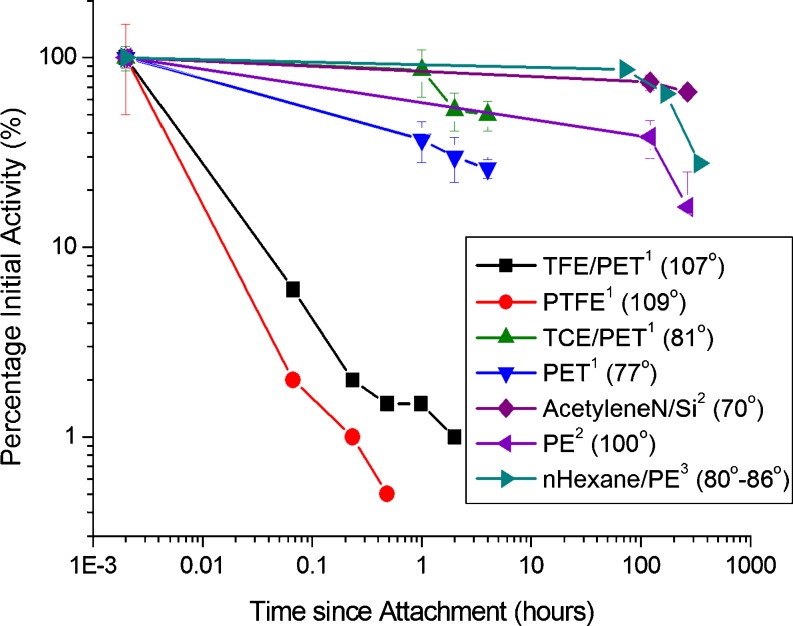
Figure 3 shows a comparison of activity reported for HRP attached to various plasma polymers and untreated polymer surfaces. The contact angle for each surface is given in parentheses in the legend on the Figure. The two surfaces which show the longest function retention were both prepared with energetic ion bombardment from n-hexane and acetylene precursor gases with self bias on the excited electrode (Kondyurin et al. 2008b) and pulsed bias of a few hundred volts (Yin et al. 2009e), respectively. The worst performance in terms of enzyme activity retention is observed on the most hydrophobic surfaces (Chen et al. 1993). The HRP on these two hydrophobic surfaces also had by far the lowest specific enzyme activity immediately after attachment by incubation in protein solution (Chen et al. 1993)—this is a point which is lost in Figure 3 due to the normalization of all initial enzyme activities to 100% and is indicative of the strong denaturation known to occur at very hydrophobic surfaces.
Fig. 3.
A comparison of activity reported for horseradish peroxidase (HRP) attached to various plasma polymers and untreated polymer surfaces. The contact angle for each surface is given in parentheses in the legend. The data are taken from (1) Chen et al. (1993) (first four datasets reading from the top of the legend), (2) Yin et al. (2009e) (next two datasets shown in the legend), and (3) Kondyurin et al. (2008b) (last dataset shown in the legend). The best retention of enzyme activity is observed on two hydrophilic surfaces deposited with energetic ion bombardment, and the two surfaces with the worst activity retention are the most hydrophobic. Where the surface is a plasma polymer, the nomenclature used in the legend shows the precursor chemical before the slash and the substrate after the slash. Where the surface is an untreated control the polymer alone is specified
Retention of activity after freeze drying
Freeze drying of proteins is a method for achieving convenient long-term storage and transportation. In many cases, removing water stabilizes the protein by removing degrees of freedom from the molecular motion and eliminating the action of proteases that break down protein in solution. The advantages of freeze drying are also applicable to surface-immobilized protein. The use of PIII has been shown to be one of four beneficial processing steps that promote the ability of the freeze-dried protein immobilized on a polymer surface to recover its activity when rehydrated after storage (Nosworthy et al. 2009).
Evidence for covalent attachment
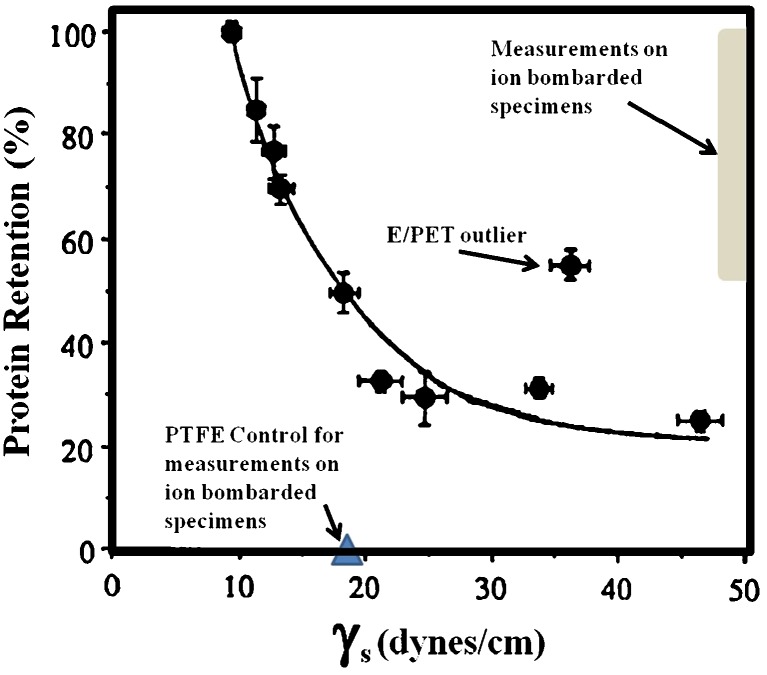
Strong protein attachment, resistant to SDS elution, was first reported to occur on plasma-deposited polymers in the early 1990s (Bohnert et al. 1990; Safranj et al. 1991; Kiaei et al. 1992; Chen et al. 1993; Danilich et al. 1992). At that time, Kiaei et al. attributed the strong attachment to physisorption due to strong hydrophobic interactions while Danilich et al. attributed it to covalent bonding. Since the majority of the plasma polymers that Kiaei et al. studied where highly hydrophobic, their assumption of physisorption appeared to be a reasonable conclusion. They, however, observed the presence of an outlier which appeared well off the curve of the surface energy versus the percentage of attached protein-resistant to SDS elution. This was a mildly hydrophilic surface deposited onto PET using plasma polymerization from an ethylene precursor (E/PET) (Kiaei et al. 1992).
The plasma polymers and plasma-modified surfaces created using energetic ion bombardment reported more recently are even more hydrophilic (Yin et al. 2009e; Kondyurin et al. 2009b) than the most hydrophilic surface studied in the earlier works, but nevertheless show much higher levels of SDS-resistant protein attachment (see, for example, MacDonald et al. 2008; Yin et al. 2009d; Nosworthy et al. 2007; Kondyurin et al. 2008b, c; Bax et al. 2009), rivalling those observed on the most hydrophobic of surfaces examined in the earlier studies. Figure 4 shows where the data obtained on the energetic ion-treated surfaces lie in relation to the adsorption curve of Kiaei et al. (1992). These data are indicative of the existence of a mechanism other than physisorption for attachment to plasma deposited and modified polymers which may also account for the outlier in the earlier data of Kiaei et al. Also shown is a point representing an untreated PTFE control used in the later works, which is also well away from the curve. The mechanism invoked to explain the deviations from the curve is the formation of a covalent bond between an active group on the surface and an exposed side chain of the protein (Bax et al. 2009; Yin et al. 2009d). This interpretation is supported by the use of a more aggressive SDS washing protocol in which the SDS (1–2% w/v) solution was heated to 70–90°C. With this protocol, all the protein adsorbed onto a highly hydrophobic surface such as PTFE is removed, indicating that the mechanism for any SDS-resistant attachment observed on more hydrophilic surfaces than PTFE cannot be associated with hydrophobic interactions. The use of a control more hydrophobic than the test sample is recommended before any conclusion of covalent attachment can be established.
Fig. 4.
Data showing SDS elution of albumin (Kiaei et al. 1992) from a range of plasma polymers and untreated polymeric materials and a curve suggested as indicative of the role of hydrophobic interactions in SDS-resistant surface attachment of proteins (Kiaei et al. 1992). The outlier observed by Kiaei et al. (1992) is indicated. The location of data obtained more recently from polymers and plasma polymers subjected to highly energetic ion bombardment is indicated by the gray rectangle while the location of measurements on a PTFE-untreated control using the same SDS washing protocol is indicated by a triangle. The fact that some data lie so far from the curve argues for a mechanism additional to physisorption contributing to high levels of SDS-resistant attachment. This mechanism is most likely to be covalent bonding
Possible mechanisms for the covalent binding
There are two aspects of a model for explaining the ability of plasma-modified and plasma-deposited surfaces to bind proteins covalently. The first is the site on the surface that binds the protein and the second is the chemical group on the protein that takes part in the bond. The binding sites on the protein are most likely to be exposed terminal groups or exposed side chains on certain amino acid residues. One candidate is the lysine residue, in view of the strong binding of poly-l-lysine onto plasma-modified polymers (Nosworthy et al. 2007).
Various binding sites on the polymer surface have been proposed including groups such as hydroxyl, carbonyl (Mitchell et al. 2005), carboxyl (Sartori et al. 2008), and amine (Martinez et al. 2000). One can speculate that these groups are created by the free radicals produced by the ion beam interactions with the polymer. Some of the radicals will react with their environment, either within the polymer or at the interface with the atmosphere or solution to produce these groups.
An alternative mechanism for the covalent attachment of protein molecules is a direct reaction of free radicals, such as the alkyl radical, with accessible side chain groups. Metsyats et al. (1999) interpreted an adhesion improvement of an epoxy adhesive to ion beam-treated PTFE as the result of a reaction with the free radicals produced by the ion beam treatment. Observations have been made very recently that correlate the number of unpaired electrons with the ability of the surface to bind bioactive protein covalently, implying that the unpaired electrons are mobile within the plasma modified or plasma deposited regions. Yin et al. (2009c) have shown in their Fig. 12 that the thickness of a plasma-deposited polymer increases both the total number of spins in the sample and the extent of covalent binding that is possible on the surface. The ability of PIII-modified surfaces to maintain their hydrophilic properties for long periods may be related to the reservoir of unpaired electrons that maintain the surface concentration of hydrophilic groups over time by supplying new radicals. It is known that energetic bombardment of the surface promotes the ability of spins to diffuse by creating overlaps in the electron wavefunctions (Wasserman et al. 1985).
Conclusions
Energetic ion bombardment during plasma modification and polymerization processes has been shown to be effective for promoting the robust surface attachment of protein molecules with extended longevity of activity compared to protein layers on untreated and plasma-treated (without ion bombardment) controls. The long-term retention of bioactivity both in solution and after freeze-dried storage appears to be correlated with the long -term retention of hydrophilic character observed on the ion-treated surfaces. These surfaces are typically observed to bind dense monolayers of protein with a high proportion of the layer being resistant to removal by vigorous SDS washing. This SDS-resistant binding onto a hydrophilic surface is indicative of covalent attachment of the protein to the surface. The covalent attachment is achieved without relying on linker molecules. The mechanism appears to involve groups on exposed termini or side chains of the protein and free radicals or active groups on the polymer surface. Recent results implicate mobile unpaired electrons in the ion-modified surface and subsurface regions as the mechanism facilitating the linker-free covalent attachment.
It is envisaged that these surface modification processes will have applications in biosensors, implantable medical devices, enzyme chemical processing, and diagnostic arrays. The ability to bind a dense layer of bioactive proteins strongly enough to resist washing by a simple incubation in a protein solution is a significant advantage as is the longevity of the binding capability for stored surfaces and the long-lived protein activity post-binding. The ease with which the processes can be used to achieve patterned surfaces and to treat complex shapes will also be significant advantages in many of these applications. Another important advantage for applications such as integrated biosensors is the process compatibility with CMOS processing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Methods for Characterising Plasma Treated surfaces and their ability to strongly attach bioactive protein layers (DOC 64 kb)
Acknowledgements
The authors wish to acknowledge the Australian Research Council for financial support. We acknowledge also Professor Hans Griesser for helpful discussions on SDS washing as a test for covalent attachment of proteins to surfaces.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-010-0028-1) contains supplementary material, which is available to authorized users.
Contributor Information
Marcela M. Bilek, Email: m.bilek@physics.usyd.edu.au
David R. McKenzie, Email: d.mckenzie@physics.usyd.edu.au
References
- Alvarez-Blanco S, Manloache S, Denes F. A novel plasma-enhanced way for surface-functionalization of polymeric substrates. Polym Bull. 2001;47:329–336. doi: 10.1007/s289-001-8189-4. [DOI] [Google Scholar]
- Azarkan M, Huet J, Baeyens-Volant D, et al. Affinity chromatography: a useful tool in proteomics studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;849:81–90. doi: 10.1016/j.jchromb.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Bax DV, McKenzie DR, Weiss AS, Bilek MM. Linker-free covalent attachment of the extracellular matrix protein tropoelastin to a polymer surface for directed cell spreading. Acta Biomater. 2009;5:3371–3381. doi: 10.1016/j.actbio.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Bax DV, McKenzie DR, Weiss AS, Bilek MMM. The linker-free covalent attachment of collagen to plasma immersion ion implantation treated polytetrafluoroethylenes and subsequent cell-binding activity. Biomaterials. 2010;31:2526–2534. doi: 10.1016/j.biomaterials.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Biederman H. Plasma polymer films. London: Imperial College Press; 2004. [Google Scholar]
- Biederman H, Osada Y. Plasma polymerisation processes. Amsterdam: Elsevier; 1992. [Google Scholar]
- Bilek M, McKenzie DR, Nosworthy NJ, Kondyurin A (2006) Activated polymers binding biological molecules. PCT/AU2007/000321 (WO2007/104107), priority 15
- Bilek M, McKenzie DR, Powles RC. Treatment of polymeric biomaterials by ion implantation. In: Chu PK, Liu X, editors. Biomaterials and surface modification. Kerala: Research Signpost; 2007. [Google Scholar]
- Bohnert JL, Fowler BC, Horbett TA, Hoffman AS. Plasma gas discharge deposited fluorocarbon polymers exhibit reduced elutability of adsorbed albumin and fibrinogen. J Biomater Sci Polym Ed. 1990;1:279–297. doi: 10.1163/156856289X00154. [DOI] [PubMed] [Google Scholar]
- Chen JP, Kiaei D, Hoffman AS. Activity of horseradish peroxide adsorbed on radio frequency glow discharge-treated polymers. J Biomater Sci Polym Ed. 1993;5:167–182. doi: 10.1163/156856294X00734. [DOI] [PubMed] [Google Scholar]
- d’Agostino R. Plasma deposition, treatment and etching of polymers. New York: Academic; 1990. [Google Scholar]
- Danilich MJ, Kandice Kottke-Marchant K, Anderson JM, Marchant RE. The immobilization of glucose oxidase onto radio-frequency plasma-modified poly(etherurethaneurea) J Biomater Polymer Sci. 1992;3:1952–216. doi: 10.1163/156856292x00123. [DOI] [PubMed] [Google Scholar]
- Frisk ML, Tepp WH, Johnson EA, Beebe DJ. Self-assembled peptide monolayers as a toxin sensing mechanism within arrayed microchannels. Anal Chem. 2009;81(7):2760–2767. doi: 10.1021/ac802707u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu RKY, Tian X, Chu PK. Enhancement of implantation energy using a conducting grid in plasma immersion ion implantation of dielectric/polymeric materials. Rev Sci Instrum. 2003;74:3697–3700. doi: 10.1063/1.1588757. [DOI] [Google Scholar]
- Gan BK, Bilek MMM, Kondyurin A, Mizuno K, McKenzie DR. Etching and structural changes in nitrogen plasma immersion ion implanted polystyrene films. Nucl Instrum Methods Phys Res, B Beam Interact Mater Atoms. 2006;247:254–260. doi: 10.1016/j.nimb.2006.01.063. [DOI] [Google Scholar]
- Gan BK, Kondyurin A, Bilek MM. Comparison of protein surface attachment on untreated and plasma immersion ion implantation treated polystyrene: protein islands and carpet. Langmuir. 2007;23:2741–2746. doi: 10.1021/la062722v. [DOI] [PubMed] [Google Scholar]
- Gan BK, Nosworthy NJ, McKenzie DR, dos Remedios CG, Bilek MMM. Plasma immersion ion implantation treatment of polyethylene for enhanced binding of active horseradish peroxidase. J Biomed Mater Res A. 2008;85:605–610. doi: 10.1002/jbm.a.31612. [DOI] [PubMed] [Google Scholar]
- Ganapathy R, Sarmadi M, Denes F. Immobilization of alpha-chymotrypsin on oxygen-RF-plasma functionalized PET and PP surfaces. J Biomater Sci Polym Ed. 1998;9:389–404. doi: 10.1080/09205063.1998.9753063. [DOI] [PubMed] [Google Scholar]
- Ganapathy R, Manolache S, Sarmadi M, Simonsick WJ, Jr, Denes F. Immobilization of active a-chymotrypsin on RF-plama functionalized polymer surfaces. J App Polymer Sci. 2000;78:1783–1796. doi: 10.1002/1097-4628(20001205)78:10<1783::AID-APP100>3.0.CO;2-#. [DOI] [Google Scholar]
- Ganapathy R, Manolache S, Sarmadi M, Denes F. Immobilization of papain on cold-plasma functionalized polyethylene and glass surfaces. J Biomater Sci Polym Ed. 2001;12:1027–1049. doi: 10.1163/156856201753252543. [DOI] [PubMed] [Google Scholar]
- George DF, Bilek MMM, McKenzie DR. Detecting and exploring partially unfolded states of proteins using a sensor with chaperone bound to its surface. Biosens Bioelectron. 2008;24:969–975. doi: 10.1016/j.bios.2008.07.076. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Roeradde J, Stoll D, Templin MS, Joos TO. Protein microarrays for diagnostic assays. Anal Bioanal Chem. 2009;393:1407–1416. doi: 10.1007/s00216-008-2379-z. [DOI] [PubMed] [Google Scholar]
- Ho JPY, Nosworthy NJ, Bilek MMM, Gan BK, McKenzie DR, Chu PK, dos Remedios CG. Plasma-treated polyethylene surfaces for improved binding of active protein. Plasma Process Polymers. 2007;4:583–590. doi: 10.1002/ppap.200600182. [DOI] [Google Scholar]
- Inagaki K. Surface modification and plasma polymerisation. Lancaster, PA, USA: Technomic; 1996. [Google Scholar]
- Itoyama K, Tokura S, Hayashi T. Lipoprotein lipase immobilization onto porous chitosan beads. Biotechnol Prog. 2008;10:225–229. doi: 10.1021/bp00026a013. [DOI] [PubMed] [Google Scholar]
- Karkhaneh A, Mirzadeh H, Ghaffariyeh AR. Simultaneous graft copolymerization of 2-hydroxyethyl methacrylate and acrylic acid onto polydimethylsiloxane surfaces using a two-step plasma treatment. J App Polymer Sci. 2007;105:2208–2217. doi: 10.1002/app.26216. [DOI] [Google Scholar]
- Kereszturi K, Tóth A, Mohai M, Bertóti I. Surface chemical and nanomechanical alterations in plasma immersion ion implanted PET. Surf Interface Anal. 2008;40:664–667. doi: 10.1002/sia.2643. [DOI] [Google Scholar]
- Kiaei D, Hoffman AS, Horbett TA. Tight binding of albumin to glow discharge treated polymers. J Biomater Sci Polym Ed. 1992;4:35–44. [PubMed] [Google Scholar]
- Kondyurin A, Bilek M. Ion beam treatment of polymers: application aspects from medicine to space. Oxford: Elsevier; 2008. [Google Scholar]
- Kondyurin A, Maitz MF (2005) Surface modification of ePTFE and implants using the same. WO2007/022174 A2, priority 18
- Kondyurin A, Gan BK, Bilek MMM, Mizuno K, McKenzie DR. Etching and structural changes of polystyrene films during plasma immersion ion implantation from argon plasm. Nucl Instrum Methods Phys Res, B Beam Interact Mater Atoms. 2006;251:413–418. doi: 10.1016/j.nimb.2006.06.027. [DOI] [Google Scholar]
- Kondyurin A, Gan BK, Bilek MMM, McKenzie DR, Wuhrer K. Argon plasma immersion ion implantation of polystyrene films. Nucl Instrum Methods Phys Res, B Beam Interact Mater Atoms. 2008;266:1074–1084. doi: 10.1016/j.nimb.2008.02.063. [DOI] [Google Scholar]
- Kondyurin A, Polonskyi O, Nosworthy N, Matousek J, Hlidek P, Biederman H, Bilek MMM. Covalent attachment and bioactivity of horseradish peroxidase on plasma-polymerized hexane coatings. Plasma Process Polymers. 2008;5:727–736. doi: 10.1002/ppap.200800010. [DOI] [Google Scholar]
- Kondyurin A, Nosworthy NJ, Bilek MMM. Attachment of horseradish peroxidase to polytetrafluorethylene (teflon) after plasma immersion ion implantation. Acta Biomater. 2008;4:1218–1225. doi: 10.1016/j.actbio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Kondyurin A, Nosworthy NJ, Bilek MMM, Jones R, Pigram PJ (2009a) Surface attachment of horseradish peroxidase to plasma immersion ion implantation modified nylon. (private communication)
- Kondyurin A, Naseri P, Fisher K, McKenzie DR, Bilek MMM. Mechanisms for surface energy changes observed in plasma immersion ion implanted polyethylene: the roles of free radicals and oxygen-containing groups. Polym Degrad Stab. 2009;94:638–646. doi: 10.1016/j.polymdegradstab.2009.01.004. [DOI] [Google Scholar]
- MacDonald C, Morrow R, Weiss AS, Bilek MM. Covalent attachment of functional protein to polymer surfaces: a novel one-step dry process. J Roy Soc Interface. 2008;5:663–669. doi: 10.1098/rsif.2007.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AJ, Manolache S, Gonzalez V, Young RA, Denes F. Immobilized biomolecules on plasma functionalized cellophane. I. Covalently attached alpha-chymotrypsin. J Biomater Sci, Polym Ed. 2000;11:415–438. doi: 10.1163/156856200743797. [DOI] [PubMed] [Google Scholar]
- Mesyats G, Klyachkin Y, Gavrilov N, Kondyurin A. Adhesion of polytetrafluorethylene modified by an ion beam. Vacuum. 1999;52:285–289. doi: 10.1016/S0042-207X(98)00300-5. [DOI] [Google Scholar]
- Mitchell SA, Davidson MR, Bradley RH. Improved cellular adhesion to acetone plasma modified polystyrene surfaces. J Colloid Interface Sci. 2005;281:122–129. doi: 10.1016/j.jcis.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Nosworthy NJ, Ho JP, Kondyurin A, McKenzie DR, Bilek MMM. The attachment of catalase and poly-l-lysine to plasma immersion ion implantation treated polyethylene. Acta Biomater. 2007;3:695–704. doi: 10.1016/j.actbio.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Nosworthy NJ, McKenzie DR, Bilek MM. A new surface for immobilizing and maintaining the function of enzymes in a freeze-dried state. Biomacromolecules. 2009;10:2577–2583. doi: 10.1021/bm900523m. [DOI] [PubMed] [Google Scholar]
- Oates TWH, Pigott J, McKenzie DR, Bilek M. Electric probe measurements of high voltage sheath collapse in cathodic arc plasmas due to surface charging of insulators. IEEE Trans Plasma Sci. 2003;3:438–443. doi: 10.1109/TPS.2003.813199. [DOI] [Google Scholar]
- Park JW, Lee DH, Kim YJ, Jang JH, Suh JY, Kim IS. Osteoconductivity of titanium implants coated with a new fusion protein containing four cell adhesion motifs. Tissue Eng Regenerative Med. 2009;6:888–892. [Google Scholar]
- Safranj A, Kiaei D, Hoffman AS. Antibody immobilization onto glow discharge treated polymers. Biotechnol Prog. 1991;7:173–177. doi: 10.1021/bp00008a012. [DOI] [PubMed] [Google Scholar]
- Sartori S, Rechichi A, Vozzi G, D'Acunto M, Heine E, Giusti P, Ciardelli G. Surface modification of a synthetic polyurethane by plasma glow discharge: preparation and characterization of bioactive monolayers. React Funct Polymers. 2008;68:809–821. doi: 10.1016/j.reactfunctpolym.2007.12.002. [DOI] [Google Scholar]
- Sugita Y, Suzuki Y, Someya K, Ogawa A, Furuhata H, Miyoshi S, Motomura T, Miyamoto H, Igo S, Nosé Y. Experimental evaluation of a new antithrombogenic stent using ion beam surface modification. Artif Organs. 2009;33:456–463. doi: 10.1111/j.1525-1594.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- Tóth A, Mohai M, Ujvári T, Bertóti I. Hydrogen plasma immersion ion implantation of ultra-high molecular weight polyethylene. Surf Interface Anal. 2006;38:898–902. doi: 10.1002/sia.2197. [DOI] [Google Scholar]
- Trevan MD, Boffey SA, Goulding KG, Stanbury PF. Enzyme applications in biotechnology: the biological principles. Milton Keynes, UK: Open University Press; 1987. [Google Scholar]
- Vallet-Regí M, Balas F, Cololla M, Manzano M. Bone-regenerative bioceramic implants with drug and protein controlled delivery capability. Prog Solid State Chem. 2008;36:163–191. doi: 10.1016/j.progsolidstchem.2007.10.002. [DOI] [Google Scholar]
- Wasserman B, Dresselhaus MS, Braunstein G, Wnek GE, Roth G. Electron spin resonance study of ion-implanted polymers. J Electron Mater. 1985;14:157–170. [Google Scholar]
- Woodward J. Immobilised cells and enzymes: a practical approach. USA: Oxford University Press; 1985. [Google Scholar]
- Woodward J. Immobilsed enzymes: adsorption and covalent coupling. In: Woodward J, editor. Immobilised cells and enzymes: a practical approach. USA: Oxford University Press; 1985. [Google Scholar]
- Yin Y, McKenzie DR, Bilek MM (2009a) Ellipsometric detection of post-drying conformational changes of surface immobilized protein monolayers at solid/air interfaces. (private communication)
- Yin Y, Nosworthy HJ, Youssef H, Gong B, Bilek MMM, McKenzie DR. Acetylene plasma coated surfaces for covalent immobilization of proteins. Thin Solid Films. 2009;517:5343–5346. doi: 10.1016/j.tsf.2009.03.045. [DOI] [Google Scholar]
- Yin Y, Fisher K, Nosworthy NJ, Bax DV, Rubanov S, Gong W, Weiss AS, McKenzie DR, Bilek MMM. Covalently bound biomimetic layers on plasma polymers with graded metallic interfaces for in vivo implants. Plasma Process Polymers. 2009c;6:658–666. [Google Scholar]
- Yin Y, Bilek MMM, McKenzie DR, Nosworthy NJ, Kondyurin A, Youssef H, Byrom MJ, Yang W. Acetylene plasma polymerised surfaces for covalent immobiization of dense bioactive protein monolayers. Surf Coatings Technol. 2009;203:1310–1316. doi: 10.1016/j.surfcoat.2008.10.035. [DOI] [Google Scholar]
- Yin Y, Nosworthy NJ, Gong B, Bax D, Kondyurin A, McKenzie DR, Bilek MMM. Plasma polymer surfaces compatible with a CMOS process for direct covalent enzyme immobilization. Plasma Process Polymers. 2009;6:68–75. doi: 10.1002/ppap.200800108. [DOI] [Google Scholar]
- Yin Y, Wise SG, Nosworthy NJ, Waterhouse A, Bax DV, Youssef H, Byrom MJ, Bilek MMM, McKenzie DR, Weiss AS, Ng MKC. Covalent immobilisation of tropoelastin on a plasma deposited interface for enhancement of endothelialisation on metal surfaces. Biomaterials. 2009;30:1675–1681. doi: 10.1016/j.biomaterials.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ziegler JF, Biersack JP. The stopping and range of ions in solids. New York: Pergamon; 1985. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods for Characterising Plasma Treated surfaces and their ability to strongly attach bioactive protein layers (DOC 64 kb)