Abstract
While the discovery of unconventional myosins raised expectations that their actions were responsible for most aspects of actin-based cell motility, few anticipated the wide range of cellular functions that would remain the purview of conventional two-headed myosins. The three nonsarcomeric, cellular myosins—M2A, M2B and M2C—participate in diverse roles including, but not limited to: neuronal dynamics, axon guidance and synaptic transmission; endothelial cell migration; cell adhesion, polarity, fusion and cytokinesis; vesicle trafficking and viral egress. These three conventional myosins each take on specific, differing functional roles during development and maturity, characteristic of each cell lineage; exact roles depend on the developmental stage of the cell, cellular location, upstream regulatory controls, relative isoform expression, orientation and associated state of the actin cytoscaffolds in which these myosins operate. Here, we discuss the separate yet related roles that characterise the actions of M2A, M2B and M2C in various cell types and show that these conventional myosins are responsible for functions as unconventional as any performed by unconventional myosins.
Keywords: Cellular myosins, M2A, M2B, M2C
Introduction
Conventional Class 2 myosins (myosin 2) have long been a research focus in animals because of their role as exquisitely organised, repetitive motors powering muscle contraction. However, when myosin 2 was detected in non-muscle cells, nearly 40 years ago, many were reluctant to relinquish the concept of the sarcomeric unit as being the basis for its mode of action. We know now that in addition to the dozen or so isoforms of myosin 2 found in muscle, most vertebrate cells express one or more of three non-muscle myosin isoforms, M2A, M2B and M2C, enzymes encoded by three distinct genes (Shohet et al. 1989; Katsuragawa et al. 1989; Simons et al. 1991; Kawamoto and Adelstein 1991; Golomb et al. 2004) which, in humans, are designated MYH9, MYH10 and MYH14, respectively. Individually, or in combination, these three isoforms are involved in diverse cellular functions including neurite outgrowth and axon guidance, wound closure and cell polarity, adhesion dynamics, synaptic transmission and vesicle trafficking, viral egress, cell migration and cytokinesis, and the mechanics of cell fusion—an incomplete listing that is sure to grow.
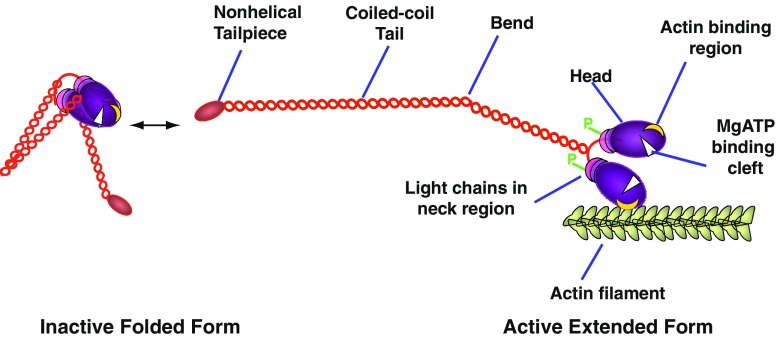
Conventional myosin molecules are hexamers, composed of three pairs of polypeptide chains—heavy chains (HCs), regulatory light chains (RLCs) and essential light chains (ELCs); there are two globular heads (each with one active site for ATP processing and one actin binding site) and a long coiled-coil tail (Fig. 1). The light chains are located at the junction between the head and tail, and play a major role in regulating activity of this enzyme. Key phosphorylatory control sites are located both within the N-terminal region of the RLC and close to or within the non-helical tail at the C-terminal end of the HC. All three non-muscle isoforms (M2A, M2B and M2C) are highly conserved (Golomb et al. 2004): the amino acid sequences of HCs from M2A and M2B show 80% identity and 89% similarity to each other while M2C shows 64% identity to the other two and about 80% similarity. Despite this level of conservation, all three myosins show differing intracellular localisations, possess different enzymatic and regulatory properties, and power distinctive cellular functions—features presumably related to regions of sequence difference found mainly at the C-terminus, N-terminus and in clusters near the head–tail junction. Additionally, M2B and M2C, but not M2A, exhibit alternatively spliced variants with additional amino acids on surface loops near ATP (B1; C1) and actin (B2; C2) binding sites (Takahashi et al. 1992; Itoh and Adelstein 1995; Golomb et al. 2004; Ma et al. 2006), which may be developmentally regulated (Takahashi et al. 1999). While it has not been established for M2A, M2B and M2C directly, it is thought that these myosins exist as compact, folded, filament-incompetent monomers—similar to structures determined for vertebrate smooth (Burgess et al. 2007) and scallop adductor (Jung et al. 2008) muscle myosins—and can switch to filament competency upon RLC phosphorylation (Fig. 1).
Fig. 1.
Schematic of Myosin 2 illustrating the phosphorylation-dependent reversible transition between the inactive folded monomer and the extended, assembly-competent, active form, the latter being able to interact with actin. Only one of two bends is shown in the extended myosin heavy chain. Phosphorylation sites (P) are located on each regulatory light chain in vertebrate myosin 2
Localisation, expression, development, disease
Comparative studies indicate that myosin isoform function varies with cell type, expression levels, cytoplasmic location, cytoskeletal organisation and prevailing upstream mechanisms of regulation. Typically, all three isoforms exhibit different, yet characteristic, localisations within a particular cell type, as in human umbilical vein endothelial cells (HUVEC) (Fig. 2a, b) where the localisation of M2A is found to be peripheral to M2B. Differences in isoform distribution may become accentuated during locomotion. For example, migrating growth cones of rat superior cervical ganglion neurons exhibit a differential distribution of M2A and M2B (Rochlin et al. 1995), M2B being the more peripheral. M2B is also found to be peripheral to M2A in lamellipodia of motile cultured Xenopus A6 cells (Kelley et al. 1996) and in Swiss 3T3 fibroblasts (Togo and Steinhardt 2004). By contrast, when monolayer cultures of bovine aortic endothelial cells migrate into a scratch wound, M2A is found at the leading edge of motile cells whereas M2B is found at the rear, moving the back end forwards (Kolega 1998, 2003). Arrangements of M2A and M2B are variable: for example, M2A and M2B can be found in association with stress fibres (Maupin et al. 1994; Vicente-Manzanares et al. 2007) where they may form sarcomere-like patterning as in fibroblasts (Rochlin et al. 1995; Lo et al. 2004). By contrast, in neuronal processes, ribbon-like arrangements of M2A have been observed (Fig. 2c). M2C, however, usually displays a punctate distribution throughout the cytoplasm, irrespective of motility (e.g. Wylie and Chantler 2008). Exact localisation for each myosin isoform is intrinsic to the structure of the C-terminal region of the myosin tail (Kolega 1998; Sandquist and Means 2008). Often, conventional myosins are found in close association with the plasma membrane and, in vitro, have been shown to associate with the anionic phospholipid, L-α-phosphatidyl-L-serine (principally found within the inner cytoplasmic leaflet of the plasma membrane) but not with the neutral phospholipid, L-α-phosphatidylcholine (Li et al. 1994; Murakami et al. 1995).
Fig. 2.
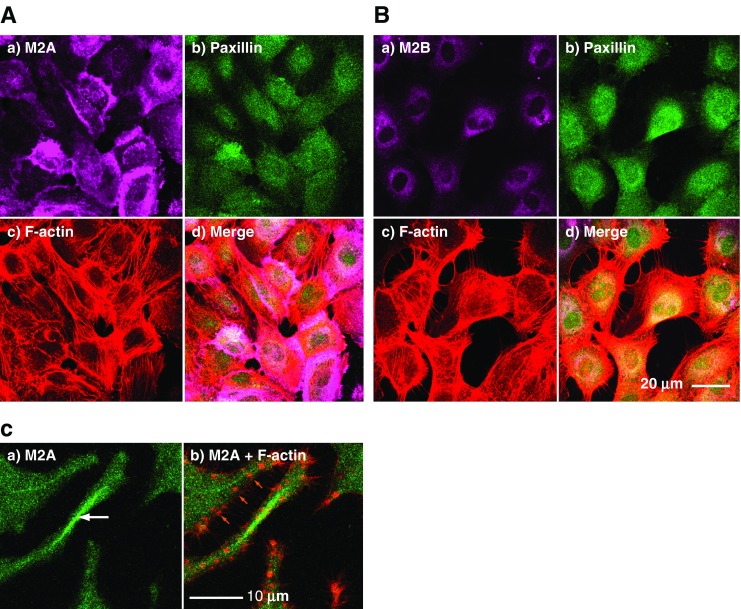
Localisation of M2A or M2B in endothelial (a,b) and neuronal (c) cell cultures. a,b Sub-confluent cultures of stationary HUVECs triple-stained to depict cytoskeletal antigens including M2A (a) and M2B (b). In each panel: a) myosin 2 (violet), b) paxillin (green), c) F-actin (red), d) merged images. Note the more peripheral distribution of M2A as compared with M2B. c High magnification image of neurites extending from cultured Neuro-2A cells indicating a ribbon-like arrangement of M2A centrally placed within a neurite, white arrow in (a), and distinct from the more particulate distribution found elsewhere in the image. In each panel: a) myosin 2A (green), b) myosin 2A (green) and F-actin (red). Note the discrete F-actin puncta which may indicate adhesion foci, orange arrows in (b)
Most mammalian cells express all three isoforms, but the relative amounts vary according to tissue and developmental stage (Murakami et al. 1993; Golomb et al. 2004). For example, M2B is highly expressed in non-vascular brain tissue, but is absent from many blood components, including lymphocytes, thymocytes and platelets. M2A is plentiful in leucocytes and organs rich in smooth muscle yet is minimal in many other tissues and absent from some cell lines, such as monkey COS-7 cells (Bao et al. 2005). M2C message is low in fetal tissues in comparison to M2A and M2B mRNA (Golomb et al. 2004). In adult tissues, M2C is absent from splenocytes yet is highly expressed in cells from the corpus callosum, for example (Golomb et al. 2004). There is a differential distribution of M2A and M2B in osteoclasts, with M2A, but not M2B, found within actin-rich podosomes and along the actin ring on which the podosomes are arranged, termed the sealing zone (Krits et al. 2002), a structure important for bone resorption.
The inserted isoforms of M2B also exhibit tissue-specific expression and are developmentally regulated. The B1 alternatively spliced isoform, possessing a 10 amino acid insert close to the ATP-binding loop, is essential to the migration of mammalian facial neurons (Ma et al. 2006) and is the most abundant isoform found in the human retina (Itoh and Adelstein 1995). The B2 isoform, possessing a 21 amino acid insert near the actin-binding region, is required for the postnatal development of cerebellar Purkinje cells (Ma et al. 2006) yet, for example, is not expressed in neurons of the olfactory lobe (Takahashi et al. 1999). While developmental roles for the inserted isoforms of M2C remain unclear, the C1 insert, consisting of 8 amino acids and found within the myosin head on the analogous loop to that of B1, has been shown to be required for cytokinesis in a human lung tumour cell line (Jana et al. 2006).
Mutations in the M2A heavy chain in humans have been linked to a family of autosomal-dominant diseases, characterised by giant platelets and thrombocytopenia, including May-Hegglin anomaly and Fechtner syndrome; patients with the latter also display sensorineural deafness, cataracts and nephritis (Hu et al. 2002); note, however, that mouse M2A knockouts display an early lethal phenotype (Conti et al. 2004). M2B knockout mice rarely survive beyond their day of birth: ablation of M2B disturbs cardiomyocyte development, leading to defects that resemble human conditions that affect the heart (e.g. tetralogy of Fallot). These mice also develop brain abnormalities, including hydrocephalus, before birth (Tullio et al. 1997, 2001). Conditional ablation of M2B in adult mice leads to cardiomyopathy and defective intercalated discs (Ma et al. 2009). Selective deletion of only M2B possessing the B1 inserts results in abnormal migration of facial neurons, which protrude into the fourth ventricle, initiating hydrocephalus (Ma et al. 2006). While M2C is largely absent during early embryonic development (Golomb et al. 2004), it has been identified in the developing mouse cochlea (Donaudy et al. 2004). In adult humans, single point mutations in M2C are known which contribute to the pathophysiology of hereditary hearing loss (Donaudy et al. 2004; Kim et al. 2005).
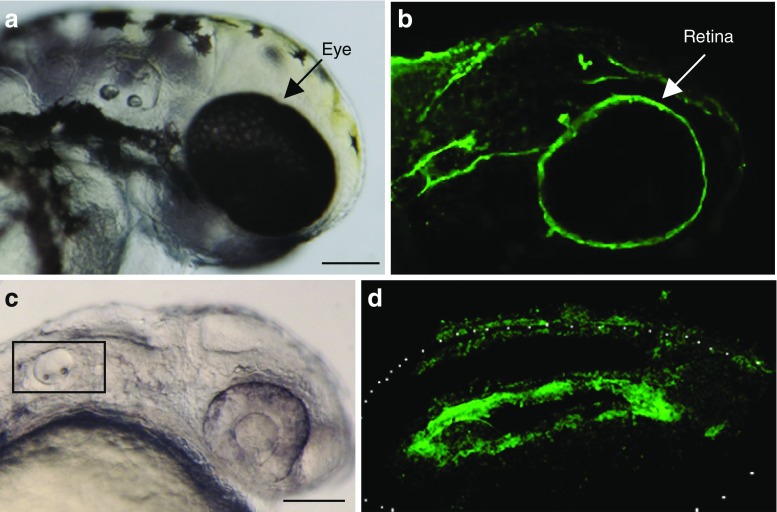
Non-mammalian vertebrates, such as zebrafish and Xenopus also possess M2A, M2B and M2C isoforms. The zebrafish embryo represents a largely untapped model for analysing myosin isoform function and its relevance to human disease in the whole animal (Lieschke and Currie 2007). Localisation studies in zebrafish demonstrate that isoform-specific myosin 2 expression is well conserved, most notably in the developing retina (Lin-Jones et al. 2009; see also Fig. 3a, b) and otic vesicle of the inner ear (Fig. 3c, d).
Fig. 3.
M2A and M2B expression in the developing zebrafish retina (a,b) and Otic Vesicle (c,d). a Zebrafish embryo at 48 h post-fertilisation (hpf). b Whole mount zebrafish embryo, 48 hpf, labelled with anti-M2B antibody and imaged by confocal laser scanning (CLS) microscopy. Note that the retina of the eye displays prominent staining for M2B. Antibodies specific for M2A did not stain the retina significantly (data not shown). Scale bar 125 μm. c Zebrafish embryo at 24 hpf. Region depicted in (d) indicated by rectangle. d Whole mount zebrafish embryo, 24 hpf, labelled with anti-M2A antibody and imaged with CLS microscopy. Note that the ventral floor of the otic vesicle displays prominent staining for M2A. Neuroblasts from this region delaminate and ultimately form the statoacoustic ganglion. Scale bar 125 μm
The players: binding partners and regulation
Many of the diverse functions performed by conventional myosins are brought about, and regulated, through their ability to interact with a heterogeneous group of binding partners—key players honing spatial and temporal aspects of myosin performance (Table 1). Actin is the universal myosin-binding partner. Detailed kinetics defining this interaction, using myosin subfragments, have been obtained for M2A (Kovacs et al. 2003), M2B (Wang et al. 2003; Rosenfeld et al. 2003) and M2C (Kim et al. 2005), as well as for murine myosins possessing key point mutations (Kim et al. 2005). All isoforms are relatively slow, M2A being the fastest, M2B the slowest; the rate of ADP release for M2B was >250 times slower than for skeletal muscle myosin (Wang et al. 2003), with none showing substantial ATPase enhancement by actin (Kim et al. 2005). Of functional importance, non-muscle myosin 2 isoforms exhibit relatively high duty ratios as compared with muscle myosins, meaning that they spend a much greater percentage of their cycling time in the attached state (Kovacs et al. 2007), a feature previously thought to be only associated with unconventional myosins; this trend is so pronounced in the case of M2B that it has been described as “unconventionally conventional” (Rosenfeld et al. 2003). These properties, coupled with appropriate localised expression, ensure that M2B is well adapted for a role supporting cortical tension or the movement of thixotropic actin meshworks (Rosenfeld et al. 2003; Chantler and Wylie 2003). In all motile cells, upstream control of the organisation of the actin cytoskeleton and onset of motility depends on opposing actions of members of the family of small GTPases: Rho, Rac and Cdc42 (Hall 1998). In neurons, for example, Rho acts upstream of M2A activation whereas Rac is upstream of M2B action (Amano et al. 1998; van Leeuwen et al. 1999; Chantler and Wylie 2003); Cdc42 controls filopodial extension (Meyer and Feldman 2002).
Table 1.
Binding partners for M2A, M2B and M2C that modify myosin function
| Binding Partner | Myosin 2 Isoform | Function of interaction | Selected reference |
|---|---|---|---|
| Actin | All | Generation of contractile force | Clark et al. (2007) |
| MLCK (myosin light chain kinase) | All | Activator - RLC (Ser19 and Thr18) phosphorylation - except for M2C-2 | Jana et al. (2009) |
| γ-PAK | M2A; M2B and M2C unknown | Activator - RLC (Ser19) phosphorylation | Chew et al. (1998) |
| Rho kinase (ROCK) | M2A > M2B; M2C unknown | Activator - RLC (direct) and MP (indirect) phosphorylation | Sandquist et al. (2006) |
| Citron kinase | Unknown specificity | Activator - through RLC phosphorylation | Yamashiro et al. (2003) |
| Zipper interacting protein (ZIP) kinase | Unknown specificity | Activator - through RLC phosphorylation | Ihara and MacDonald (2007) |
| MP (myosin phosphatase) | All | Inhibitor - dephosphorylation of phosphorylated RLC | Matsumura and Hartshorne (2008) |
| MRCK | Unknown specificity | Activator - RLC (direct) and MP (indirect) phosphorylation | Wilkinson et al. (2005) |
| S100A4 (Mts1) | M2A | Inhibitor - MHC (maps to 1909–1924); promotes filament disassembly | Li and Bresnick (2006) |
| PKCα/β | All | Modulator - RLC (Ser1, Ser2 and Thr9) - decreases affinity for MLCK | Komatsu and Ikebe (2007) |
| PKCβII | M2A and M2B | Activator - MHC (Ser1917 -M2A; multiple sites -M2B) phosphorylation | Ludowyke et al. (2006) |
| PKCγ | M2B | Activator - MHC (Ser1937 and Ser1952 + others) phosphorylation | Rosenberg and Ravid (2006) |
| aPKCζ | M2B | Inhibitor - MHC (Ser1937) phosphorylation | Even-Faitelson and Ravid (2006) |
| Casein kinase (CK) II | All | Activator - MHC (Ser1943) phosphorylation | Murakami et al. (1995) |
| CAMK II | M2A | Activator - MHC (Thr1940) phosphorylation | Buxton and Adelstein (2000) |
| TRPM7 | All | Inhibitor - through MHC phosphorylation at several sites | Clark et al. (2006) |
| cathepsin B | M2A | Proteolytic cleavage of M2A: enhances osteoclast fusion | McMichael et al. (2009) |
In each case, a single citation has been selected (often from among many) to facilitate entry to the relevant literature
CAMK calcium-calmodulin-dependent protein kinase, CK casein kinase, MHC myosin heavy chain, MLCK myosin light chain kinase, MP myosin phosphatase, MRCK myotonic dystrophy related-kinase cdc42 binding kinase, Mts1 metastasis-associated protein 1, PAK p21-activated kinase, PKC protein kinase C, RLC regulatory light chain, ROCK Rho-associated coiled-coil containing kinase, TRPM7 transient receptor potential melastatin 7, ZIP zipper interacting protein
The RLCs of all three myosin isoforms can be phosphorylated on Ser19 (numbering as for smooth muscle myosin), increasing actin-activated MgATPase activity and filament stability—hence referred to as an “activation site” (Komatsu and Ikebe 2007). A recent exception has been found, however: although the C2-inserted isoform of M2C can undergo RLC phosphorylation, the unphosphorylated molecule is constitutionally active (Jana et al. 2009). Exactly what benefits this confers on the action of the C2 isoform is unclear, but whatever these may be, they are restricted to neuronal cells, the only cells that express this particular isoform. While vertebrate myosin RLCs can be phosphorylated on Ser19 (or equivalent), Thr18 may also be phosphorylated—and the diphosphorylated product maximises filament stability (Ikebe et al. 1988). Myosin light chain kinase (MLCK) operates in a calcium-calmodulin-dependent manner and can activate myosin 2 through phosphorylation at both sites (Somlyo and Somlyo 2003). By contrast, Rho kinase (ROCK)(Amano et al. 1996), p21-activated kinase (PAK)(Chew et al. 1998), citron kinase (Yamashiro et al. 2003), zipper-interacting protein (ZIP) kinase (Ihara and MacDonald 2007) and myotonic dystrophy related-kinase cdc42 binding kinase (MRCK) (Leung et al. 1998) catalyse the same reactions but operate through diverse calcium-independent pathways. ZIP kinase, ROCK, PAK and MRCK also activate myosin indirectly, through direct phosphorylation of myosin phosphatase (MP), switching it off (Wilkinson et al. 2005; Matsumura 2005). MLCK appears to be the principal activator in pathways concerned with cell motility, morphology and division (Bresnick 1999). MRCK regulates nuclear positioning in migrating cells (Gomes et al. 2005) whereas PAK, localised at the centrosome, controls positioning of the microtubule organising centre (MTOC) (Zhao et al. 2005) (Table 1).
In addition to the RLC “activation sites” Thr18 and Ser19, “inhibitory sites” are also known: these include Ser1, Ser2 and Thr9 and were originally shown, in vitro, to require phosphorylation by unknown isoforms of PKC, which decreased RLC affinity for subsequent phosphoryation at activation sites by MLCK (Nishikawa et al. 1984). Recent work has shown that platelet-derived growth factor (PDGF) stimulation of NIH3T3 fibroblasts leads to activation of PKCα/β isoforms and phosphorylation of RLC Ser1 and Ser2, with subsequent reorganisation of myosin filaments and stress fibres (Komatsu and Ikebe 2007). However, PKC isoforms exert their most profound effects through HC phosphorylation.
Atypical PKCζ has been shown to be capable of phosphorylating M2B specifically on Ser1937 (Table 1) within the non-helical tailpiece of the HC (Even-Faitelson and Ravid 2006), leading to a decreased rate of filament assembly and, presumably, a decline in force-generating activity. This action is downstream of PKCζ phosphorylation by PAK1, and these authors suggest that PAK1 itself does not phosphorylate M2B HC directly. However, it may be noted that another PAK isoform, γ-PAK, has been shown to phosphorylate the myosin RLC on Ser19, leading to activation in vitro and endothelial cell retraction in vivo (Chew et al. 1998). While PKCζ phosphorylation of M2B is downstream of EGF stimulation, this is not the case for PAK1 phosphorylation and the activation of PKCζ (Even-Faitelson and Ravid 2006).
PKCγ (Table 1), but not PKCβ2, phosphorylates M2B HC at a number of sites including Ser1937 and Ser1952 (Rosenberg and Ravid 2006). This EGF-dependent phosphorylation leads to a redistribution of M2B within the cell margin (TSU-pr1 prostate carcinoma) and broadening of the lamellipodia. Exactly how these observations fit alongside the expected decline in activity following specific Ser1937 phosphorylation by PKCζ (Even-Faitelson and Ravid 2006) remains unclear, but the observed differences presumably relate to the consequences of phosphorylation at additional sites by PKCγ (Rosenberg and Ravid 2006).
PKCβII (Table 1) phosphorylates the HCs of both M2A and M2B; the former on Ser1917, a site close to the non-helical tailpiece but still within the helical domain (Ludowyke et al. 2006), and the latter at multiple sites within the nonhelical tailpiece (Murakami et al. 1998). PKCβII-dependent phosphorylation of the M2A HC in RBL-2H3 Mast cells occurs as a consequence of antigen binding to IgE and the phosphorylation time-course parallels degranulation (Ludowyke et al. 2006).
Although the dynamics of myosin 2 filament formation are undoubtedly regulated by RLC phosphorylation (Watanabe et al. 2007), binding partners of myosin 2 HCs modulate this interplay in an isoform-specific manner. While HC phosphorylations at multiple sites modulate M2B filament formation (Murakami et al. 2000; Even-Faitelson and Ravid 2006), metastasis-associated protein, S100A4 (a.k.a. Mts1), binds to the C-terminal end of the α-helical tail of M2A in a calcium-dependent manner and blocks the critical filament assembly site including the site for PKCβII phosphorylation at Ser1917, directly inhibiting filament assembly and leading to an inhibition of MgATPase activity (Ford et al. 1997; Kriajevska et al. 1998; Li and Bresnick 2006). S100A4 binding is itself blocked by casein-kinase II-dependent phosphorylation of M2A HC on Ser1943, thereby preventing S100A4 inhibition of filament assembly (Dulyaninova et al. 2005). Because S100A4 protein is a marker associated with human cancer, its binding to M2A is seen to be critical to onset of metastasis (Garrett et al. 2006).
A number of other binding partners also interact with myosin 2 isoforms. While further discussion is beyond the scope of this review, several are tabulated (Table 1) and key references given. Some aspects of their actions have been discussed in recent reviews (Bresnick 1999; Haystead 2005; Clark et al. 2007; Vicente-Manzanares et al. 2009).
Myosin 2 motors: the movers and shakers of cell function
Throughout the animal kingdom, during development, cells migrate considerable distances either individually (e.g. neuroblasts) or collectively (e.g. sheets of epithelial cells). In maturity, some cell types (e.g. phagocytes) maintain their ability to perform independent locomotion. In otherwise stationary cells, onset of malignancy leads to activation of cell motility and transformation to metastatic cancer cells, which invade previously undamaged tissues. Myosin 2 activation is critical to all these events, contractile forces being required to drive movement of the actin cytoskeleton, whether it is to pull the rear of a fibroblast forwards or to power neuronal dynamics.
Neurite outgrowth and axonal guidance
Neuronal outgrowth is a specialised form of cell motility by which nerve processes extend to form long axons or shorter dendrites during development, ultimately making contact with target neurons or muscle cells. Such movement occurs in response to external guidance cues by means of growth cones; vectorial progress is not uniform but consists of periods of outgrowth, stasis and retraction—elaboration and disassembly of adhesive contacts being important to each phase. At the leading edge of the growth cone is a broad, relatively thin, veil-like lamellipodium, actin polymerisation pushing the plasma membrane forwards (Pantaloni et al. 2001; Pollard and Borisy 2003); actin filaments, thus formed, are translocated rearwards, initiating a phenomenon known as retrograde actin flow. The forces required to move the bulk of the growth cone forwards are much greater than can be achieved through actin polymerisation alone, hence a requirement for myosin 2 molecular motors, which act vectorially and power forward outgrowth (Wylie et al. 1998; Bridgman et al. 2001) and rearward retraction (Amano et al. 1998; Wylie and Chantler 2003), as well as retrograde actin flow (Lin et al. 1996; Cai et al. 2006; Medeiros et al. 2006). Similar actions underpin the generation of protrusions in non-neuronal cells.
Isoform-specific knockdown by antisense oligonucleotides is a powerful technique that allows cellular functional deficit to be established within a normal protein expression background (Chantler and Wylie 2003). Knockdown of the specified message attenuates expression of the target protein (Fig. 4b, d, f) and leads to characteristic changes in cell morphology as compared with sense-treated controls (Fig. 4a, c, e). Using this technique in a mouse neuroblastoma cell line (Neuro-2A), we have shown that M2B and M2C are key drivers of neuronal outgrowth (Wylie et al. 1998; Wylie and Chantler 2008), a role that may be functionally coupled to guidance cues (Lo et al. 2004); antisense depletion of M2B or M2C leads to a compact phenotype (Wylie et al. 1998; Wylie and Chantler 2008) lacking neuritic processes (Fig. 4d, f). Similar conclusions have been reached using cultured neurons obtained from M2B knockout mice (Bridgman et al. 2001; Ma et al. 2006). Thrombin- or lysophosphatidate (LPA)-induced retraction of neuritic processes in Neuro-2A cells were blocked by either M2A knockdown or Y27632 (a specific inhibitor of Rho-kinase (ROCK)) but not by M2B-depletion (Wylie and Chantler 2003, 2008), implicating M2A as the functional motor driving neuronal retraction, downstream of Rho (Wylie and Chantler 2003; Chantler and Wylie 2003).
Fig. 4.
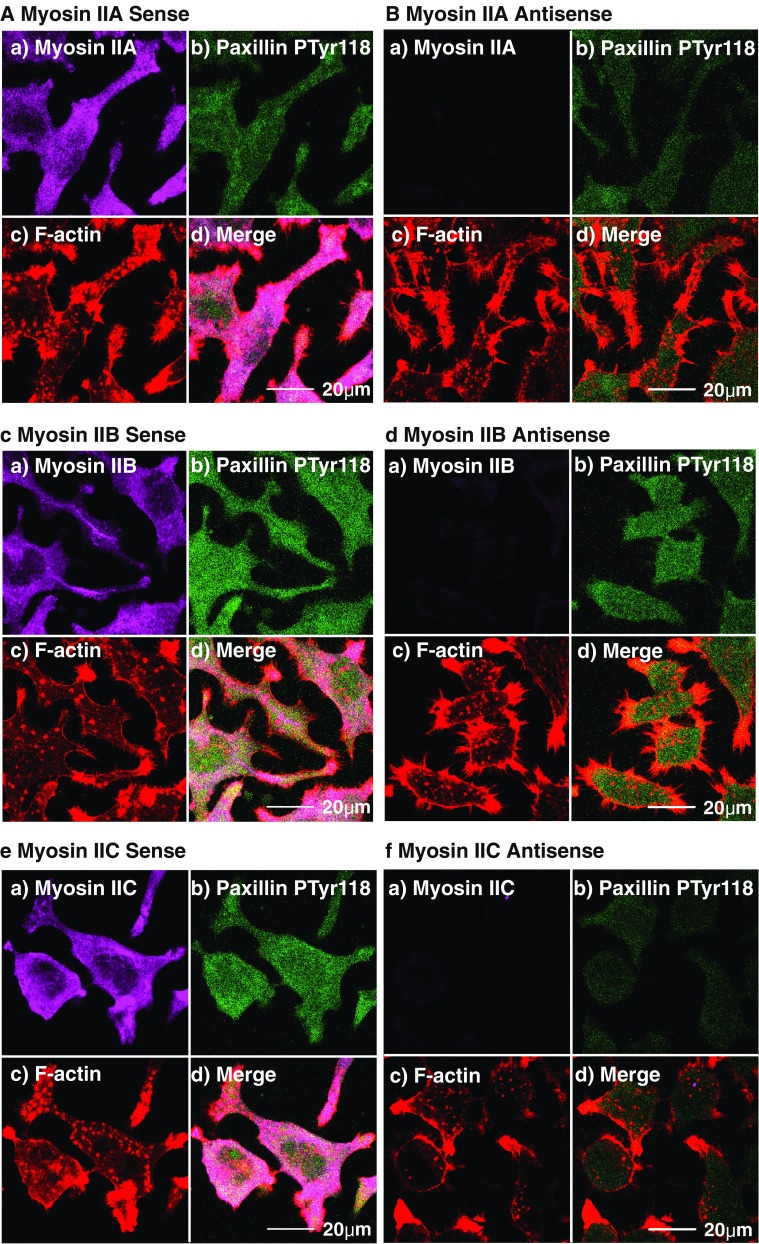
Targeted knockdown of M2A, M2B or M2C alters cell shape and/or cell adhesion. Paxillin phosphorylated on Tyr118 (an indicator of active adhesion) was observed relative to F-actin, M2A (a,b), M2B (c,d) or M2C (e,f) in Neuro-2A cells subsequent to 96-h exposure to either sense (a,c,e) or antisense (b,d,f) oligonucleotides targeting M2A (a,b), M2B (c,d) or M2C (e,f). All images obtained by CLS microscopy and are from confocal slices taken within 2 μm of the substratum to ensure inclusion of all structures associated with adhesion. Myosins pseudo-coloured in violet (Alexa-Fluor 633-labelled secondary) (a), phosphoTyr118-paxillin appears green (FITC-labelled secondary) (b), rhodamine-phalloidin F-actin is red (c) and merged images and scalebars are shown in (d). Note phenotypic changes as a result of M2B (d) and M2C (f) knockdowns, and corresponding attenuation of phosphoTyr118-paxillin expression with M2A (b) and M2C (f) knockdown
M2B has a role to play in neuronal guidance. As noted above, deletion of the B1 insert results in abnormal migration of facial neurons, which then protrude into the fourth ventricle, initiating hydrocephalus (Ma et al. 2006). Such defective migration may result not only from motor deficit but also as a consequence of faulty guidance. In support of this, it may be noted that M2B null fibroblasts exhibit defective directional movement in Boyden chambers while developing substantial traction forces, although no apparent response to the rigidity of the substratum could be detected (Lo et al. 2004). As a consequence, these authors view the action of M2B as one of coordinating protrusion and guidance. Further support comes from observing neurite outgrowth from embryonic day (13.5) mouse neuronal explant cultures growing on coverslips coated with stripes of poly-l-ornithine (PLO) and PLO plus laminin (Turney and Bridgman 2005). Normally, outgrowth from the laminin-enriched area is diverted along the boundary line with PLO alone. However, in the case of cells cultured from M2B knockout mice, signals at the boundary are ignored and turning is greatly reduced (Turney and Bridgman 2005).
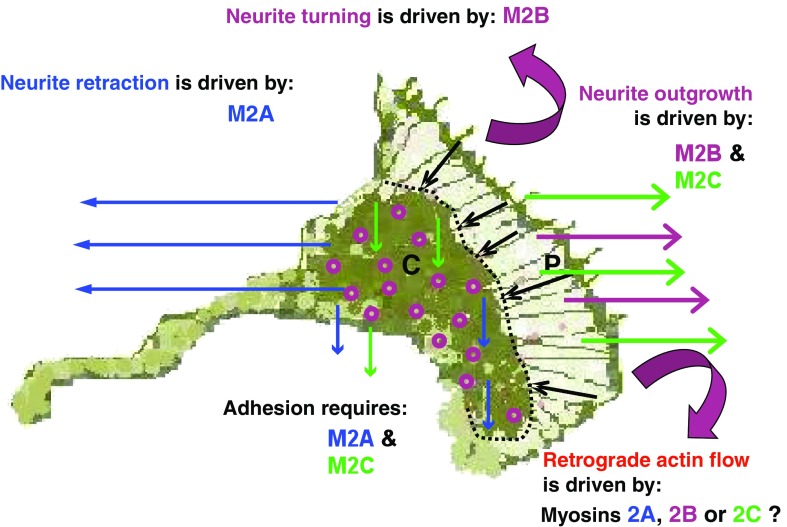
There is an inverse relationship between neuronal growth cone advance and retrograde actin flow (Lin and Forscher 1995), which is driven by one or more myosin 2 isoforms (Lin et al. 1996; Medeiros et al. 2006). Although it is clear that retrograde actin flow is integral to forward propulsion of the central domain of the growth cone, the exact identities of the isoforms responsible remain unknown. In an earlier model (Chantler and Wylie 2003), we presented arguments in support of M2B as the retrograde motor. However, the observation that the rate of actin retrograde flow increased in superior cervical ganglion neurons isolated from M2B knockout mice (Brown and Bridgman 2003) suggests otherwise. Nevertheless, in human MDA-MB-231 breast cancer cells, both M2A and M2B have been implicated (Betapudi et al. 2006). Additionally, time-lapse photography of magnetic bead transport over the surface of mouse embryonic fibroblast lamellipodia has implicated M2A (Cai et al. 2006). The observations that M2C can perform functions similar to M2B (Wylie and Chantler 2008) would suggest that M2C may also be considered a candidate for this role, consistent with our earlier reasoning (Chantler and Wylie 2003). Specific functional roles for the myosin 2 isoforms during nerve growth are adumbrated in Fig. 5.
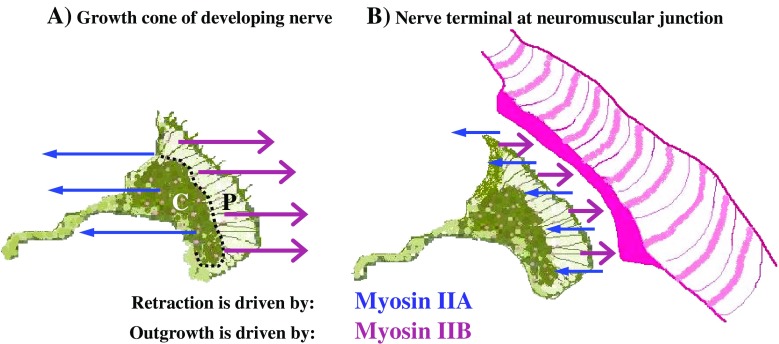
Fig. 5.
Schematic detailing the actions of M2A, M2B and M2C within a neuronal growth cone during development. M2A action is indicated by blue arrows, depicting M2A involvement in retraction and adhesion; M2B action is indicated by magenta arrows, depicting M2B involvement in outgrowth and turning; M2C action is indicated by green arrows, depicting M2C involvement in outgrowth and adhesion. Focal contacts are shown as purple “doughnuts”. P defines the peripheral zone of growth cone, separated from C, the central zone, by a dotted line. Black arrows indicate the direction of retrograde actin flow, known to be powered by one or more of the three myosin 2 motors
Wound closure, and cell polarity in migration
Many non-neuronal cells also exhibit myosin 2-driven locomotion. siRNA knockdown of M2B in the lung carcinoma A549 cell line compromised scratch wound closure within hours after scoring confluent cell layers (Sandquist et al. 2006). Analogous to conclusions from our neuronal studies, these authors found M2B-depleted A549 cells to be retractile. Cell shape contracted to simulate small polyhedrons; stress fibres and adhesion foci became confined to the periphery. By contrast, M2A-depleted cells accomplished wound closure at twice the rate of control cells (Sandquist et al. 2006), reminiscent of the enhanced rates of neurite outgrowth in adherent Neuro-2A cells after M2A knockdown (Wylie and Chantler 2003, 2008). Treatment of A549 cells with Y27632 led to results similar to those obtained by M2A-depletion, including increased cell spreading and increased rate of wound closure (Sandquist et al. 2006). Thrombin-induced cell rounding was also blocked following M2A depletion or Y27632 treatment, but not by M2B depletion—a comparable situation to that observed with regard to neurite retraction (Wylie and Chantler 2003). These targeted knockdown studies (Wylie and Chantler 2003; Sandquist et al. 2006), as well as Rho-kinase overexpression studies (Amano et al. 1998), define separate, yet linked, functions for M2A and M2B and indicate that M2A is downstream of Rho-ROCK signalling, while M2B is downstream of Rac (van Leeuwen et al. 1999; Wylie and Chantler 2003; Chantler and Wylie 2003; Sandquist et al. 2006). M2C is functionally distinct, complementing the actions of M2B with respect to neurite outgrowth, yet operating alongside M2A with respect to adhesion (Wylie and Chantler 2008); however, its upstream control pathways are not well defined at present.
Additionally, M2B may define cell polarity. Nuclear positioning and polarisation of the microtubule organising centre (MTOC) are downstream of kinases that target myosin 2 in migrating cells (Gomes et al. 2005; Zhao et al. 2005). Knockdown of M2B in cells from the chinese hamster ovary epithelial cell line (CHO.K1) (Vincente-Manzaneres et al. 2007) generated clockwise rotation of the nucleus coupled to the centrosome; this slow spin (∼2 h per cycle) led to the Golgi apparatus being smeared around the nuclear surface rather than occupying a polarised position (Vincente-Manzaneres et al. 2007), suggesting a role for M2B in achieving cell polarity.
Adhesion
Adhesion foci, comprising well over 100 different proteins (Zaidel-Bar et al. 2007), act as physical contact points and signalling conduits between a cell and its surroundings. Key to adhesion are proteins such as integrin, vinculin or paxillin, which are found in adherent cells (Zamir and Geiger 2001) including those of neuronal origin, where they mediate adhesive interactions of the growth cone (Gomez et al. 1996; de Curtis and Malanchini 1997; Renaudin et al. 1999). These adhesive foci are also sites for signal transmission between the extracellular matrix and the cell interior, using mechanisms that require myosin 2-generated contractility (Burridge and Chrzanowska-Wodnicka 1996). At these sites, the universal myosin partner, actin, is usually present in the form of complex bundles, known as stress fibres; each stress fibre terminates at an adhesion focus and interacts with a plethora of associated proteins including adaptors, regulators and kinases amongst others, collectively known as the “integrin adhesome” (Zaidel-Bar et al. 2007). Each adhesion focus acts as a mechanosensor, its size increasing in direct proportion to the forces sensed (Bershadsky et al. 2003); typically, mammalian cells exert stress forces of the order 2–10 nN/μm2 at these sites (Bershadsky et al. 2003). As a cell migrates, adhesion sites mature, beginning as small point contacts near the leading edge and developing to larger focal adhesions distal to the lamellipodia (Renaudin et al. 1999; Rottner et al. 1999). While the formation of nascent adhesions within the lamellipodium appears to be independent of myosin 2 activity (Choi et al. 2008), maturation of these nascent sites, behind the lamellipodium, requires myosin 2 action. As part of the maturation process, focal adhesion kinase (FAK) (Bellis et al. 1997) phosphorylates paxillin, the integrin assembly adaptor protein, on Tyr118, initiating active adhesion (Nakamura et al. 2000; Tsubouchi et al. 2002), a process involving conventional myosin activation. Interestingly, cell lineage specification of stem cells appears to be dependent on detection of extracellular matrix stiffness through a mechanism that is also dependent on myosin 2 and must therefore involve mature adhesions: soft matrices are neurogenic whereas rigid matrices are osteogenic (Engler et al. 2006).
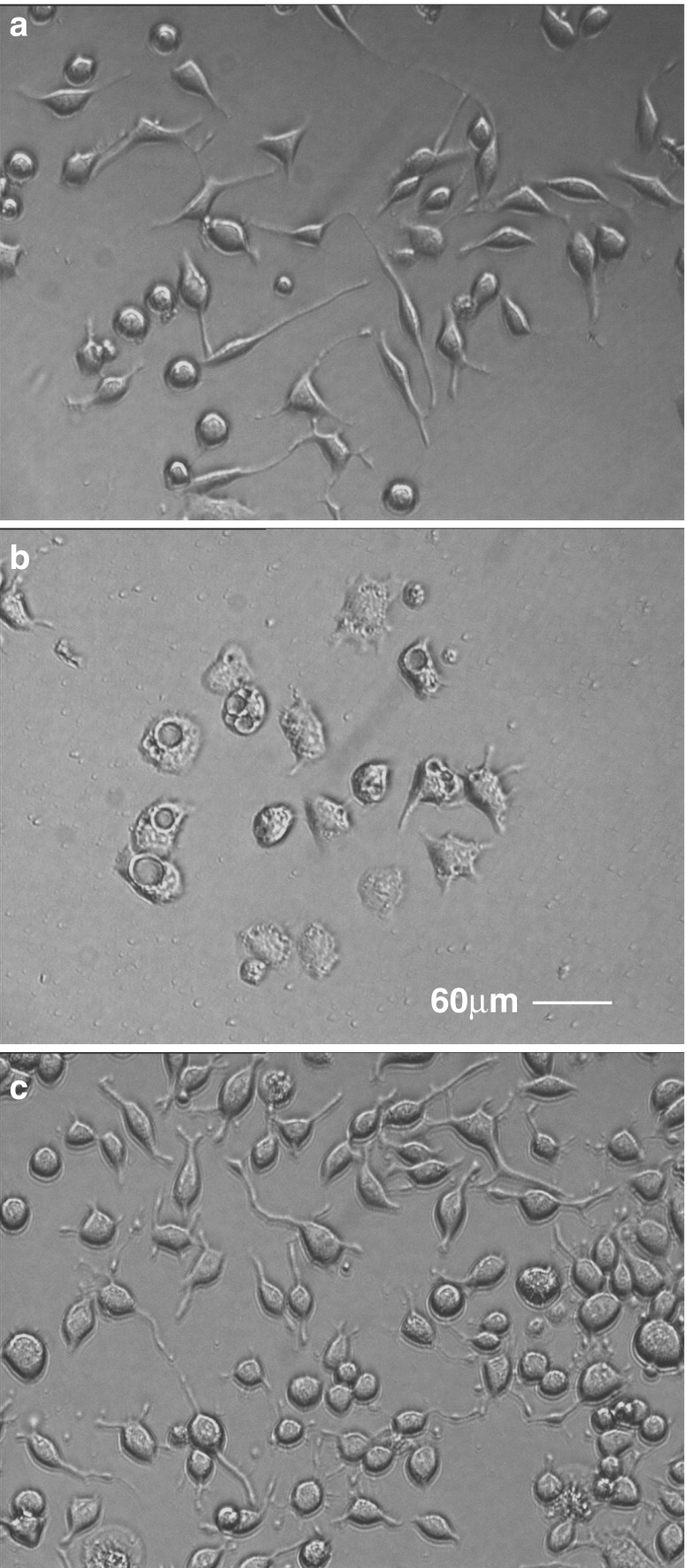
M2A and M2C play important roles in cell adhesion (Wylie and Chantler 2001, 2008; Conti et al. 2004). Antisense, but not sense, targeting of M2A or M2C in Neuro-2A cells (Wylie and Chantler 2001, 2008) leads to coincident attenuation of paxillin phosphoTyr118 fluorescence (Fig. 4b, f), a functional indicator of adhesion (Nakamura et al. 2000; Tsubouchi et al. 2002). Cells, so treated, display variable cell body diameters (decreasing in size following M2A-depletion; increasing with M2C-depletion) and detach easily from normally adherent surfaces (Wylie and Chantler 2001, 2008); in the case of M2C-depletion, pronounced vacuolisation ensues (Fig. 6) (Wylie and Chantler 2008). These results suggest that both M2A and M2C are required for adhesion (Fig. 5). However, despite the plethora of proteins defining the “integrin adhesome” (Zaidel-Bar et al. 2007), myosins were not identified as intrinsic components. This implies that conventional myosins “act at a distance”, a conclusion also reached by others (Vincente-Manzaneres et al. 2007) who have used isoform-specific knockdown to examine the roles of M2A and M2B in migrating CHO.K1 cells. Here, despite different cellular localisations, knockdown of either M2A or M2B prevented maturation of point contacts into larger adhesions, and these immature foci clustered into a band close to the leading edge (Vincente-Manzaneres et al. 2007). Action at a distance could be accommodated by adhesome components such as the adaptor proteins, paxillin or vinculin. It is therefore noteworthy that paxillin expression is also diminished as a consequence of either M2A or M2C knockdown in Neuro-2A cells, yet message levels remain unaffected (Wylie and Chantler 2001, 2008). Vinculin expression is also affected, albeit to a lesser degree (Chantler and Wylie 2003). Taken together, these results suggest that M2A and M2C may actively recruit key adhesome proteins, including paxillin and vinculin, to form focal contacts.
Fig. 6.
M2C-depletion dramatically alters the phenotype of Neuro-2A cells. Neuro-2A cells were treated with oligonucleotides targeting M2C for 96 h and observed by differential interference contrast (DIC) microscopy. Note that normal cell phenotype (a) is altered upon depletion of M2C causing an increase in cell body diameter and profuse vacuolisation (b); this aberrant phenotype is reversed following a 72-h recovery period in the absence of oligonucleotide (c). Scale bar 60 μm
Synaptic transmission, vesicle trafficking and viral egress
Actin-based vesicle trafficking is mainly the province of unconventional myosin motors, such as myosin 5a, and as such is beyond the scope of this review. Nevertheless, conventional myosins do have roles to play in this area. Early reports, for example, identified myosin 2 in isolated synaptic junctions (Beach et al. 1981), suggesting the possibility of presynaptic or postsynaptic roles, or both. Functional blockade of cholinergic synapses in cultured rat superior cervical ganglion neurons suggested a role for myosin 2 in presynaptic neurotransmitter release (Mochida et al. 1994). The morphology of synaptic architecture in these same neurons, but as seen in myosin 5a null mice, appeared normal (Takagishi et al. 2005). Furthermore, in wild-type animals, only M2B (not M2A or M5) could be localised on the presynaptic side of the terminal, suggestive of a role in presynaptic vesicle trafficking (Takagishi et al. 2005). Interestingly, the morphology of dendritic spines on rat hippocampal neurons, major sites for receipt of excitatory signals in central neuronal dendrites, were found to change dramatically following M2B knockdown—from a classic “mushroom” profile to filopodial-like protrusions that led to impaired synaptic function (Ryu et al. 2006). This implies that the postsynaptic localisation of conventional myosins in postsynaptic densities (Miller et al. 1992) may also play a functional role in synaptic transmission. Cerebellar Purkinje cells lacking the B2 isoform manifest reduced numbers of dendritic spines and branches (Ma et al. 2006).
Both M2A and M2B are present within the terminals of motor nerves from adult murine neuromuscular junctions (NMJs) (Vega-Riveroll et al. 2005). These specialised synapses are known to exhibit plasticity throughout life. The actions of M2A and M2B may contribute to de-nervation (retraction) and re-inervation (outgrowth) throughout life, thereby accounting for the observed plasticity. Because re-innervation is not 100% efficient, and involves muscle fibres distributed over a greater area, changes to this balance may provide a plausible explanation for the gradual decline in motor control with age, often accompanied by a decrease in muscle mass (sarcopenia) (Fig. 7).
Fig. 7.
Schematic showing proposed antagonistic actions of M2A and M2B in a neuronal growth cone during development (a) and within a neuromuscular junction (NMJ) at maturity (b). M2A retraction is indicated by blue arrows; M2B outgrowth is indicated by magenta arrows. P defines the peripheral zone of growth cone, separated from C, the central zone, by a dotted line. The NMJ is stylised for simplicity: neural input depicted by the same growth cone cartoon; muscle end-plate and fibre indicated in pink
Myosin 2 has been identified in vesicles leaving the trans-Golgi network (Ikonen et al. 1997; Musch et al. 1997). Calcium-dependent vesicle exocytosis occurs in the vicinity of breaks to the plasma membrane, an essential process that promotes resealing because it decreases membrane tension (Togo et al. 2000). In Swiss 3T3 cells, M2B-depletion suppressed wound-induced exocytosis and the membrane resealing process (Togo and Steinhardt 2004), an effect not seen following M2A knockdown. Consistent with these observations, S91 melanoma cells (which lack myosin 5a) showed a normal membrane resealing process (Togo and Steinhardt 2004). These results suggest that M2B plays a distinct role in vesicle trafficking.
In a different process related to vesicle trafficking and exocytosis, M2A was shown to facilitate egress of Herpes Simplex virus Type 1 through interaction with the major tegument protein, VP22 (van Leeuwen et al. 2002). Viral infection of Vero cells resulted in relocation of M2A from its pre-infection peripheral location to an arrangement of radial spokes surrounding the nucleus.
Cytokinesis
While extensive evidence has defined an involvement of myosin 2 in cytokinesis (Zang et al. 1997; Straight et al. 2003), much was derived using organisms, such as Dictyostelium, that possess a single myosin 2 isoform, or through the use of inhibitors that do not discriminate between isoforms, such as blebbistatin. Myosin 2, operating downstream of Rho-kinase, has been shown to play a role in furrow maturation in the early zebrafish embryo (Urven et al. 2006). Although regulation of myosin light chain phosphorylation during vertebrate cytokinesis is complex (Matsumura 2005), phosphorylation of the RLC at both Ser19 and Thr18 by citron kinase (Yamashiro et al. 2003) is important for contraction of the cleavage furrow. Yet relatively little is known of the contribution of individual myosin 2 isoforms. M2A and M2B were shown to localise to the cleavage furrow and midbody of dividing HeLa cells (Wei and Adelstein 2000), whereas a truncated construct of M2A lacking the N-terminal 591 amino acids did not, suggesting a requirement for N-terminal sequence in order for a cell to be competent for cytokinesis. Neither M2A nor M2B is absolutely essential for cytokinesis, there being considerable variability between different cell lines. For example, in cardiac myocytes (which lack M2A), complete ablation of M2B results in cell enlargement and binucleation, yet the presence of M2B at only 6% of wild-type levels confers normal phenotype (Takeda et al. 2003). Further, in monkey COS-7 cells (which also lack M2A), knockdown of M2B significantly inhibited cell proliferation and led to the generation of multi-nucleated cells (Bao et al. 2005), a situation rescued by exogenous M2A or M2C, but not by the less abundant endogenous M2C. This demonstrates that functional redundancy can occur between myosin 2 isoforms in some situations. By contrast, human A549 lung carcinoma cells, which possess all three isoforms, exhibited slow proliferation in response to knockdown of the C1 inserted isoform of M2C (Jana et al. 2006), a situation that could not be rescued by application of exogenous M2A or M2B. The C1 isoform was localised, during cell division, to the intercellular bridge linking dividing cells. Clearly, while myosin 2 isoforms are essential for cytokinesis, considerable variation exists depending on cell type and isoform composition.
Cell fusion
Cell fusion is essential for development and is the pivotal event during fertilisation. A crucial step in mammalian spermatozoa, en-route to fertilisation, is a form of specialised exocytosis known as the acrosome reaction. Initial contact with the zona pellucida of the oocyte leads to calcium-dependent multiple point fusions of the outer acrosomal membrane with the plasma membrane. This is followed by a coordinated fusion event in which the proteolytic contents of the acrosomal vesicle is released and can degrade the outer regions of the egg, allowing the spermatozoan to penetrate and achieve nuclear fusion. Although exact details remain obscure, M2A and M2B, as well as the microtubular molecular motor, kinesin, are required to pull the outer acrosomal membrane towards the plasma membrane (Oikonomopoulou et al. 2009). Once these membranes are in close proximity, SNARE-family proteins on either membrane are able to interact to achieve membrane fusion (De Blas et al. 2005; Oikonomopoulou et al. 2009).
Myoblast fusion to form multinucleated skeletal muscle myotubes represents another critical developmental step. Paired myoblasts change shape, from polyhedral to bipolar, as they align with each other; simultaneously, cortical actin walls form beneath the plasma membranes of these juxtaposed cells. As fusion progresses and vesicles appear, gaps emerge in the actin wall; vesicles pair across the aligned myoblasts, leading to the formation of fusion pores. M2A-depletion (Swailes et al. 2006; Duan and Gallagher 2009), or inhibition (Duan and Gallagher 2009), have been shown to prevent the required morphological change, formation of the cortical actin wall, appearance of vesicles and consequent myoblast fusion, indicating the importance of M2A action with respect to cell shape and actin bundle formation during myotube formation. M2B-depletion inhibited myoblast tail retraction, resulting in a doubling of cell length (Swailes et al. 2006).
By contrast, an alternative role for M2A has been defined during osteoclastogenesis, the final stage of osteoclast development involving the fusion of monocyte/macrophage precursor cells to produce multinucleated cells capable of bone resorption. Throughout the period of cell fusion, M2A expression was substantially reduced despite constant message levels (McMichael et al. 2009). Using a battery of protease inhibitors, these authors established that expressed M2A is specifically degraded by cathepsin B, which suppresses its action during cell fusion. Application of specific inhibitors to cathepsin B suppressed osteoclast fusion and led to diminished bone resorption. M2A knockdown led to large cells with increased multinucleation; these cells also exhibited increased spreading and decreased motility (McMichael et al. 2009).
Conclusions
The three mammalian conventional myosin isoforms, M2A, M2B and M2C, exhibit distinct localisations, separate yet linked functions and characteristic mechanisms of regulation; they are responsible for performing many of the mechanochemical actions that underlie cellular and intracellular movements. Exactly how they do so defines a range of, as yet unsolved, biophysical problems. It is no longer possible to view these myosins as degenerate alternatives, covering for each other through simple repetitive actions akin to sarcomeric contraction cycles undertaken by their myofibrillar counterparts. The actions defined by this group of non-sarcomeric conventional myosins are seen to be as unconventional as any of the functions undertaken by unconventional myosins.
Acknowledgements
We thank Brian Cox and Peter Nunn for invaluable assistance with figure preparation. Work described from the P.D.C. laboratory was originally supported by project grants from the Biotechnology and Biological Sciences Research Council (B.B.S.R.C.) and the Wellcome Trust.
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Bao J, Jana SS, Adelstein RS. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J Biol Chem. 2005;280:19594–19599. doi: 10.1074/jbc.M501573200. [DOI] [PubMed] [Google Scholar]
- Beach RL, Kelly PT, Babitch JA, Cotman CW. Identification of myosin in isolated synaptic junctions. Brain Res. 1981;225:75–93. doi: 10.1016/0006-8993(81)90319-X. [DOI] [PubMed] [Google Scholar]
- Bellis SL, Perrotta JA, Curtis MS, Turner CE. Adhesion of fibroblasts to fibronectin stimulates both serine and tyrosine phosphorylation of paxillin. Biochem J. 1997;325:375–381. doi: 10.1042/bj3250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11:26–33. doi: 10.1016/S0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME, Bridgman PC. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J Cell Sci. 2003;116:1087–1094. doi: 10.1242/jcs.00335. [DOI] [PubMed] [Google Scholar]
- Burgess SA, Yu S, Walker ML, Hawkins RJ, Chalovich JM, Knight PJ. Structures of smooth muscle myosin and heavy meromyosin in the folded, shutdown state. J Mol Biol. 2007;372:1165–1178. doi: 10.1016/j.jmb.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Buxton DB, Adelstein RS. Calcium-dependent threonine phosphorylation of nonmuscle myosin in stimulated RBL-2H3 mast cells. J Biol Chem. 2000;275:34772–34779. doi: 10.1074/jbc.M004996200. [DOI] [PubMed] [Google Scholar]
- Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, Sheetz MP. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler PD, Wylie SR. Elucidation of the separate roles of myosins IIA and IIB during neurite outgrowth, adhesion and retraction. IEE Proc Nanobiotechnol. 2003;150:111–125. doi: 10.1049/ip-nbt:20031076. [DOI] [PubMed] [Google Scholar]
- Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK) J Muscle Res Cell Motil. 1998;19:839–854. doi: 10.1023/A:1005417926585. [DOI] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–186. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- De Blas GA, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. 2005;3(10):e323. doi: 10.1371/journal.pbio.0030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I, Malanchini B. Integrin-mediated tyrosine phosphorylation and redistribution of paxillin during neuronal adhesion. Exp Cell Res. 1997;230:233–243. doi: 10.1006/excr.1996.3423. [DOI] [PubMed] [Google Scholar]
- Donaudy F, Snoeckx R, Pfister M, Zenner HP, Blin N, Di Stazio M, Ferrara A, Lanzara C, Ficarella R, Declau F, Pusch CM, Nürnberg P, Melchionda S, Zelante L, Ballana E, Estivill X, Van Camp G, Gasparini P, Savoia A. Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4) Am J Hum Genet. 2004;74:770–776. doi: 10.1086/383285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Gallagher PJ. Dependence of myoblast fusion on a cortical actin wall and nonmuscle myosin IIA. Dev Biol. 2009;325:374–385. doi: 10.1016/j.ydbio.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulyaninova NG, Malashkevich VN, Almo SC, Bresnick AR. Regulation of myosin-IIA assembly and Mts1 binding by heavy chain phosphorylation. Biochemistry. 2005;44:6867–6876. doi: 10.1021/bi0500776. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Even-Faitelson L, Ravid S. PAK1 and aPKCζ regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol Biol Cell. 2006;17:2869–2881. doi: 10.1091/mbc.E05-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford HL, Silver DL, Kachar B, Sellers JR, Zain SB. Effect of Mts1 on the structure and activity of nonmuscle myosin II. Biochemistry. 1997;36:16321–16327. doi: 10.1021/bi971182l. [DOI] [PubMed] [Google Scholar]
- Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–680. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Roche FK, Letourneau PC. Chick sensory neuronal growth cones distinguish fibronectin from laminin by making substratum contacts that resemble focal contacts. J Neurobiol. 1996;29:18–34. doi: 10.1002/(SICI)1097-4695(199601)29:1<18::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Haystead AJ. ZIP kinase, a key regulator of myosin protein phosphatase 1. Cell Signal. 2005;17:1313–1322. doi: 10.1016/j.cellsig.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Hu A, Wang F, Sellers JR. Mutations in human nonmuscle myosin IIA found in patients with May-Hegglin anomaly and Fechtner syndrome result in impaired enzymatic function. J Biol Chem. 2002;277:46512–46517. doi: 10.1074/jbc.M208506200. [DOI] [PubMed] [Google Scholar]
- Ihara E, MacDonald JA. The regulation of smooth muscle contractility by zipper-interacting protein kinase. Can J Physiol Pharmacol. 2007;85:79–87. doi: 10.1139/Y06-103. [DOI] [PubMed] [Google Scholar]
- Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988;263:6432–6437. [PubMed] [Google Scholar]
- Ikonen E, de Almeida JB, Fath KR, Burgess DR, Ashman K, Simons K, Stow JL. Myosin II is associated with Golgi membranes: identification of p200 as nonmuscle myosin II on Golgi-derived vesicles. J Cell Sci. 1997;110:2155–2164. doi: 10.1242/jcs.110.18.2155. [DOI] [PubMed] [Google Scholar]
- Itoh K, Adelstein RS. Neuronal cell expression of inserted isoforms of vertebrate nonmuscle myosin heavy chain II-B. J Biol Chem. 1995;270:14533–14540. doi: 10.1074/jbc.270.24.14533. [DOI] [PubMed] [Google Scholar]
- Jana SS, Kawamoto S, Adelstein RS. A specific isoform of nonmuscle myosin II-C is required for cytokinesis in a tumor cell line. J Biol Chem. 2006;281:24662–24670. doi: 10.1074/jbc.M604606200. [DOI] [PubMed] [Google Scholar]
- Jana SS, Kim KY, Mao J, Kawamoto S, Sellers JR, Adelstein RS. An alternatively spliced isoform of non-muscle myosin II-C is not regulated by myosin light chain phosphorylation. J Biol Chem. 2009;284:11563–11571. doi: 10.1074/jbc.M806574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Burgess SA, Billington N, Colegrave M, Patel H, Chalovich JM, Chantler PD, Knight PJ. Conservation of the regulated structure of folded myosin 2 in species separated by at least 600 million years of independent evolution. Proc Natl Acad Sci USA. 2008;105:6022–6026. doi: 10.1073/pnas.0707846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragawa Y, Yanagisawa M, Inoue A, Masaki T. Two distinct nonmuscle myosin-heavy-chain mRNAs are differentially expressed in various chicken tissues. Identification of a novel gene family of vertebrate non-sarcomeric myosin heavy chains. Eur J Biochem. 1989;184:611–616. doi: 10.1111/j.1432-1033.1989.tb15057.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Adelstein RS. Chicken nonmuscle myosin heavy chains: differential expression of two mRNAs and evidence for two different polypeptides. J Cell Biol. 1991;112:915–924. doi: 10.1083/jcb.112.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CA, Sellers JR, Gard DL, Bui D, Adelstein RS, Baines IC. Xenopus nonmuscle myosin heavy chain isoforms have different subcellular localizations and enzymatic activities. J Cell Biol. 1996;134:675–687. doi: 10.1083/jcb.134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Kovács M, Kawamoto S, Sellers JR, Adelstein RS. Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J Biol Chem. 2005;280:22769–22775. doi: 10.1074/jbc.M503488200. [DOI] [PubMed] [Google Scholar]
- Kolega J. Cytoplasmic dynamics of myosin IIA and IIB: spatial ‘sorting’ of isoforms in locomoting cells. J Cell Sci. 1998;111:2085–2095. doi: 10.1242/jcs.111.15.2085. [DOI] [PubMed] [Google Scholar]
- Kolega J. Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction. Mol Biol Cell. 2003;14:4745–4757. doi: 10.1091/mbc.E03-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Ikebe M. The phosphorylation of myosin II at the Ser1 and Ser2 is critical for normal platelet-derived growth factor induced reorganization of myosin filaments. Mol Biol Cell. 2007;18:5081–5090. doi: 10.1091/mbc.E06-12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács M, Wang F, Hu A, Zhang Y, Sellers JR. Functional divergence of human cytoplasmic myosin II: kinetic characterization of the non-muscle IIA isoform. J Biol Chem. 2003;278:38132–38140. doi: 10.1074/jbc.M305453200. [DOI] [PubMed] [Google Scholar]
- Kovács M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA. 2007;104:9994–9999. doi: 10.1073/pnas.0701181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriajevska M, Tarabykina S, Bronstein I, Maitland N, Lomonosov M, Hansen K, Georgiev G, Lukanidin E. Metastasis-associated Mts1 (S100A4) protein modulates protein kinase C phosphorylation of the heavy chain of nonmuscle myosin. J Biol Chem. 1998;273:9852–9856. doi: 10.1074/jbc.273.16.9852. [DOI] [PubMed] [Google Scholar]
- Krits I, Wysolmerski RB, Holliday LS, Lee BS. Differential localization of myosin II isoforms in resting and activated osteoclasts. Calcif Tissue Int. 2002;71:530–538. doi: 10.1007/s00223-001-1112-0. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Miller M, Chantler PD. Association of a cellular myosin II with anionic phospholipids and the neuronal plasma membrane. Proc Natl Acad Sci USA. 1994;91:853–857. doi: 10.1073/pnas.91.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Bresnick AR. The S100A4 metastasis factor regulates cellular motility via a direct interaction with myosin-IIA. Cancer Res. 2006;66:5173–5180. doi: 10.1158/0008-5472.CAN-05-3087. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14:763–771. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/S0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J, Sohlberg L, Dosé A, Breckler J, Hillman DW, Burnside B. Identification and localization of myosin superfamily members in fish retina and retinal pigmented epithelium. J Comp Neurol. 2009;513:209–223. doi: 10.1002/cne.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludowyke RI, Elgundi Z, Kranenburg T, Stehn JR, Schmitz-Peiffer C, Hughes WE, Biden TJ. Phosphorylation of nonmuscle myosin heavy chain IIA on Ser1917 is mediated by protein kinase C beta II and coincides with the onset of stimulated degranulation of RBL-2H3 mast cells. J Immunol. 2006;177:1492–1499. doi: 10.4049/jimmunol.177.3.1492. [DOI] [PubMed] [Google Scholar]
- Ma X, Kawamoto S, Uribe J, Adelstein RS. Function of the neuron-specific alternatively spliced isoforms of nonmuscle myosin II-B during mouse brain development. Mol Biol Cell. 2006;17:2138–2149. doi: 10.1091/mbc.E05-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Takeda K, Singh A, Yu ZX, Zerfas P, Blount A, Liu C, Towbin JA, Schneider MD, Adelstein RS, Wei Q (2009) Conditional ablation of nonmuscle myosin II-B delineates heart defects in adult mice. Circ Res [Epub ahead of print] PubMed PMID: 19815823 [DOI] [PMC free article] [PubMed]
- Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: many roles in cell function. Biochem Biophys Res Commun. 2008;369:149–156. doi: 10.1016/j.bbrc.2007.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin P, Phillips CL, Adelstein RS, Pollard TD. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J Cell Sci. 1994;107:3077–3090. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- McMichael BK, Wysolmerski RB, Lee BS. Regulated proteolysis of nonmuscle myosin IIA stimulates osteoclast fusion. J Biol Chem. 2009;284:12266–12275. doi: 10.1074/jbc.M808621200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Meyer G, Feldman EL. Signaling mechanisms that regulate actin-based motility processes in the nervous system. J Neurochem. 2002;83:490–503. doi: 10.1046/j.1471-4159.2002.01185.x. [DOI] [PubMed] [Google Scholar]
- Miller M, Bower E, Levitt P, Li D, Chantler PD. Myosin II distribution in neurons is consistent with a role in growth cone motility but not synaptic vesicle mobilization. Neuron. 1992;8:25–44. doi: 10.1016/0896-6273(92)90106-N. [DOI] [PubMed] [Google Scholar]
- Mochida S, Kobayashi H, Matsuda Y, Yuda Y, Muramoto K, Nonomura Y. Myosin II is involved in transmitter release at synapses formed between rat sympathetic neurons in culture. Neuron. 1994;13:1131–1142. doi: 10.1016/0896-6273(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Murakami N, Trenkner E, Elzinga M. Changes in expression of nonmuscle myosin heavy chain isoforms during muscle and nonmuscle tissue development. Dev Biol. 1993;157:19–27. doi: 10.1006/dbio.1993.1108. [DOI] [PubMed] [Google Scholar]
- Murakami N, Singh SS, Chauhan VPS, Elzinga M. Phospholipid binding, phosphorylation by protein kinase C, and filament assembly of the COOH terminal heavy chain fragments of nonmuscle myosin II isoforms MIIA and MIIB. Biochemistry. 1995;34:16046–16055. doi: 10.1021/bi00049a019. [DOI] [PubMed] [Google Scholar]
- Murakami N, Chauhan VPS, Elzinga M. Two non-muscle myosin heavy heavy chain isoforms expressed in rabbit brains: filament forming properties, the effects of phosphorylation by protein kinase C and casein kinase II, and the location of the phosphorylation sites. Biochemistry. 1998;37:1989–2003. doi: 10.1021/bi971959a. [DOI] [PubMed] [Google Scholar]
- Murakami N, Koyula L, Hwang YW. Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry. 2000;39:11441–11451. doi: 10.1021/bi000347e. [DOI] [PubMed] [Google Scholar]
- Musch A, Cohen D, Rodriguez-Boulan E. Myosin II is involved in the production of constitutive transport vesicles from the TGN. J Cell Biol. 1997;138:291–306. doi: 10.1083/jcb.138.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Yano H, Uchida H, Hashimoto S, Schaefer E, Sabe H. Tyrosine phosphorylation of paxillin alpha is involved in temporospatial regulation of paxillin-containing focal adhesion formation and F-actin organization in motile cells. J Biol Chem. 2000;275:27155–27164. doi: 10.1074/jbc.M000679200. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Sellers JR, Adelstein RS, Hidaka H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase. J Biol Chem. 1984;259:8808–8814. [PubMed] [Google Scholar]
- Oikonomopoulou I, Patel H, Watson PF, Chantler PD. Relocation of myosin and actin, kinesin and tubulin in the acrosome reaction of bovine spermatozoa. Reprod Fertil Dev. 2009;21:364–377. doi: 10.1071/RD08166. [DOI] [PubMed] [Google Scholar]
- Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292:1502–1506. doi: 10.1126/science.1059975. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- Renaudin A, Lehmann M, Girault J, McKerracher L. Organization of point contacts in neuronal growth cones. J Neurosci Res. 1999;55:458–471. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Ravid S. Protein kinase Cgamma regulates myosin IIB phosphorylation, cellular localization, and filament assembly. Mol Biol Cell. 2006;17:1364–1374. doi: 10.1091/mbc.E05-07-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld SS, Xing J, Chen LQ, Sweeney HL. Myosin IIb is unconventionally conventional. J Biol Chem. 2003;278:27449–27455. doi: 10.1074/jbc.M302555200. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/S0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Ryu J, Liu L, Wong TP, Wu DC, Burette A, Weinberg R, Wang YT, Sheng M. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 2006;49:175–182. doi: 10.1016/j.neuron.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Sandquist JC, Means AR. The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells. Mol Biol Cell. 2008;19:5156–5167. doi: 10.1091/mbc.E08-05-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem. 2006;281:35873–35883. doi: 10.1074/jbc.M605343200. [DOI] [PubMed] [Google Scholar]
- Shohet RV, Conti MA, Kawamoto S, Preston YA, Brill DA, Adelstein RS. Cloning of the cDNA encoding the myosin heavy chain of a vertebrate cellular myosin. Proc Natl Acad Sci USA. 1989;86:7726–7730. doi: 10.1073/pnas.86.20.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Wang M, McBride OW, Kawamoto S, Yamakawa K, Gdula D, Adelstein RS, Weir L. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ Res. 1991;69:530–539. doi: 10.1161/01.res.69.2.530. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Swailes NT, Colegrave M, Knight PJ, Peckham M. Non-muscle myosins 2A and 2B drive changes in cell morphology that occur as myoblasts align and fuse. J Cell Sci. 2006;119:3561–3570. doi: 10.1242/jcs.03096. [DOI] [PubMed] [Google Scholar]
- Takagishi Y, Futaki S, Itoh K, Espreafico EM, Murakami N, Murata Y, Mochida S. Localization of myosin II and V isoforms in cultured rat sympathetic neurones and their potential involvement in presynaptic function. J Physiol. 2005;569:195–208. doi: 10.1113/jphysiol.2005.095943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Kawamoto S, Adelstein RS. Evidence for inserted sequences in the head region of nonmuscle myosin specific to the nervous system. Cloning of the cDNA encoding the myosin heavy chain-B isoform of vertebrate nonmuscle myosin. J Biol Chem. 1992;267:17864–17871. [PubMed] [Google Scholar]
- Takahashi M, Hirano T, Uchida K, Yamagishi A. Developmentally regulated expression of a nonmuscle myosin heavy chain IIB inserted isoform in rat brain. Biochem Biophys Res Commun. 1999;259:29–33. doi: 10.1006/bbrc.1999.0717. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ Res. 2003;93:330–337. doi: 10.1161/01.RES.0000089256.00309.CB. [DOI] [PubMed] [Google Scholar]
- Togo T, Steinhardt RA. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol Biol Cell. 2004;15:688–695. doi: 10.1091/mbc.E03-06-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T, Krasieva TB, Steinhardt RA. A decrease in membrane tension precedes successful cell membrane repair. Mol Biol Cell. 2000;11:4339–4346. doi: 10.1091/mbc.11.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi A, Sakakura J, Yagi R, Mazaki Y, Schaefer E, Yano H, Sabe H. Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J Cell Biol. 2002;159:673–683. doi: 10.1083/jcb.200202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci USA. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- Urven LE, Yabe T, Pelegri F. A role for non-muscle myosin II function in furrow maturation in the early zebrafish embryo. J Cell Sci. 2006;119:4342–4352. doi: 10.1242/jcs.03197. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- van Leeuwen H, Elliott G, O’Hare P. Evidence of a role for nonmuscle myosin II in herpes simplex virus type 1 egress. J Virol. 2002;76:3471–3481. doi: 10.1128/JVI.76.7.3471-3481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Riveroll LJ, Wylie SR, Loughna PT, Parson SH, Chantler PD. Nonmuscle myosins IIA and IIB are present in adult motor nerve terminals. Neuroreport. 2005;16:1143–1146. doi: 10.1097/00001756-200508010-00002. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AR. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J Biol Chem. 2003;278:27439–27448. doi: 10.1074/jbc.M302510200. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hosoya H, Yonemura S. Regulation of myosin II dynamics by phosphorylation and dephosphorylation of its light chain in epithelial cells. Mol Biol Cell. 2007;18:605–616. doi: 10.1091/mbc.E06-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Adelstein RS. Conditional expression of a truncated fragment of nonmuscle myosin II-A alters cell shape but not cytokinesis in HeLa cells. Mol Biol Cell. 2000;11:3617–3627. doi: 10.1091/mbc.11.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- Wylie SR, Chantler PD. Separate but linked functions of conventional myosins modulate adhesion and neurite outgrowth. Nat Cell Biol. 2001;3:88–92. doi: 10.1038/35050613. [DOI] [PubMed] [Google Scholar]
- Wylie SR, Chantler PD. Myosin IIA drives neurite retraction. Mol Biol Cell. 2003;14:4654–4666. doi: 10.1091/mbc.E03-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SR, Chantler PD. Myosin IIC: a third molecular motor driving neuronal dynamics. Mol Biol Cell. 2008;19:3956–3968. doi: 10.1091/mbc.E07-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SR, Wu PJ, Patel H, Chantler PD. A conventional myosin motor drives neurite outgrowth. Proc Natl Acad Sci USA. 1998;95:12967–12972. doi: 10.1073/pnas.95.22.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Totsukawa G, Yamakita Y, Sasaki Y, Madaule P, Ishizaki T, Narumiya S, Matsumura F. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol Biol Cell. 2003;14:1745–1756. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- Zang JH, Cavet G, Sabry JH, Wagner P, Moores SL, Spudich JA. On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol Biol Cell. 1997;8:2617–2629. doi: 10.1091/mbc.8.12.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]