Abstract
Benzophenanthridine alkaloids represent a very interesting and significant group of natural products that exhibit a broad range of biological and pharmacological properties. Among this group of alkaloids, sanguinarine, nitidine, fagaronine, and chelerythrine have the potential to form molecular complexes with DNA structures and have attracted recent attention for their possible clinical and pharmacological utility. This review focuses on the interaction of these alkaloids with polymorphic DNA structures (B-form, Z-form, HL-form, and triple helical form) reported by several research groups employing various physical techniques such as spectrophotometry, spectrofluorimetry, circular dichroism, NMR spectroscopy, thermal melting, viscometry as well as thermodynamic analysis by isothermal titration calorimetry and differential scanning calorimetry to elucidate the mode and mechanism of action at the molecular level to determine the structure-activity relationship. DNA binding properties of these alkaloids are interpreted in relation to their biological activity.
Keywords: Sanguinarine, Nitidine, Fagaronine, Chelerythrine, Polymorphic DNA structures, Alkaloid-DNA interactions
Introduction
Alkaloids are nitrogen-containing bases produced mostly by plants during metabolism. They occupy an important position in applied chemistry and also play an indispensable role in medicinal chemistry. People were using alkaloids as drugs even before their chemistry and biological mode of action were understood. With the development of science, chemists, physicists, and biologists are not only isolating more and more naturally occurring alkaloids but are also trying to prepare synthetic alkaloids with more potential biological activity. The mode of action of many alkaloids in current clinical use for the treatment of cancer, genetic disorders, and microbial and viral diseases is believed to be based on their highly specific but noncovalent and reversible intercalative binding to nucleic acid structures and subsequent modification of the genetic material (Denny 1989; Hurley 2002).
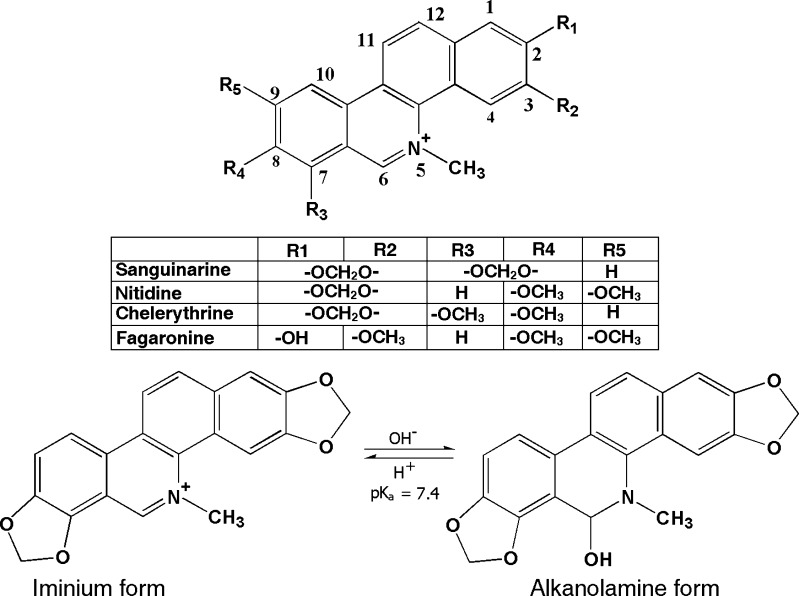
The physical and molecular basis of interaction of natural alkaloids with nucleic acid structures have been a subject of extensive study in the recent past (Waring 1981; Ren and Chaires 1999; Maiti and Kumar 2007a, b). In this context, the quaternary benzophenanthridine group of alkaloids has been the focus of increasing attention for their potential clinical and pharmacological utility. In this group of alkaloids, sanguinarine, nitidine, fagaronine, and chelerythrine (Fig. 1) are the most important compounds noted for their low toxicity and diverse biological activities. This review focuses on the biophysical aspects and the biological implications of the complex formation of these alkaloids with various DNA structures. These aspects of the alkaloids have been extensively investigated in the laboratory of the authors as well as in many others for the last two decades in order to assess their structure-activity relationships.
Fig. 1.
Chemical structures of four quaternary benzophenanthridine alkaloids and the two forms of sanguinarine
Interaction of benzophenanthridine alkaloids with DNA structures
The anticancer activity of many small molecules is believed to be due to their interaction with DNA. The elucidation of the physicochemical characteristics of such interactions is of considerable interest from the standpoints of chemistry and biology. A wide variety of physical techniques such as UV-VIS absorption and fluorescence spectroscopy, circular dichroism (CD), mass spectroscopy, NMR spectroscopy, X-ray crystallography, electron microscopy, isothermal titration calorimetry (ITC), differential scanning calorimetry (DSC), and viscometry have been employed for determining binding mode, stoichiometry, affinity, and selectivity of noncovalent complexes between small organic molecules and nucleic acid structures.
Sanguinarine (iminium)–B-DNA interaction
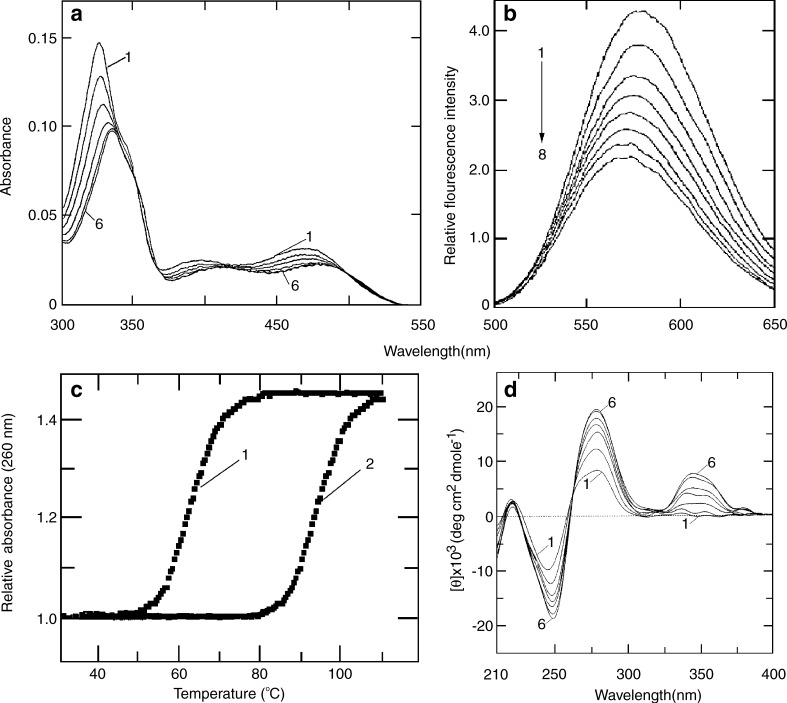
The physical and molecular basis of the interaction of sanguinarine (iminium) with several naturally occurring and synthetic DNAs of different base compositions and sequences was first demonstrated by Maiti and colleagues (Maiti et al. 1982, 1984; Nandi and Maiti 1985; Maiti and Nandi 1987). Further, they reported the complex formation of sanguinarine (iminium) with DNA by a mechanism of intercalation. On binding to DNA, the absorption spectrum of sanguinarine (iminium) undergoes bathochromic and hypochromic changes exhibiting a clear isosbestic point (Fig. 2a), quenching of the steady-state fluorescence emission intensity (Fig. 2b), stabilization of the thermal melting temperature of DNA against thermal strand separation (Fig. 2c), and strong perturbation of the CD pattern of DNA (Fig. 2d). Although sanguinarine is achiral, it exhibited extrinsic CD signals when bound to DNA as a result of effective coupling of its electronic dipole allowing transitions with the chirally organized nucleobases (Maiti and Nandi 1987). Sanguinarine increases the contour length of sonicated rod-like duplex B-DNA depending on base composition and induces the unwinding-rewinding process of covalently closed superhelical DNA by 27°, conclusively proving the intercalative binding mode. The intercalative binding of sanguinarine to B-DNA was subsequently reported and confirmed by several groups (Faddejeva et al. 1984; Smekal et al. 1984; Nafao et al. 1985).
Fig. 2.
Interaction of sanguinarine with calf thymus DNA as obtained from a spectrophotometric, b spectrofluorimetric, c optical thermal melting, and d circular dichroism [reprinted from the PhD Thesis of S. Das (2000)]
The binding parameters for the interaction of sanguinarine with various naturally occurring and synthetic DNAs presented in Table 1 show that its interaction with poly(dG-dC).poly(dG-dC) was the strongest; with other polymers the interaction differed in the order poly(dG).poly(dC) > Micrococcus lysodeikticus DNA > Escherichia coli DNA > calf thymus DNA > Clostridium perfringens DNA > poly(dA-dT).poly(dA-dT) > poly(dA).poly(dT). Essentially sanguinarine exhibits GC base-pair specificity. The sequence selective binding of sanguinarine to DNA was studied by the Waring group (Bajaj et al. 1990) through the use of DNase I footprinting techniques. The footprinting pattern established a mixed nucleotide sequence for binding of sanguinarine to DNA.
Table 1.
Various binding parameters of the interaction of sanguinarine (iminium) with DNAsa
| DNA | GC (mole %) | Kba (×106 M-1) | ∆Tcm (°C at r max) | βd | Lpe (%) at r max |
|---|---|---|---|---|---|
| Clostridium perfringens | 30 | 0.63 | 22.5 | 1.80 | 22.5 |
| Calf thymus | 42 | 0.94 | 21.3 | 1.86 | 26.5 |
| Escherichia coli | 50 | 1.08 | 18.0 | 1.87 | 30.0 |
| Micrococcus lysodeikticus | 72 | 1.44 | na | 1.95 | 33.15 |
| Poly(dG-dC).poly(dG-dC) | 100 | 2.40 | na | 2.38 | 41.65 |
| Poly(dG).poly(dC) | 100 | 1.45 | na | 2.00 | 34.7 |
| Poly(dA-dT).poly(dA-dT) | 0 | 0.37 | 25.15 | 1.77 | 7.08 |
| Poly(dA).poly(dT) | 0 | 0.27 | 28.0 | 1.63 | 4.58 |
na Not available
aData obtained from Maiti et al. (1984) and Nandi and Maiti (1985)
bKa is the binding constant
c∆Tm is the difference between the Tm of the DNA in the presence of alkaloid and the Tm of the free DNA
dβ is the slope of the equation 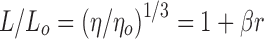 where L and Lo are the contour length of the helix in the presence and absence of ligand and η and ηo are the intrinsic viscosities (approximated by the reduced viscosity) of DNA solution in the presence and absence of the ligand
where L and Lo are the contour length of the helix in the presence and absence of ligand and η and ηo are the intrinsic viscosities (approximated by the reduced viscosity) of DNA solution in the presence and absence of the ligand
eLp% is the percent helix length calculated from 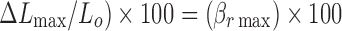 where r is the number of ligand molecules bound per mole of nucleotide
where r is the number of ligand molecules bound per mole of nucleotide
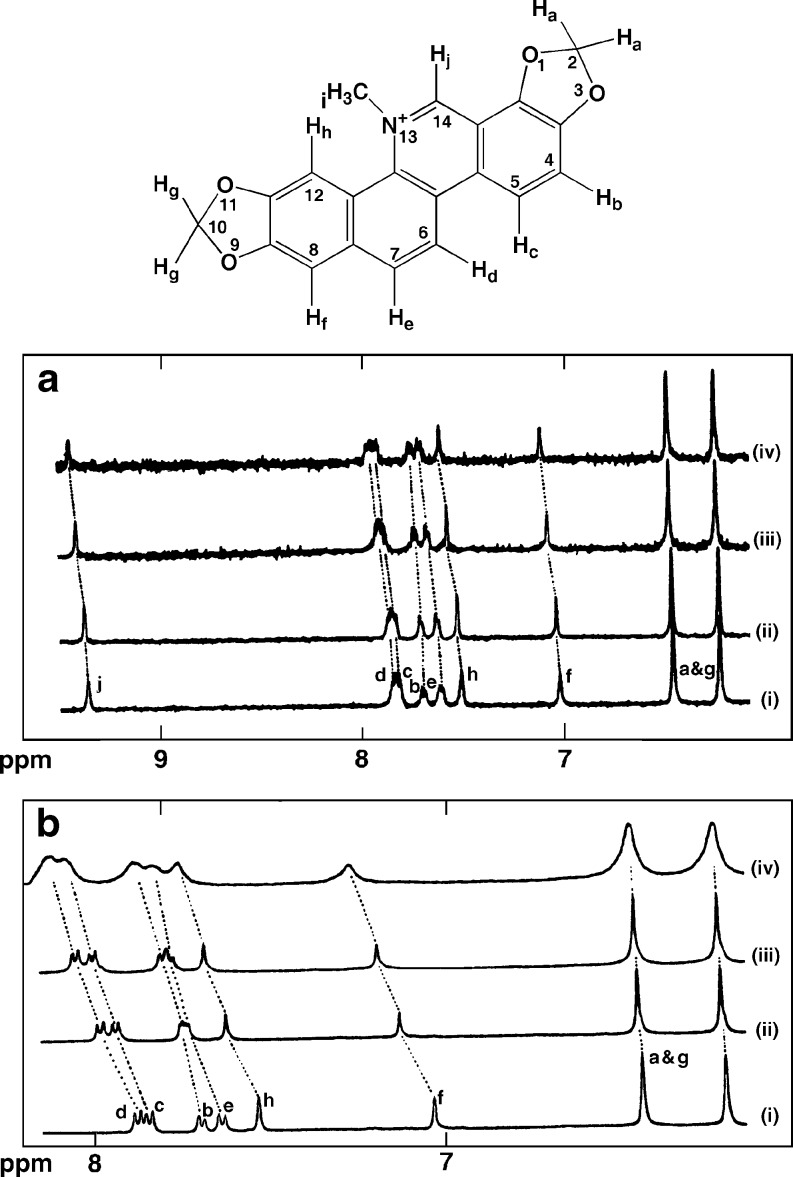
Saran et al. (1995) first studied the interaction of sanguinarine with calf thymus DNA by using high field 1H NMR and reported the assignment of all the proton resonances in the molecule through a combination of 2D-COSY, NOESY, and ROESY techniques. The proton NMR spectra of sanguinarine with increasing concentrations of calf thymus DNA at 298 K revealed that almost all of the resonances were shifted downfield on binding to DNA (Fig. 3a). The protons Hc, Hd, He, Hf, and Hh all experienced a shift of as much as 0.09–0.1 ppm. Smaller shifts of 0.05 and 0.07 ppm were noted for protons Hb and Hj suggesting intercalation of the entire molecule into DNA. On titration of sanguinarine with DNA at high temperatures, the changes in the chemical shifts of almost all protons were found to be very large. The results indicated that protons Hb and Hd shifted downfield by as much as 0.11 ppm, while protons Hc, He, Hf and Hb moved downfield by 0.08 to 0.09 ppm.
Fig. 3.
Chemical structure of sanguinarine with atom numbering and proton labels. a 1H NMR spectra of sanguinarine titrated with increasing concentrations of calf thymus DNA at 298 K. i 0, ii 5, iii 50, and iv 100 μL of 1% DNA solution. b Effect of temperature on a fixed sanguinarine-DNA molar ratio at i 300, ii 315, iii 325, and iv 350 K [reprinted from Saran et al. (1995) with permission from the publisher]
Again, for a fixed ratio of sanguinarine to calf thymus DNA, significant changes in the chemical shifts with increasing temperature showed the strong binding of sanguinarine to the DNA helix (Fig. 3b) by intercalation. It is known that the thermodynamic aspects of small molecule binding to DNA structures provide a wealth of information such as the energetics, binding affinity, stoichiometry, and also hydration/dehydration effects that occur in a binding reaction (Gallagher and Sharp 1998). Using a combination of spectrophotometric and spectrofluorimetric techniques, Maiti and his group (Sen et al. 1996) first reported the thermodynamics of binding of sanguinarine to a series of natural and synthetic DNA duplexes of differing base compositions and sequences over a wide range of temperatures and salt concentrations. The sign and magnitude of the thermodynamic parameters of sanguinarine complexation were dependent on the base composition being characterized by negative enthalpy and positive entropy change for all natural DNAs and AT polymers and negative enthalpy and entropy changes for GC polymer.
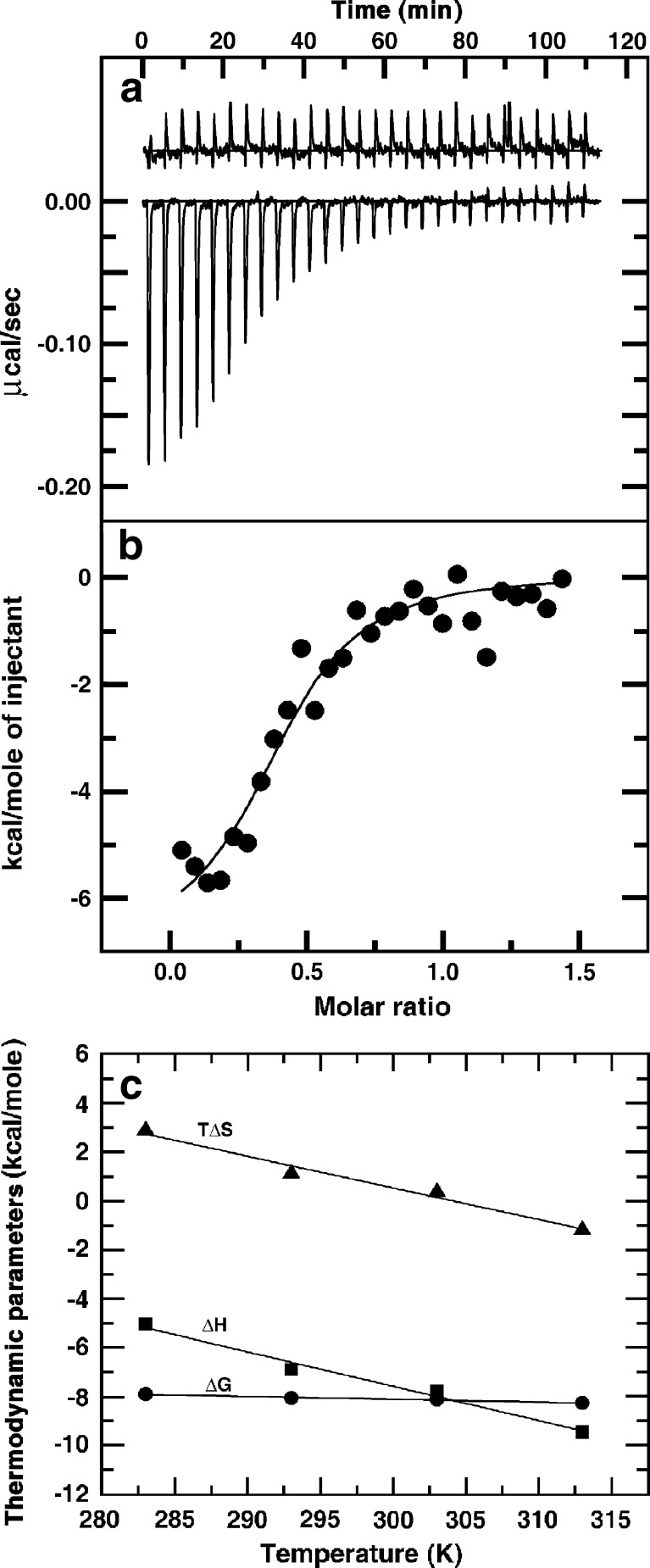
Recently Adhikari et al. (2008) investigated the thermodynamic aspects of sanguinarine on binding to natural and synthetic DNAs of differing base-pair sequences by using the ITC technique under various environmental conditions. The ITC profile for the binding to calf thymus DNA is presented in Fig. 4a,b. Sanguinarine showed high affinity to an alternating GC polymer with binding constant of 6.03 × 106 M-1 (Table 2). Except for the homopolymer of AT that produced endothermic heats, in all the other systems, the bindings showed exothermic heat evolution. Thermodynamic parameters suggested the binding to be exothermic and enthalpy driven, which is typical for intercalative binding with significant hydrophobic contribution. The binding affinity to calf thymus DNA was found to be 0.95 × 105 M-1, which decreased with an increase in ionic strength and temperature. The free energy (∆G) of the interaction more or less remained between –7.56 to –9.62 kcal/mol for all DNAs.
Fig. 4.
Isothermal titration calorimetry (ITC) profile for the binding of sanguinarine to calf thymus DNA. a The raw data for sequential injection of a constant amount of sanguinarine into DNA (curves on the bottom) and dilution of sanguinarine into buffer under identical conditions (curves on the top offset for clarity). b The integrated heat data after correction of heat of dilution of sanguinarine against the molar ratio of sanguinarine to DNA. c Temperature dependence of the thermodynamic parameters (∆G, ∆H, and T∆S) for the binding of sanguinarine to calf thymus DNA. [Reprinted from the PhD Thesis of M. Hossain (2009)]
Table 2.
Isothermal titration calorimetry (ITC)-derived thermodynamic parameters for the binding of sanguinarine (iminium) to various DNAs at 20°Ca
| DNA | Ka (×106 M-1) | ∆G (kcal/mol) | ∆H (kcal/mol) | T∆S (kcal/mol) |
|---|---|---|---|---|
| Calf thymus DNA | 0.95 | -8.02 | -6.91 | 1.11 |
| Poly(dA-dT).poly(dA-dT) | 3.01 | -8.69 | -7.16 | 1.53 |
| Poly(dG-dC).poly(dG-dC) | 6.03 | -9.62 | -9.06 | 0.058 |
| Poly(dG).poly(dC) | 0.46 | -7.56 | -4.37 | 3.19 |
| Poly(dA).poly(dT) | 1.36 | -8.18 | 5.76 | 13.94 |
aThe ITC profile was fit to a model of single binding sites (Adhikari et al. 2008)
Further studies on the temperature dependence of the binding enthalpy were performed to determine the change in heat capacity (∆Cp) with the equation 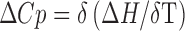 . The temperature dependence of the thermodynamic parameters of sanguinarine on binding of calf thymus DNA is presented in Fig. 4c, and the value of ∆Cp was found to be -589.94 mol-1 K-1. The binding of sanguinarine with calf thymus DNA was further characterized using the DSC technique (Hossain and Kumar 2009), showing a strong stabilization of as much as 30°C with a binding constant of 1.41 × 106 M-1.
. The temperature dependence of the thermodynamic parameters of sanguinarine on binding of calf thymus DNA is presented in Fig. 4c, and the value of ∆Cp was found to be -589.94 mol-1 K-1. The binding of sanguinarine with calf thymus DNA was further characterized using the DSC technique (Hossain and Kumar 2009), showing a strong stabilization of as much as 30°C with a binding constant of 1.41 × 106 M-1.
Sanguinarine (alkanolamine)–B-DNA interaction
Sanguinarine shows very interesting characteristics in aqueous buffers of various pH values from the standpoint of its absorption and fluorescence spectral behavior. Using a combination of spectrophotometric and spectrofluorimetric measurements, Maiti and colleagues (Maiti et al. 1983) first demonstrated that sanguinarine has a pH-dependent structural equilibrium between the iminium (charged) and alkanolamine (neutral) forms with pKa value of 7.4. Sanguinarine remains almost exclusively as iminium and alkanolamine forms in the pH range 1.0–6.0 and 8.5–11.0, respectively. The pH-dependent structural transition between sanguinarine iminium and alkanolamine forms is represented in Fig. 1. The stability of the two forms was later confirmed by Jones et al. (1986) and by Moterich et al. (2007) from the analysis of absorption, fluorescence, and NMR spectroscopy as well as by HPLC analysis and surface-enhanced Raman scattering spectral characteristics. It was suggested that conversion from iminium to alkanolamine form may enhance the antimicrobial activity by increasing cellular availability of the alkaloid due to the greater lipophilicity of the alkanolamine form (Jones et al. 1986).
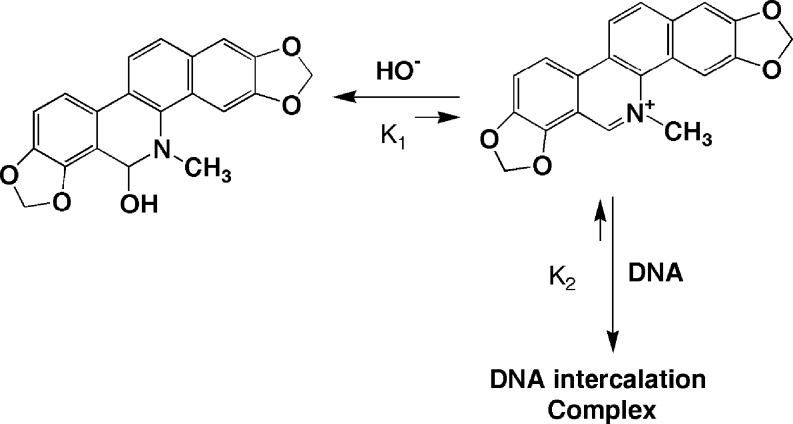
Smekal et al. (1984) investigated the interaction of sanguinarine (alkanolamine) with duplex DNA and suggested that the alkanolamine form formed a partially intercalated complex. This observation was contradicted by Sen and Maiti (1994) based on results obtained from extensive spectrophotometry, fluorescence polarization anisotropy, thermal melting, circular dichroism, and viscometric analysis. It was reported (Sen and Maiti 1994; Maiti et al. 2002) that the alkanolamine form of sanguinarine does not bind to duplex DNA. It is pertinent to point out that the alkanolamine form is neutral and its structure has a partial saturation leading to nonplanarity and is unable to bind to DNA. However, the alkanolamine form can be transferred to the charged iminium form–DNA complex at a very high concentration of DNA (Fig. 5b). This conversion of alkanolamine form to iminium form can be explained by a shift in the equilibrium shown in Scheme 1.
Fig. 5.
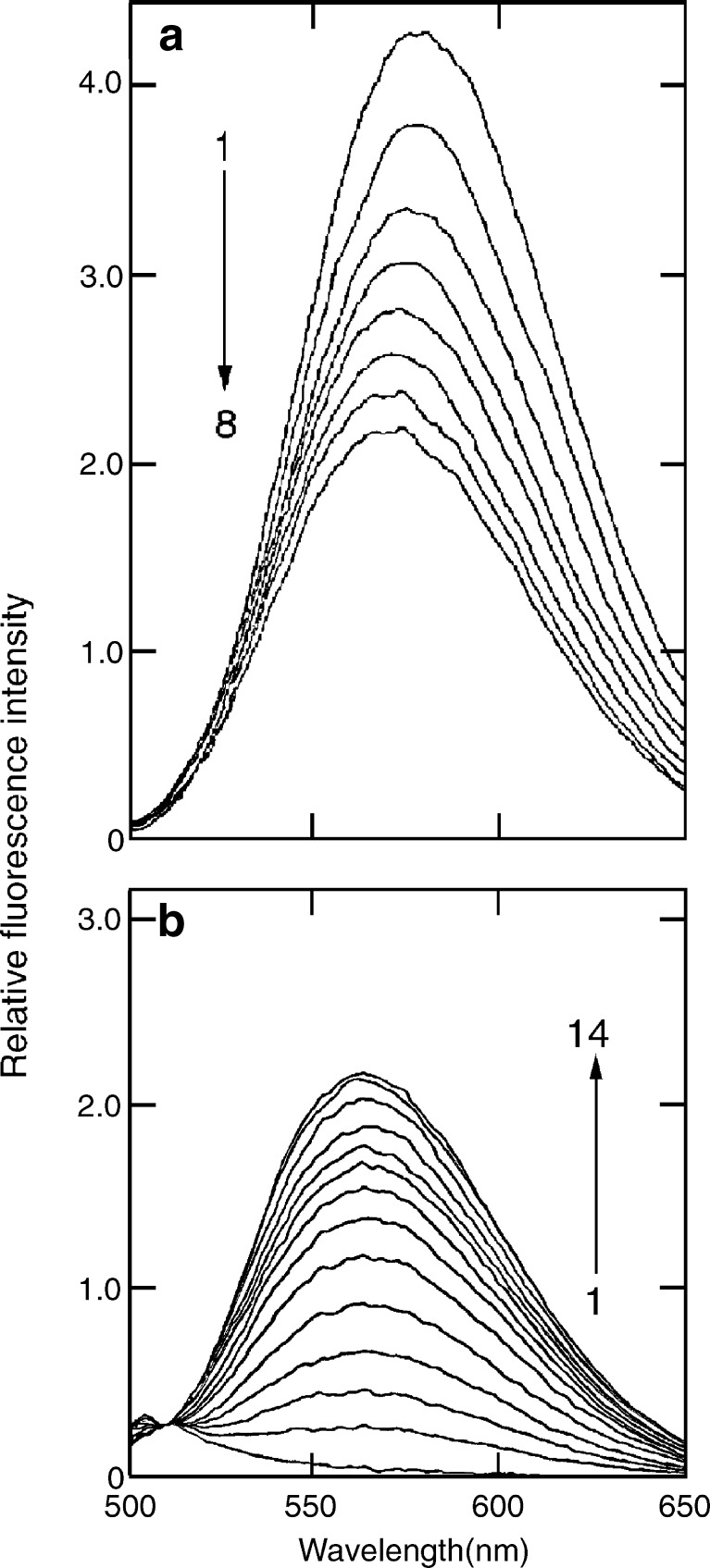
Fluorescence emission spectrum of sanguinarine iminium form (a) and alkanolamine form (b) treated with increasing concentrations of calf thymus DNA [reprinted in part from Maiti et al. (2002) with permission from Adenine Press, © 2002]
Scheme 1.
Conversion of alkanolamine form to iminium form of sanguinarine
Sanguinarine (iminium)–Z-DNA interaction
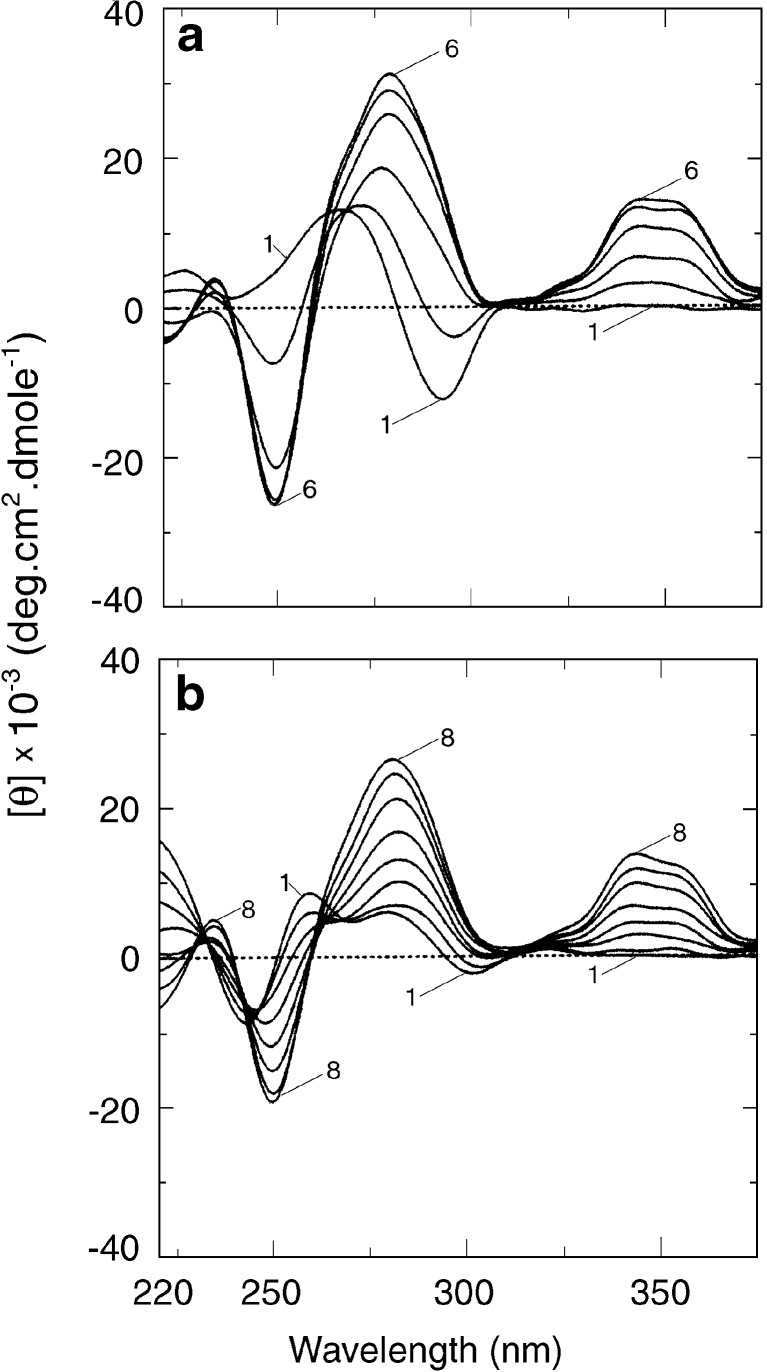
It is known that B form DNA can adopt a variety of polymorphic conformations depending on the nucleotide sequence and environmental conditions (Saenger 1984). Of the structures so far known, perhaps the most striking example is the Z-DNA, which is a left-handed duplex conformation with Watson-Crick base pairs that can be generated easily in alternating purine-pyrimidine sequences in high sodium salt concentrations (Pohl and Jovin 1972). It was further established that Z-DNA can be generated easily in solutions of poly(dG-dC).poly(dG-dC) and more easily in its methylated analogue in the presence of divalent cations, such as Mg2+, Ca2+, and Ni2+, micromolar concentrations of hexamine cobalt chloride (Co(NH3)6]Cl3), or millimolar concentrations of polyamines (Jovin et al. 1987). To evaluate the affinity and mode of binding of sanguinarine to Z-DNA, Das et al. (1999) first reported that on addition of the alkaloid to the left-handed conformation of poly(dG-dC).poly(dG-dC) and poly(dGme5-dC).poly(dGme5-dC), the alkaloid does not bind to Z-form but converts the Z-form back to the bound right-handed form as evidenced from CD (Fig. 6a) and absorption spectroscopy studies. Sanguinarine inhibits the rate of B to Z transition under ionic conditions that otherwise favor the left-handed confirmation of the polynucleotides. It was established that about two to three base pairs of Z-form switched to the right-handed form for each bound molecule of sanguinarine (Das et al. 1999).
Fig. 6.
Circular dichroic spectra of a Z-form and b HL-form of poly(dG-dC).poly(dG-dC) treated with increasing concentrations of sanguinarine iminium form [reprinted from the PhD Thesis of S. Das (2000)]
Sanguinarine (iminium)–HL-form DNA complexation
Protonation of DNA has been studied for several years (Maiti and Kumar 2007b and references therein) but has gained considerable significance from the observation of Chen (1984) that a left-handed (Z-like) conformation could be generated in poly(dG-dC).poly(dG-dC) structure at acid pH. B-form duplex poly(dG-dC).poly(dG-dC) and its methylated cytosine analogue can form a variety of unique structures depending on ionic strength, pH, and various other environmental conditions (Saenger 1984). From extensive UV absorption, circular dichroic spectroscopic, and thermal melting studies, the author’s laboratory has advanced a model of left-handed structure with the formation of Hoogsteen base pairing at low pH and low molarity (Kumar and Maiti 1994). It was determined to be yet another polymorphic form of DNA, and its name was coined as HL-form (Maiti 2001). The molecular architecture of HL-form DNA appears to be unique, stable, and distinctly different from that of the left-handed Z-DNA. This model of HL-DNA has further been confirmed by FTIR studies (Tajmir-Riahi et al. 1995) and by the Raman spectroscopic studies of Otto and coworkers (Segers-Nolten et al. 1997). The interaction of sanguinarine with HL-DNA showed that it does not bind to HL-DNA, but it converts the HL-DNA back to the bound right-handed B-form as revealed from CD spectroscopy ([Fig.6b). It was revealed that approximately two to three base pairs of HL-DNA switched to the bound right-handed B-form for each bound sanguinarine molecule (Das et al. 1999; Maiti 2001). Binding isotherms obtained from spectrophotometric data showed that sanguinarine binds strongly to the B-form poly(dG-dC).poly(dG-dC) structure in a noncooperative manner in sharp contrast to the highly cooperative interaction with HL-DNA.
Sanguinarine (iminium)-triplex DNA interaction
In recent years, increasing interest has been focused on triple helical nucleic acid structures due to the high selectivity of the third strand, which illustrates a variety of potential applications in molecular biology, diagnostics, and therapeutics (Hélène 1991). Several intercalating molecules have now been reported that can preferentially stabilize the third strand of triplex structures (Ren and Chaires 1999; Ray et al. 1999). Latimer et al. (1995) first demonstrated that sanguinarine binds strongly to poly(dT).poly(dA)xpoly(dT) but weakly to poly(dC).poly(dG)xpoly(dC+) triplex (“dot” Watson-Crick pairing and “cross” Hoogsteen base pairing) from thermal melting studies. The author’s laboratory (Das et al. 2003) studied the binding of sanguinarine to DNA triplexes more quantitatively and reported that sanguinarine binds to poly(dT).poly(dA)xpoly(dT) and poly(dC).poly(dG)xpoly(dC+) triplexes by intercalation in a noncooperative manner and that the binding was stronger to DNA triplexes than to their corresponding duplex structures. It was further revealed (Das et al. 2003) that sanguinarine stabilized the Hoogsteen base-paired third strand of DNA triplexes more tightly compared to their Watson-Crick strands (Fig. 7a,b). A thermodynamic study revealed (Das et al. 2003) that the process of binding of sanguinarine to the poly(dC).poly(dG)xpoly(dC+) triplex was exothermic and enthalpy driven while that to poly(dT).poly(dA)xpoly(dT) triplex was exothermic and entropy driven.
Fig. 7.
Thermal melting profiles (a) and CD spectra (b) of poly(dT).poly(dA)xpoly(dT) with increasing concentrations of the iminium form of sanguinarine [reprinted in part from Das et al. (2003) with permission from Adenine Press, © 2002]
DNA binding aspects of nitidine, fagaronine, and chelerythrine
Nitidine, fagaronine, and chelerythrine, which are strictly different from sanguinarine in the type and position of substituents in their aromatic rings (Fig. 1), exhibit pronounced cytotoxity (Cabrespine et al. 2005) and anticancer activity (Ahmad et al. 2000). The potential anticancer activities of these alkaloids may be attributed to their binding to chromosomal DNA. Prado et al. (2004) reported that nitidine and fagaronine bind to duplex DNA by the mechanism of intercalation. Recently a comparative study on the binding of sanguinarine, nitidine, and chelerythrine with calf thymus DNA, poly(dG-dC).poly(dG-dC), poly(dA-dT).poly(dA-dT), and several sequence-designed double-stranded oligonucleotides was performed using spectrophotometric and spectrofluorimetric techniques to elucidate their sequence selectivity for DNA binding (Bai et al. 2006; Urbanova et al. 2009). Fluorescence titration of DNA with three alkaloids was performed in acidic buffer at pH 5.30 to ensure that these alkaloids exist exclusively as the iminium form (Bai et al. 2006). Upon binding to DNA, the steady-state fluorescence of sanguinarine and nitidine was greatly quenched, while that of chelerythrine was sharply enhanced. The binding affinities of these alkaloids with calf thymus DNA, poly(dG-dC).poly(dG-dC), poly(dA-dT).poly(dA-dT), and duplex oligodeoxyribonucleotides are listed in Table 3.
Table 3.
Comparative binding constants (Ka) of the iminium forms of benzophenanthridine alkaloids with CT DNA, polydeoxynucelotides, and double-stranded oligonucleotides at 18°C
| Polymer | Sanguinarinea (×106 M-1) | Nitidinea (×106 M-1) | Chelerythrinea (×106 M-1) | Fagaronineb (×106 M-1) |
|---|---|---|---|---|
| Calf thymus DNA | 1.0 | 0.31 | 0.22 | 0.21 |
| Poly(dA-dT).poly(dA-dT) | 0.42 | 0.60 | 0.30 | - |
| Poly(dG-dC).poly(dG-dC) | 2.24 | 1.03 | 0.71 | - |
| d(TGGGGA)2 | 0.1 | 0.14 | 1.04 | - |
| d(TGGGCA)2 | 0.11 | 0.052 | 0.198 | - |
| d(TGGCGA)2 | 0.14 | 0.042 | 0.055 | - |
| d(TGCGCA)2 | 0.57 | 0.20 | 0.134 | - |
| d(ATGCGCAT)2 | 10.1 | 1.10 | 2.86 | - |
The binding constants of three alkaloids with CT DNA were found to be in the order sanguinarine > nitidine > chelerythrine. Sanguinarine showed much higher selectivity towards poly(dG-dC).poly(dG-dC) than chelerythrine and nitidine. For the sequence selectivity, both sanguinarine and nitidine bind preferentially to 6-mer d(TGCGCA)2 and 8-mer d(ATGCGCAT)2 (alternating GC base pairs), exhibiting quite distinct sequence selectivity of sanguinarine and nitidine. Comparison of the binding constants also suggests that sanguinarine, nitidine, and chelerythrine show a remarkable structure-activity correlation when bound to duplex DNAs (Table 3). The more planar molecule sanguinarine in general has much higher binding constants than nitidine and chelerythrine towards CT DNA, poly(dG-dC).poly(dG-dC), and poly(dA-dT).poly(dA-dT). Further studies (Bai et al. 2006) showed that all three alkaloids bind to duplex DNA through the mechanism of intercalation similarly to sanguinarine as evidenced from spectrophotometric titration and competitive ethidium bromide displacement assays.
Fagaronine has been shown to bind calf thymus DNA with a binding constant of 2.1 × 105 M-1 by intercalation (Pezzuto et al. 1983). Recently Raman, surface-enhanced scattering (SERS), and flow linear dichroism (FLD) spectroscopy techniques were employed to study the interaction of fagaronine with duplex DNA (Ianoul et al. 1999). The FLD data suggest that fagaronine is a strong major groove intercalator with stoichiometry of one fagaronine molecule per two DNA base pairs. The SERS study of the fagaronine-DNA complex showed that the OH group of fagaronine plays a key role in DNA binding by formation of an H-bond with DNA. It was suggested that molecular planarity plays an important role for DNA-binding activities of all three alkaloids and may provide some important guidance for the quaternary benzophenanthridine alkaloid-based anticancer drug design (Todor 2003).
Biological implications
Alkaloids are known to have an important role in medicinal chemistry due to their extensive biological activity. Especially noteworthy is the quaternary benzophenanthridine alkaloids that are widely distributed in several botanical families (Papaveraceae, Rutaceae, Fumaraceae, Ranunculaceae, etc.) exhibiting extensive therapeutic applications (Chen et al. 2005). The most important members of this group are sanguinarine, nitidine, fagaronine, and chelerythrine, which are the subject of practical and research interest because of their broad range of biological applications, both beneficial and adverse due to their quaternary nitrogen and their polycyclic planar structure. In fact plants containing sanguinarine have been used as Chinese and folk medicines for the treatment of human cancer and other diseases (Chen et al. 2005; Qin et al. 2006). In the U.K. and U.S.A., sanguinarine is used in mouthwashes and toothpastes to inhibit dental plaque and to protect gingival cells (Southard et al. 1987).
The alkaloid sanguinarine exhibits excellent antimicrobial, antioxidant, anti-inflammatory, and proapoptotic properties (Malikova et al. 2006). It has been shown to have multiple cellular targets, for example, it inhibits microtubule assembly better than colchicines (Wolff and Knipling 1993), has effects on membrane permeability, and inhibits a wide variety of enzymes that are involved in signal transduction pathways leading to cell proliferation and/or cell death (Hussain et al. 2007). At the same time, sanguinarine has been found to be less toxic towards normal cells, including normal human epidermal keratinocytes (Ahmad et al. 2000). Several mechanisms have been proposed to explain its antiproliferative activities (Adhami et al. 2004; Vogt et al. 2005). More recent results clearly correlate the cytotoxicity of sanguinarine to DNA intercalation and subsequent ability to induce strand breaks and other damage (Kaminskyy et al. 2008).
The in vitro cytotoxicity of sanguinarine was studied using cell lines and primary cells from oral human tissues (Babich et al. 1996). The results established the potential use of sanguinarine for the inhibition of growth of SG gingival epithelial cells and KB carcinoma cells due to its complex formation with DNA. Sanguinarine has, on the other hand, been identified as the principal constituent responsible for argemone oil toxicity causing epidemic dropsy syndrome (Das and Khanna 1997). Recently Serafim et al. (2008) reported that sanguinarine is primarily accumulated by cell nuclei, causing inhibition of cell proliferation and inducing cell death. At low concentrations, sanguinarine induces mitochondrial depolarization in a sub-population of melanoma cells. These findings along with the reported covalent modification after in vitro metabolic activation of the alkaloid by the microsomal fraction of rat hepatocytes underscore the possibility of biotargets other than DNA in the cell (Stiborova et al. 2002).
Nitidine and chelerythrine, which are planar but differ slightly from sanguinarine, are the active constituents of herbal medicines Radis Zanthoxyli Nitidi and Herba chelidonii, respectively. Nitidine displays antileukemic activity and exhibits pronounced cytotoxic activity (Janin et al. 1993; Prado et al. 2004) and also anticancer activity, which may result from its capacity to intercalate into DNA (Chang et al. 2003). Chelerythrine possesses a broad spectrum of pharmacological activities including antimicrobial (Giuliana et al. 1997), antitumor (Ahmad et al. 2000), and antileukemic (Wang et al. 1993) activity. It was shown that its antitumor activity is mediated via cell viability and DNA damage effect in mouse spleen cells and mouse lymphocytic leukemic cells (L1210). The results suggest that cytotoxic and DNA damaging effects of chelerythrine are more selective in these cells (Prado et al. 2004).
Fagaronine has been found to possess antimalarial (Kassim et al. 2005) and antileukemic properties (Barret and Samvaire 2006). It exhibits antitumor and anticancer activity (Benoist et al. 1989). Fagaronine has been demonstrated to be a reverse transcriptase inhibitor in an in vitro screening system for HIV antiviral activity in cell culture (Novak et al. 1991). It appears to be active at very low concentrations (nanogram) in cell culture, which suggests its utility as an antiviral agent in the therapy of HIV infection. Sanguinarine, fagaronine, nitidine, and chelerythrine display antileukemic and antitumor activities. Indeed, these alkaloids have been shown to act as topoisomerase inhibitors. Sanguinarine has been reported to have Topo II poisoning properties in mammalian cells. More recent immunocytological studies in MCF-7 breast cancer cells showed that sanguinarine induces a striking disruption of nucleocytoplasma trafficking of cyclin D1 and Topo II in mammalian cells (Holy et al. 2006). Fagaronine and nitidine have been shown to be cytotoxic and act as Topo I inhibitors at low concentrations and Topo II inhibitors at higher concentrations (Holden et al. 1999).
Conclusions and perspectives
The use of plants as medicines by humans dates back several thousand years. Alkaloids of plant origin are natural products that play an indispensable role in chemistry, biology, and medicine. Among the alkaloids, the benzophenanthridine group exhibits significant diverse biological and pharmacological properties. In view of their extensive occurrence in various plants and their very low toxicities, prospective development of these alkaloids as effective anticancer and antileukemic agents is a matter of significant current interest. The interaction of sanguinarine, nitidine, fagaronine, and chelerythrine with various DNA structures and their relation to biological activity indicate detailed binding mechanisms at the molecular level for structure-activity relationships. Review of the large amounts of published data suggests that the iminium form of each of these alkaloids binds to duplex B-form DNA very strongly by intercalation.
The alkanolamine form was reported to have greater cellular availability than the iminium form due to its greater lipophilicity (Jones et al. 1986), while the iminium form of these alkaloids is expected to play an important role in the biological activities such as cancer and topoisomerase inhibition, due to a positive charge on the polyaromatic nucleus, which facilitates intercalative binding with DNA. Thus the DNA binding results for sanguinarine, nitidine, fagaronine, and chelerythrine presumably shed light on the molecular mechanism of their anticancer activities. The structure-activity relationships of these alkaloids may provide some important guidance for the design of quaternary benzophenanthridine alkaloid-based anticancer drugs. Again, the differential binding of alkaloid to Z-DNA, HL-DNA, and triplex DNA structures may convey some specific meaning for their regulatory role in biological systems.
Acknowledgements
Thanks are due to the Council of Scientific and Industrial Research, Government of India, for the Emeritus Scientist award to M. Maiti. The authors are grateful to all the present and past colleagues at the Biophysical Chemistry Laboratory for their help and cooperation and in particular to those who have contributed over the years to the understanding of sanguinarine binding to nucleic acid structures.
References
- Adhami VM, Aziz MH, Reagan-Shaw SR, Nihal M, Mukhtar H, Ahmad N. Sanguinarine causes cell cycle blockade and apoptosis of human prostate carcinoma cells via modulation of cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery. Mol Cancer Ther. 2004;3:933–940. [PubMed] [Google Scholar]
- Adhikari A, Hossain M, Maiti M, Kumar GS. Energetics of the binding of phototoxic and cytotoxic plant alkaloid sanguinarine to DNA: isothernmal titration calorimetric studies. J Mol Struct. 2008;899:54–63. doi: 10.1016/j.molstruc.2008.01.016. [DOI] [Google Scholar]
- Ahmad N, Gupta S, Husain MM, Heiskanen KM, Mukhtar H. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524–1528. [PubMed] [Google Scholar]
- Babich H, Zukerbraun HL, Barber IB, Babich SB, Borenfreund E. Cytotoxicity of sanguinarine chloride to cultured human cells from oral tissue. Pharmacol Toxicol. 1996;78:397–403. doi: 10.1111/j.1600-0773.1996.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Bai LP, Zhao ZZ, Cai Z, Jiang ZH. DNA binding affinities and sequence selectivity of quaternary benzophenanthrine alkaloids sanguinarine, chelerythrine, and nitidine. Bioorg Med Chem. 2006;14:5439–5445. doi: 10.1016/j.bmc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bajaj NP, McLean MJ, Waring MJ, Smekal E. Sequence-selective, pH-dependent binding to DNA of benzophenanthridine alkaloids. J Mol Recog. 1990;3:48–54. doi: 10.1002/jmr.300030106. [DOI] [PubMed] [Google Scholar]
- Barret Y, Samvaire Y. Fagaronine a novel antileukemic alklaoid. Phytother Res. 2006;6:59–63. doi: 10.1002/ptr.2650060202. [DOI] [Google Scholar]
- Benoist H, Comoe L, Joly P, Carpenter Y, Desplaces A, Dufer J. Comparative effects of fagaronine, adriamycin and aclacinomycin on K562 cell sensitivity to natural-killer-mediated lysis. Lack of agreement between alteration of transferrin receptor and CD15 antigen expressions and induction of resistance to natural killer. Cancer Immunol Immunother. 1989;30:289–294. doi: 10.1007/BF01744896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrespine A, Bay JO, Barthomeuf C, Curé H, Chollet P, Debiton E. In vitro assessment of cytotoxic agent combinations for hormone-refractory prostate cancer treatment. Anticancer Drug. 2005;16:417–422. doi: 10.1097/00001813-200504000-00008. [DOI] [PubMed] [Google Scholar]
- Chang YC, Chang FR, Khalil AT, Hsiesh PW, Wu YC. Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z Naturforsch. 2003;58:521–526. doi: 10.1515/znc-2003-7-813. [DOI] [PubMed] [Google Scholar]
- Chen FM. Base protonation facilitates B-Z interconversions of poly(dG-dC).poly(dG-dC) Biochemistry. 1984;23:6159–6165. doi: 10.1021/bi00320a041. [DOI] [PubMed] [Google Scholar]
- Chen WH, Qin Y, Cai Z, Chan CL, Luo GA, Jiang ZH. Spectrometric studies of cytotoxic protoberberine alkaloids binding to double-stranded DNA. Bioorg Med Chem. 2005;13:1859–1866. doi: 10.1016/j.bmc.2004.10.049. [DOI] [PubMed] [Google Scholar]
- Das S (2000) Biophysical studies on the interactions of benzophenanthridine alkaloids with polymorphic nucleic acid structures. PhD Thesis, Jadavpur University, Kolkata
- Das M, Khanna SK. Clinicoepidemiological, toxicological, and safety evaluation studies on argemone oil. Crit Rev Toxicol. 1997;27:273–297. doi: 10.3109/10408449709089896. [DOI] [PubMed] [Google Scholar]
- Das S, Kumar GS, Maiti M. Conversions of the left-handed and protonated form of DNA back to the bound right-handed form by sanguinarine and ethidium: a comparative study. Biophys Chem. 1999;76:199–218. doi: 10.1016/S0301-4622(98)00238-5. [DOI] [PubMed] [Google Scholar]
- Das S, Kumar GS, Ray A, Maiti M. Spectroscopic and thermodynamic studies on the binding of sanguinarine and berberine to triple and double helical DNA and RNA structures. J Biomol Struct Dyn. 2003;20:703–714. doi: 10.1080/07391102.2003.10506887. [DOI] [PubMed] [Google Scholar]
- Denny WA. DNA-intercalating ligands as anti-cancer drugs: prospects for future design. Anticancer Drug Des. 1989;4:241–263. [PubMed] [Google Scholar]
- Faddejeva MD, Belyaeva TN, Rosanov Yu M, Sedova M, Sokolovskaya EL. Studies on the complex formation with DNA and the effect on DNA hydrolysis, RNA syhthesis and cellular membrane ATPase system of some antitumor alkaloids. Stud Biophys. 1984;104:267–269. [Google Scholar]
- Gallagher K, Sharp K. Electrostatic contributions to heat capacity changes of DNA-ligand binding. Biophys J. 1998;75:769–776. doi: 10.1016/S0006-3495(98)77566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliana G, Pizzo G, Milici ME, Musotto GC, Giangreco R. In vitro antifungal properties of mouthrinses containing antimicrobial agents. J Periodontol. 1997;68:729–733. doi: 10.1902/jop.1997.68.8.729. [DOI] [PubMed] [Google Scholar]
- Hélène C. The anti-gene strategy: control of gene expression by triplex-forming-oligonucleotides. Anticancer Drug Des. 1991;6:569–584. [PubMed] [Google Scholar]
- Holden JA, Wall ME, Wani MC, Manikumar G. Human DNA topoisomerase I: quantitative analysis of the effects of camptothecin analogs and the benzophenanthridine alkaloids nitidine and 6-ethoxydihydronitidine on DNA topoisomerase I-induced DNA strand breakage. Arch Biochem Biophys. 1999;370:66–76. doi: 10.1006/abbi.1999.1355. [DOI] [PubMed] [Google Scholar]
- Holy J, Lamont G, Perkins E. Disruption of nucleocytoplasmic trafficking of cyclin D1 and topoisomerase II by sanguinarine. BMC Cell Biol. 2006;7:13–26. doi: 10.1186/1471-2121-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M (2009) Biophysical studies on the interaction of intercalating and groove binding molecules with deoxyribonucleic acids. PhD Thesis, Jadavpur University, Kolkata
- Hossain M, Kumar GS. DNA binding of benzophenanthridine compounds sanguinarine versus ethidium: comparative binding and thermodynamic profile of intercalation. J Chem Thermodyn. 2009;41:764–774. doi: 10.1016/j.jct.2008.12.008. [DOI] [Google Scholar]
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- Hussain AR, Al-Jomah NA, Siraj AK, Manogaran P, Al-Hussein K, Abubaker J, Platanias LC, Al-Kuraya KS, Uddin S. Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells. Cancer Res. 2007;67:3888–3897. doi: 10.1158/0008-5472.CAN-06-3764. [DOI] [PubMed] [Google Scholar]
- Ianoul A, Fleuny F, Dural O, Waigh R, Jardiller JC, Alix AJP, Nabier I. DNA binding by fagaronine and ethoxidine, inhibitors of human DNA topoisomerases I and II, probes by SERS and flow linear dichroism spectroscopy. J Phys Chem B. 1999;103:2008–2013. doi: 10.1021/jp984291y. [DOI] [Google Scholar]
- Janin YL, Croisy A, Riou JF, Bisagni E. Synthesis and evaluation of new 6-amino-substituted benzo[c]phenanthridine derivatives. J Med Chem. 1993;36:3686–3692. doi: 10.1021/jm00075a025. [DOI] [PubMed] [Google Scholar]
- Jones RP, Harkrader RJ, Southard GL. The effect of pH on sanguinarine iminium ion form. J Nat Prod. 1986;49:1109–1111. doi: 10.1021/np50048a025. [DOI] [Google Scholar]
- Jovin TM, Soumpasis DM, McIntosh LP. The transition between B-DNA and Z-DNA. Annu Rev Phys Chem. 1987;38:521–558. doi: 10.1146/annurev.pc.38.100187.002513. [DOI] [Google Scholar]
- Kaminskyy VO, Lin KW, Filyak Y, Stoika RS. Differential effect of sanguinarine, chelerythrine and chelidonine on DNA damage and cell viability in primary mouse spleen cells and mouse leukemic cells. Cell Biol Int. 2008;32:1271–277. doi: 10.1016/j.cellbi.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Kassim OO, Loyevsky M, Elliott B, Geall A, Amonoo H, Gordeuk VR. Effects of root extracts of Fagara zanthoxyloides on the in vitro growth and stage distribution of Plasmodium falciparum. Antimicrob Agents Chemother. 2005;49:264–268. doi: 10.1128/AAC.49.1.264-268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GS, Maiti M. DNA polymorphism under the influence of low pH and low temperature. J Biomol Struct Dyn. 1994;12:183–201. doi: 10.1080/07391102.1994.10508096. [DOI] [PubMed] [Google Scholar]
- Latimer LJP, Payton N, Forsyth G, Lee JS. The binding of analogues of coralyne and related heterocyclics to DNA triplexes. Biochem Cell Biol. 1995;73:11–18. doi: 10.1139/o95-002. [DOI] [PubMed] [Google Scholar]
- Maiti M. Molecular aspects on the interaction of sanguinarine with B-form, Z-form and HL-form structures. Indian J Biochem Biohys. 2001;38:20–26. [PubMed] [Google Scholar]
- Maiti M, Kumar GS. Molecular aspects on the interaction of protoberberine, benzophenanthridine, and aristolochia group of alkaloids with nucleic acid structures and biological perspectives. Med Res Rev. 2007;27:649–695. doi: 10.1002/med.20087. [DOI] [PubMed] [Google Scholar]
- Maiti M, Kumar GS. Protoberberine alkaloids: physicochemical and nucleic acid binding properties. Top Heterocycl Chem. 2007;10:155–209. doi: 10.1007/7081_2007_071. [DOI] [Google Scholar]
- Maiti M, Nandi R. Circular dichroism of sanguinarine-DNA complexes: effect of base composition, pH and ionic strength. J Biomol Struct Dyn. 1987;5:159–175. doi: 10.1080/07391102.1987.10506383. [DOI] [PubMed] [Google Scholar]
- Maiti M, Nandi R, Chaudhuri K. Sanguinarine: a monofunctional intercalating alkaloid. FEBS Lett. 1982;142:280–284. doi: 10.1016/0014-5793(82)80152-X. [DOI] [PubMed] [Google Scholar]
- Maiti M, Nandi R, Chaudhuri K. The effect of pH on the absorption and fluorescence of sanguinarine. Photochem Photobiol. 1983;38:245–249. doi: 10.1111/j.1751-1097.1983.tb03869.x. [DOI] [Google Scholar]
- Maiti M, Nandi R, Chaudhuri K. Interaction of sanguinarine with natural and synthetic deoxyribonucleic acids. Indian J Biochem Biophys. 1984;21:158–165. [PubMed] [Google Scholar]
- Maiti M, Das S, Sen A, Kumar GS, Nandi R. Influence of DNA structures on the conversion of sanguinarine alkanolamine form to iminium form. J Biomol Struct Dyn. 2002;20:455–464. doi: 10.1080/07391102.2002.10506864. [DOI] [PubMed] [Google Scholar]
- Malikova J, Zdarilova A, Hlobikova A. Effect of sanguinarine and chelerythrine on cell cycle and apoptosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:5–12. doi: 10.5507/bp.2006.001. [DOI] [PubMed] [Google Scholar]
- Moterich IG, Strekal ND, Nowicky JW, Maskerich SA. Absorption, fluorescence and SERS spectra of sanguinarine at different pH values. J Appl Spectrosc. 2007;74:666–672. doi: 10.1007/s10812-007-0107-7. [DOI] [Google Scholar]
- Nafao Y, Sano S, Miyasaka T, Ochiai M, Fiji K, Fujita E, Ishii H. Evaluation of the affinity of new N atom-containing tumor inhibitors for nucleic acids. Nucleic Acids Sym Ser. 1985;16:37–40. [PubMed] [Google Scholar]
- Nandi R, Maiti M. Binding of sanguinarine to deoxyribonucleic acids of differing base composition. Biochem Pharmacol. 1985;34:321–324. doi: 10.1016/0006-2952(85)90038-3. [DOI] [PubMed] [Google Scholar]
- Novak RM, Keyer KA, Kinghorn AD, Pezzuto JM. Antiviral activity of the plant compound fagaronine chloride, a reverse transcriptase inhibitor in HIV-infected peripheral blood mononuclear cells and T cell lines. Int Conf AIDS. 1991;7:11–12. [Google Scholar]
- Pezzuto JM, Antosiak SK, Messmer WM, Slaytor MB, Honig GR. Interaction of the antileukemic alkaloid, 2-hydroxy-3,8,9-trimethoxy-5-methylbenzo[c]phenanthridine (fagaronine), with nucleic acids. Chem Biol Interact. 1983;43:323–329. doi: 10.1016/0009-2797(83)90116-3. [DOI] [PubMed] [Google Scholar]
- Pohl FM, Jovin TM. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC) J Mol Biol. 1972;67:375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Prado S, Michel S, Tillequin F, Koch M, Pfeiffer B, Pierre A, Leonce S, Colson P, Baldeyrou B, Lansiaux A, Bailly C. Synthesis and cytotoxic activity of benzo[c][1,7] and [1,8]phenanthrolines analogues of nitidine and fagaronine. Bioorg Med Chem. 2004;12:3943–3953. doi: 10.1016/j.bmc.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Qin Y, Pang J, Chen WH, Cai Z, Jiang ZH. Synthesis, DNA-binding affinities, and binding mode of berberine dimers. Bioorg Med Chem. 2006;14:25–32. doi: 10.1016/j.bmc.2005.07.069. [DOI] [PubMed] [Google Scholar]
- Ray A, Kumar GS, Das S, Maiti M. Spectroscopic studies on the interaction of aristololactam-β-D glucoside with DNA and RNA double and triple helices: a comparative study. Biochemistry. 1999;38:6239–6247. doi: 10.1021/bi982128n. [DOI] [PubMed] [Google Scholar]
- Ren J, Chaires JB. Sequence and structural selectivity of nucleic acid binding ligands. Biochemistry. 1999;38:16067–16075. doi: 10.1021/bi992070s. [DOI] [PubMed] [Google Scholar]
- Saenger W. Principles of nucleic acids structure. New York: Springer-Verlag; 1984. [Google Scholar]
- Saran A, Srivastava S, Coutinho E, Maiti M. 1H NMR investigation of the interaction of berberine and sanguinarine with DNA. Indian J Biochem Biophys. 1995;32:74–77. [PubMed] [Google Scholar]
- Segers-Nolten GM, Sijtsema NM, Otto C. Evidence for Hoogsteen GC base pairs in the proton-induced transition from right-handed to left-handed poly(dG-dC).poly(dG-dC) Biochemistry. 1997;36:13241–13247. doi: 10.1021/bi971326w. [DOI] [PubMed] [Google Scholar]
- Sen A, Maiti M. Interaction of sanguinarine iminium and alkanolamine form with calf thymus DNA. Biochem Pharmacol. 1994;48:2097–2102. doi: 10.1016/0006-2952(94)90510-X. [DOI] [PubMed] [Google Scholar]
- Sen A, Ray A, Maiti M. Thermodynamics of the interactions of sanguinarine with DNA: influence of ionic strength and base composition. Biophys Chem. 1996;59:155–170. doi: 10.1016/0301-4622(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Serafim TL, Matos JAC, Sardão VA, Pereira GC, Branco AF, Pereira SL, Parke D, Perkins EL, Moreno AJM, Holy J, Oliveira PJ. Sanguinarine cytotoxicity on mouse melanoma K1735–M2 cells—nuclear vs. mitochondrial effects. Biochem Pharmacol. 2008;76:1459–1475. doi: 10.1016/j.bcp.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Smekal E, Kubova N, Kleinwatcher V. Interaction of benzophenanthridine alkaloid sanguinarine with DNA. Stud Biophys. 1984;114:125–132. [Google Scholar]
- Southard GL, Boulware RT, Walborn DR, Groznik WJ, Thorne EE, Yankell SL. Sanguinarine, a new antiplaque agent: retention and plaque specificity. J Am Dent Assoc. 1987;108:338–341. doi: 10.14219/jada.archive.1984.0022. [DOI] [PubMed] [Google Scholar]
- Stiborova M, Simanek V, Frei E, Hobza P, Ulrichova J. DNA adduct formation from quaternary benzo[c]phenanthridine alkaloids sanguinarine and chelerythrine as revealed by the 32P-postlabeling technique. Chem Biol Interact. 2002;140:231–242. doi: 10.1016/S0009-2797(02)00038-8. [DOI] [PubMed] [Google Scholar]
- Tajmir-Riahi HA, Neault JF, Naoui M. Does DNA acid fixation produce left-handed Z structure? FEBS Lett. 1995;370:105–108. doi: 10.1016/0014-5793(95)00802-G. [DOI] [PubMed] [Google Scholar]
- Todor IN. The effect of the antineoplastic drug Ukrain on the electrokinetic potential of malignant and normal cells. Int J Immunother. 2003;19:159–167. [Google Scholar]
- Urbanova J, Lubal P, Slaninova I, Taborska E, Taborsky P (2009) Fluorescence properties of selected benzo[c]phenanthridine alkaloids and studies of their interaction with CT DNA. Anal Bioanal Chem. doi:10.1007/s00216-009-2601-7 [DOI] [PubMed]
- Vogt A, Tamewitz A, Skoko J, Sikorski PP, Giuliano KA, Lazo JS. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem. 2005;280:19078–19086. doi: 10.1074/jbc.M501467200. [DOI] [PubMed] [Google Scholar]
- Wang LK, Johnson RK, Hecht SM. Inhibition of topoisomerase I function by nitidine and fagaronine. Chem Res Toxicol. 1993;6:813–818. doi: 10.1021/tx00036a010. [DOI] [PubMed] [Google Scholar]
- Waring MJ. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- Wolff J, Knipling L. Antimicrotubule properties of benzophenanthridine alkaloids. Biochemistry. 1993;32:13334–13339. doi: 10.1021/bi00211a047. [DOI] [PubMed] [Google Scholar]