Abstract
The orientation of a cross-bridge is widely used as a parameter in determining the state of muscle. The conventional measurements of orientation, such as that made by wide-field fluorescence microscopy, electron paramagnetic resonance (EPR) or X-ray diffraction or scattering, report the average orientation of 1012–109 myosin cross-bridges. Under conditions where all the cross-bridges are immobile and assume the same orientation, for example in normal skeletal muscle in rigor, it is possible to determine the average orientation from such global measurements. But in actively contracting muscle, where a parameter indicating orientation fluctuates in time, the measurements of the average value provide no information about cross-bridge kinetics. To avoid problems associated with averaging information from trillions of cross-bridges, it is necessary to decrease the number of observed cross-bridges to a mesoscopic value (i.e. the value affected by fluctuations around the average). In such mesoscopic regimes, the averaging of the signal is minimal and dynamic behavior can be examined in great detail. Examples of mesoscopic analysis on skeletal and cardiac muscle are provided.
Keywords: Myosin cross-bridge orientation, Fluorescence polarization, Mesoscopic measurements
Introduction
The orientation of a cross-bridge is widely used as a parameter of the state of muscle. The conventional measurements of orientation, such as the ones made by wide-field fluorescence microscopy (Aronson and Morales 1969; Borejdo et al. 1979; Dos Remedios et al. 1972a, b) or by electron paramagnetic resonance (EPR) (Cooke et al. 1982; Thomas and Cooke 1980), report the average orientation of 1012–109 myosin cross-bridges. When all the cross-bridges are immobile and assume the same orientation, like in normal skeletal muscle in rigor, it is possible to determine the average orientation from such global measurements (Thomas and Cooke 1980; Borejdo et al. 1982; Berger et al. 1996; Hopkins et al. 2002; Sabido-David et al. 1998). But in actively contracting muscle, where a parameter indicating orientation fluctuates in time, the averaging destroys all the kinetic information. For example, only 10−4 % (√N/N) of a signal from muscle fiber containing n = 1012 randomly oriented cross-bridges carries kinetic information. The rest is an average and carries no information about cross-bridge kinetics. When the number of observed molecules is small enough so that the result is affected by fluctuations around the average (Qian et al. 2002), the measurements are called mesoscopic. In a mesoscopic regime, the averaging of the signal is minimal and the dynamic and steady-state behavior can be examined in great detail. While it is possible to obtain kinetic information by imposing transients (Huxley and Simmons 1971; Goldman et al. 1984a, b), it is non-steady-state information.
The ultimate way to avoid averaging information from trillions of myosins or other motor molecules is the observation of fluorescence from a single molecule (fluorescence is the only signal strong enough to be used). This is possible with a conventional confocal microscope in in vitro experiments, where the background signal can be effectively eliminated. Thus, Warshaw et al. were able to determine conformational states of smooth myosin using fluorescence of rhodamine incorporated into smooth myosin (Warshaw et al. 1998). Quinlan et al. used total internal reflection fluorescence (TIRF) to measure the orientation of myosin light chain (Quinlan et al. 2005). Lu et al. used quantum dots to observe the diffusive movement of processive kinesin-1 on microtubules (Lu et al. 2009). Yildiz et al. determined that the measured motion of a single molecule of kinesin labeled with Cy3 occurred in hand-over-hand fashion (Yildiz et al. 2004a), and the same mechanism was observed in myosin VI labeled with GFP or Cy3 (Yildiz et al. 2004b). Myosin V, because of its large tail domain, has been extensively investigated. Forkey using single-molecule fluorescence polarization determined the three-dimensional structural dynamics of myosin V (Forkey et al. 2003), and Lu et al. observed processive motion of the head of myosin V simultaneously with the motion of the lever arm (Lu et al. 2010). Finally, Sun et al. determined stepping and structural dynamics of unconventional myosin X (Sun et al. 2010).
To observe single molecules in vivo is much more complex, mainly because of high local protein concentration and complicating effects of light scattering and autofluorescence. Particularly successful methods for decreasing the effective concentration involved the temporal separation of fluorophores. Central to these methods is the idea that the center of a point spread function (PSF) of the microscope objective can be determined with higher precision than its full width at half maximum (FWHM), and thus as long as only one molecule at a time exists in the detection volume, its precise location may be determined from the center of the diffraction-expanded PSF. The effective concentration of fluorophores within a living cell is reduced to the necessary level by randomly activating/deactivating them with light pulses. The practical realization of this idea resulted in different flavors of PALM and STORM microscopy, where fluorescent images of a cell are obtained when fluorophores are randomly activated. The images are recorded and every molecule is analyzed computationally to determine the center of its PSF. This results in beautiful images with resolution far below the diffraction limit of conventional microscopes but the time resolution of the method is low.
A related method of overcoming the diffraction limit involves minimizing the observational volume. In this technique, like in conventional confocal microscopy, fluorescent probes are attached to the molecules of interest and are excited by a focused laser beam and observed through a confocal aperture. However, unlike PALM, fluorophores are not randomly activated, but most of their fluorescence is depleted by a second, red-shifted, doughnut-shaped laser beam which is made to illuminate the sample before spontaneous emission of fluorescence occurs. This second beam stimulates the transition of excited molecules back to their ground state. Stimulated emission has the same red-shifted wavelength as the doughnut-shaped beam. A bandpass filter rejects the stimulated photons and allows collection of the shorter wavelength fluorescence photons, which originate from the center of the doughnut. The second beam effectively quenches a subset of fluorophores at the periphery of the PSF, thereby reducing the effective PSF to the diameter of the “doughnut hole”. The PSF produced by the non-quenched fluorophores is ∼10 times smaller than that in a conventional or confocal microscope (Hell and Wichmann 1994; Klar et al. 2000). Therefore, STED microscopes produce spectacular images of the insides of cells (Willig et al. 2006; Urban et al. 2011; Tian et al. 2011), but like PALM, STORM and related methods are too slow to follow rapid conformational changes.
To achieve the required time resolution in experiments on skeletal or cardiac muscle, we have adopted a different approach. The number of observed molecules is equal to the detection volume (DV) multiplied by the concentration of the fluorophore. Our approach has been to simultaneously limit the DV and to minimize the concentration of the fluorophore. The effective way to minimize the volume has been to use TIRF microscopy to minimize the height of the DV and to use a confocal aperture to minimize its lateral dimension (Borejdo et al. 2006a; Burghardt et al. 2006a; Muthu et al. 2008). Alternatively, around-the-objective total internal reflection fluorescence microscopy can be used (Burghardt et al. 2009). The height of DV could be further minimized by observing surface plasmon coupled emission (SPCE) fluorescence (Borejdo et al. 2006b, c; Gryczynski et al. 2006; Burghardt et al. 2006b), by two photon fluorescence (Borejdo et al. 2004) or by reverse Kretchmann illumination (Mettikolla et al. 2010). Ultimately, we found that the most effective way to decrease DV was to use the confocal microscope with single molecule detection (SMD) capability. The advantage of this is that one does not have to rely on wide-field detection such as TIRF, which gives higher background due to autofluorescence from the glass elements of the objective where the exciting beam is focused.
The most effective way to minimize the concentration is to use isolated myofibrils as a sample. It is possible to add an external dye to demembrenated muscle that binds specifically and rigidly to various sarcomeric proteins. In the example provided here, it is myosin that is labeled. It is exchanged very inefficiently with the fluorescent essential light chain 1 (LC1) or with the regulatory light chain (RLC). The smallest practical concentration of the dye was found to be ∼10 nM. This makes the smallest number of myosin molecules in the DV 6–8.
Briefly, the height of the DV is equal to the height of a myofibril, which is typically 0.5–1 μm. The lateral dimension of DV is limited to ∼0.5 μm by using confocal detection with small pinhole. This method does not yet allow observing a single cross-bridge, but the number of observed molecules is in the mesoscopic range (Qian et al. 2002). We measure conformation by recording the polarization of fluorescence from a few molecules located in a single half-sarcomere by recording parallel (I║) and perpendicular (I┴) components of the fluorescent light emitted by a fluorophore bound to myosin light chain. The normalized difference between these components, polarized fluorescence (PF), is a sensitive indicator of the orientation of the transition dipole of the fluorophore (Dos Remedios et al. 1972a, b; Berger et al. 1995, 1996; Burghardt et al. 2006b; Hopkins et al. 1998; Goldman 1998; Morales 1995). The technique allows the observation of only a few myosin molecules in contracting muscle with ms time resolution. Using this technique, we show that the lever arms of cross-bridges in isometrically contracting muscle rotate on a millisecond time scale, and that the probability of their distributions indicates relatively narrow range of orientations. This point is made schematically in Fig. 1.
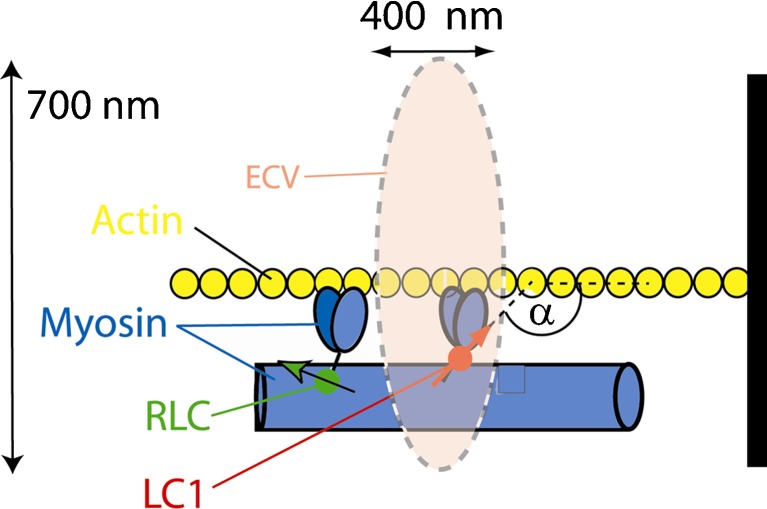
Fig. 1.
The principle of observing few molecules in muscle. ECV elliptical confocal volume. A small fraction of fluorescently labeled myosin is in ECV (here it is myosin that has been exchanged with LC1), which is characterized by a single transition dipole (red arrow). The dynamics of the fluorophore is determined by polarized fluorescence(polarized fluorescence) = [normalized difference between parallel (║) and perpendicular (┴) components the fluorescent light]. The probability distribution of polarized fluorescence is a measure of the extent of dispersion of orientations of lever arm orientations (Δα)
The exciting light beam is focused to the diffraction limit on the overlap band of a myofibril. The axial and lateral dimensions of the elliptical confocal volume (ECV) (dashed line) are estimated by measuring the FWHM of an image of 20 nm fluorescent beads. They are 700 nm and 400 nm, respectively. ECV is equal to (π/2)3/2 × (0.400 μm)2 × (0.700 μm) = 0.6 μm3. This makes DV = ECV/0.35 =1.7 μm3 (Buschmann et al. 2009). The concentration of myosin in muscle is 0.1 mM (Bagshaw 1982), and therefore there are 105 myosin molecules in the detection volume. We show below that the procedure labels only 0.02 % out of this number.
A PicoQuant MT 200 confocal system (PicoQuant, Berlin, Germany) coupled to an Olympus IX 71 microscope is used to acquire the fluorescent data. This instrument operates in the time-resolved mode and is capable of lifetime imaging with SMD sensitivity. Each photon is recorded individually by the time-correlated single photon counting electronics in time-tagged time-resolved mode. A 635-nm pulsed laser provided linearly polarized excitation parallel to the myofibrillar axis. After collecting fluorescence by Olympus ×60, 1.2-NA water immersion objective, fluorescent light was passed through a 30-μm pinhole and split by a 50:50 birefringent prism. Avalanche photodiodes (APDs) detected separated light beams through orthogonally oriented analyzers and 650 LP filter. It is made certain that APD's give identical readings for isotropic solution of dye with long fluorescence lifetime (50 nM rhodamine 700). To smooth the data it was binned by combining 1,000 measurements. This decreased time resolution from 10 μs to 10 ms.
If the first subscript of the fluorescent intensity (I) signifies the direction of orientation (either ║ or ┴ to myofibrillar axis) of excitation light , and the second subscript signifies the direction of orientation (either ║ or ┴ to myofibrillar axis) of emitted light, than the polarization (PF) is defined as PF = P║ = (║I║-║I┴ )/(║I║ + ║I┴ ) (Tregear and Mendelson 1975). Myofibrils were always excited with light ║to its axis (║I) . Channels 2 and 1 were used to detect ║I┴ and ║I║, respectively.
Skeletal muscle
Here, we applied the novel method described above to skeletal myofibrils exchanged with SeTau-647-mono-maleimide (SeTau) dye-labeled LC1. SeTau has several important advantages over a single isomer of tetramethylrhodamine-5-iodoacetamide dihydroiodide used earlier (Midde et al. 2011a). Most importantly, SeTau is excited in the red and thus reduces the contribution of autofluorescence (Lakowicz 2006). Further, it is well suited for excitation with 635-nm diode lasers, it has a large Stokes shift (44 nm), has much higher photostability than Cy5 or Alexa647, has a high extinction coefficient (230,000), and has a several times longer fluorescent lifetime than Cy5 or Alexa647 or SETA dyes. Because it has relatively a long fluorescence lifetime, it has low polarization, and a contribution of the unbound fraction of fluorophores to the observed polarization of fluorescence is negligible. SeTau is a unique dye with high excitation in red and a relatively long lifetime, two properties that are usually mutually exclusive.
Figure 2 shows an example of implementatation of the scheme in skeletal muscle. The laser is focused to a diffraction limited spot on the A-band of a sarcomere. The projection of this spot on the image plane is a shown as a red circle.
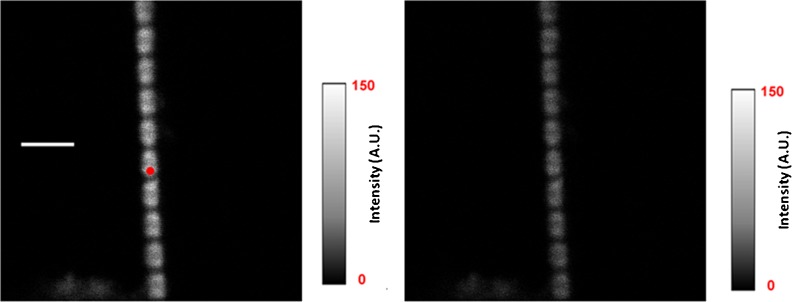
Fig. 2.
Intensity images of a rigor myofibril. The red circle is a projection of the confocal aperture on the sample plane (diameter 0.5 μm). Left image direction of polarization of exciting light is parallel (║I║, left image) and perpendicular (║I┴ , right image) to the axis of a myofibril. The intensity scales are in arbitrary units with 0 corresponding to black and 150 to white. Native myofibrillar LC1 was exchanged with 10 nM SeTau-LC1 . Scale bar 5 μm, sarcomere length 2.1 μm. Images were acquired on a PicoQuant Micro Time 200 confocal lifetime microscope. The sample was excited with a 635-nm pulsed laser and observed through a LP 650 filter
The left image is more intense than the right image indicating that that the fluorescence is highly polarized as expected from the anisotropic sample.
The number of myosin molecules in DV is determined experimentally by Fluorescence Correlation Spectroscopy (FCS) from the number fluctuations of the free SeTau dye. The number of molecules (N) contributing to the autocorrelation function of these fluctuations is equal to the inverse of the value of the autocorrelation function at delay time 0 [G(0)], N = 1/G(0) (Magde et al. 1974; Elson 1985; Elson 2007). The ACF's were obtained for solutions of fluorophore in the range 5-20 nM. To eliminate contributions from random events and afterpulsing, we compute the cross-correlation function between ch1 and ch2. The 1/G(0) is plotted vs. number of SeTau molecules in the DV in Fig. 3. Extrapolation reveals that the concentration contributed by one molecule of the dye corresponds to ∼75 counts per channel, i.e. the total fluorescence (Itotal = I║ + 2* I┴) from one molecule of SeTau is 225 counts/s.
Fig. 3.
Estimating the number of molecules in the detection volume. The numbers of molecules in the DV were obtained from the inverse of the autocorrelation function, such as shown in the inset, extrapolated to a lag time 0. Average diffusion coefficient of SeTau and its correlation time were 250 μm2/s and 10.5 ms, respectively. Inset autocorrelation function of 10 nM SeTau solution giving 6 molecules. The laser power (before objective) = 0.1 μW
The actual number of SeTau-LC1 molecules in the DV can now be estimated from the photon rate collected in a typical experiment. A signal obtained in a typical experiment is shown in Fig. 4. Myofibrils have been cross-linked with the water-soluble reagent 1-ethyl-3-[3-(dimethylamino)-propyl]-carbodiimide (EDC) (Herrmann et al. 1993; Tsaturyan et al. 1999) to prevent shortening. The intensities decrease because of photobleaching, but the mean polarization of fluorescence (bottom panel, blue) remains constant, because both intensities bleach at the same rate.
Fig. 4.
Typical time course of polarized intensity of contracting psoas muscle myofibril. This is a bar plot, where the vertical scale is the number of counts during 10 msec. Ch1 (black) and Ch 2 (red) are the fluorescence intensities polarized perpendicular (I┴) and parallel (I║) to the myofibrillar axis, respectively. The direction of excitation polarization is ║ to the myofibrillar axis. Laser power = 0.1 μW
The polarized intensities in ║ and ┴ channels were 3,400 and 1,300 counts/s giving total intensity Itotal = I║ + 2* I┴ = 6,000 counts/s. The background was 1,200 counts/s indicating that the number of myosin molecules in the DV was ∼20. It should be emphasized, however, that as long as the number of cross-bridges is mesoscopic, i.e. the exact number does not matter, then 20 molecules should give the same result as 80 molecules, etc.
Results
The kinetics of the conformational changes of cross-bridges during contraction of skeletal muscle
To examine the nature of PF fluctuations we measured the autocorrelation function (ACF) of polarized fluorescence. The autocorrelation function of PF's is the normalized time average of PF's multiplied by the value of PF's a delay time later. This is a powerful method of extracting individual contributions from a signal (Magde et al. 1974; Elson 1985, 2007). The ACF of polarization of the fluorescence signal (bottom of Fig. 4) from contracting muscle is shown in Fig. 5. A decay of ACF characterizes the rapidity of rotational motions of the lever arm. During contraction, the lever arms undergo power-stroke cycles and the PF changes cyclically. It is not at all obvious that lever arms rotate at all during isometric contraction. Isometric conditions are enforced by cross-linking and this may prevent cross-bridges from reaching neighboring actin target zones while detached. Moreover, even if cross-bridges manage to reach target zones, they may undergo a single cycle while stretching a series elastic component, and remain in immobile afterwards. Analyzing the shape of autocorrelation function can tell whether the signal has a periodic component; the autocorrelation function of a periodic signal is periodic (Bracewell 1965). The ACF of contracting muscle was not periodic. This finding is not surprising in view of the fact that synchronization would have imposed periods in which all the cross-bridges in a half-sarcomere would have been dissociated from actin causing extension by its neighbors. We note that the other possible source of fluctuations, i.e., fluctuations in the number of fluorophores in the DV (Magde et al. 1974; Elson and Magde 1974) are not significant because PF is only sensitive to rotations and myofibrils were unable to twist.
Fig. 5.
Autocorrelation function of polarization of fluorescence of contracting skeletal myofibril of Fig. 4. The fact that the correlation decays in time indicates that the orientation of absorption/emission dipoles change in time
It is possible to relate the decay of the correlation function to cross-bridge kinetics (Mettikolla et al. 2011). For a simple 2-state model of cross-bridge cycle, where cross-bridges can assume only two orientations (rigor or detached from actin) the decay parameters are related in a simple way to the rate of cross-bridge attachment (k1) and dissociation (k2) from actin
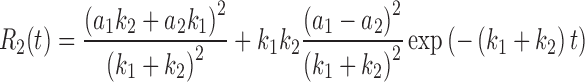 |
where a1 and a2 are fluorescence intensities associated with conformational change. Substituting a1 = 0 and a2 = 1, we get from a three-parameter exponential fit k1 = 10.82 s-1 and k2 = 0.21 s-1.
During rigor, the lever arms were stationary and the autocorrelation of fluctuations was flat. A flat correlation function arises when there are no correlations between fluorescence intensities at any time within the time of the measurement. We conclude that the transition dipoles of the dye attached to rigor cross-bridges do not change orientation at all (Magde et al. 1974; Elson and Magde 1974). This suggests that LC1 moieties are close enough to the myosin heads to be immobilized by the binding of heads to thin filaments. The autocorrelation function of polarization of fluorescence of the lever arms during relaxation was non-zero and non-flat.
The fact that cross-bridges rotate in cross-linked muscle indicates that they must also rotate in intact muscle. Moreover, exclusion volume effects must play a significant role during contraction (Minton 2001) because muscle is an exceptionally dense environment (Bagshaw 1982). Therefore, kinetics obtained here are different than those obtained before in in vitro in solution experiments (Jacobs et al. 2011), by X-ray (Brown et al. 2011; Minoda et al. 2011), by AFM (Kodera et al. 2010), by electron tomography (Wu et al. 2010), by laser trap (Sleep et al. 2006), and in single molecule experiments in myosin II (Warshaw et al. 1998; Quinlan et al. 2005), myosin V (Forkey et al. 2003), myosin VI (Reifenberger et al. 2009; Spudich 2008; Sun et al. 2007), and in myosin X (Sun et al. 2010).
Distribution of cross-bridge orientations during contraction of skeletal muscle
Mesoscopic measurements also provide useful information about the degree of local order of cross-bridges. The degree of order, whether in contracting, relaxed, or rigor muscle, is an indicator of the state of the muscle. Again, the global measurements are inadequate because they average out the information about the disorder. The observable fluctuations in global measurements are contributed mainly by the noise and carry no information about cross-bridge disorder. The mesoscopic measurements, in contrast, provide such information. We examined the distribution of lever arm orientations in hundreds of haf-sarcomeres of contracting, relaxing, and rigor muscle. In order to make a statistically valid comparison between hundreds of cross-bridges from different sarcomeres, it must be recognized that myofibrils in each state give rise to fluorescent signals of different strengths. Since the signal fluctuations are random, the width of a probability distribution depends on the square root of the signal strength (Bracewell 1965; Elson 2004). The absolute value of the FWHM of the probability distribution is large for strong signals and small for weak signals (the relative value of FWHM is opposite - it is small for large signals and big for large signals). Therefore, to make meaningful comparisons between the signals originating from different sarcomeres have to be normalized with respect to the total fluorescence intensity. Normalization involves multiplying FWHM of the weaker signal by the square root of the ratio between larger and smaller signals.
The reason the signals from rigor, relaxed, and contracting muscles vary in strength is that the properties of the fluorophore vary in different environments. Properties such as fluorescence quantum yield, extinction coefficient, and fluorescence lifetime each depend on the environment (Lakowicz 2006). Another critical determinant of fluorescence intensity is the density of fluorophores. This may vary because some solvent extrusion may occur in muscle during contraction. The extrusion of solvent from contracting myofibrils was proposed earlier as a mechanism of muscle contraction (Szent-Gyorgyi 1974). Szent-Gyorgi believed that contraction is brought about by ATP-induced shedding of the water envelope surrounding the S2 segment of myosin in rigor. Although extrusion is no longer believed to be responsible for muscle contraction, extrusion of solvent can be seen by nuclear magnetic as an increase of relaxation times of water protons in living skeletal frog muscle during isometric contraction (Bratton et al. 1965). This led to the suggestion that water in muscle is organized (Cope 1969; Hazlewood et al. 1969; Damadian 1976) and aligned along the myofilaments, and that the state of the intracellular water changes with physiological conditions (Yamada 2001). Oplatka and collaborators suggested that hydrolysis of ATP causes directional flow of water away from the Z-discs forcing buildup of pressure in the center of a sarcomere, thus causing sliding of filaments (Oplatka et al. 1974). A similar mechanism was proposed for the cytoplasmic streaming in Physarum polycephallum (Tirosh et al. 1973), thrombostenin (Cohen et al. 1974), and actomyosin solutions (Oplatka and Tirosh 1973). Solvent extrusion is massive enough to cause tightening of sarcomere structure and an increase in the density of muscle, and so cause increase in the number of molecules in the DV.
The order is best represented by the probability distribution of orientations. Probability distribution are plots of polarization values versus the number of times that a given orientation occurs during a 20-s experiment. A narrow probability distribution indicates that the cross-bridges are relatively uniformly oriented. Conversely, a broad probability distribution indicates that cross-bridges are relatively disordered. In relaxation, the myosin heads are free to oscillate, driven by thermal fluctuations. The dispersion of orientations arises from the variability of the orientations the lever arm due to thermal motions. It is believed to be completely disorganized. In contraction, in contrast, the lever arms undergo power-stroke cycles. The dispersion of orientations arises from the changes of orientations of LC1 and from stochastic noise. Therefore, the comparison of FWHMs of probability distributions during contraction and relaxation is a measure of cross-bridge order. Figure 6 compares normalized probability distribution of contracting and relaxing myofibrils.
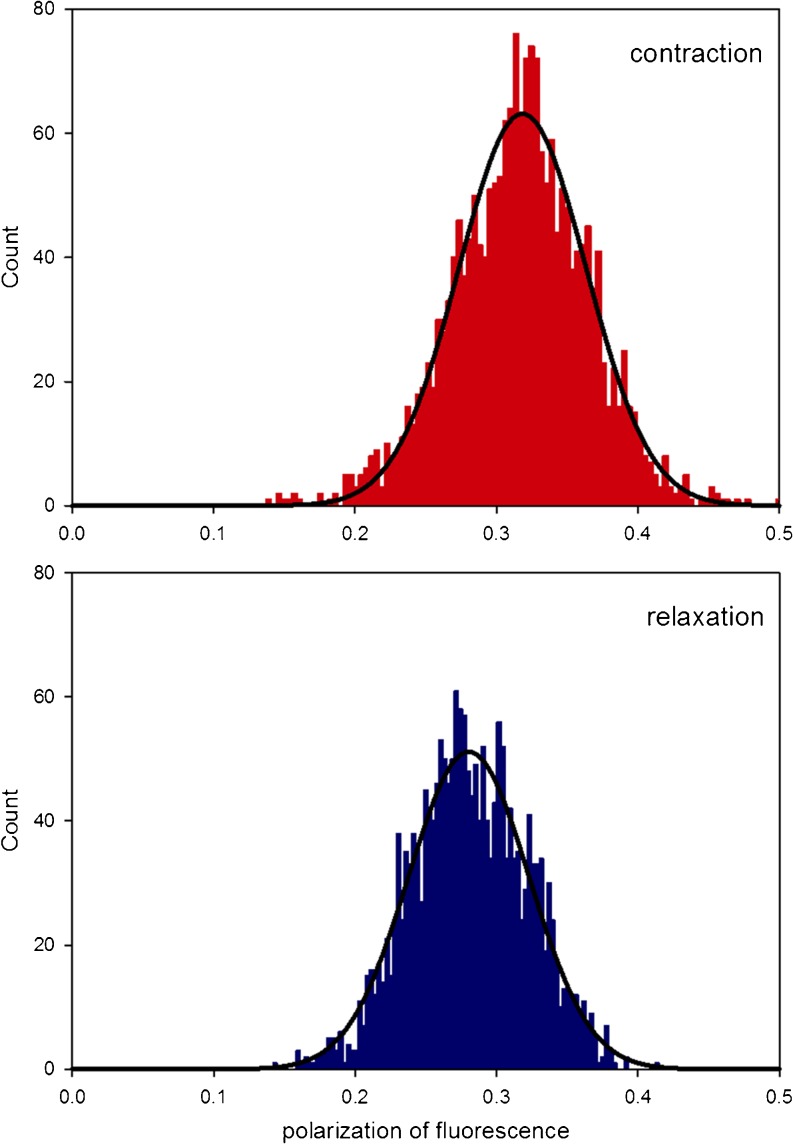
Fig. 6.
Probability distribution of orientations of cross-bridge lever arms during contraction (red) and relaxation (blue) of myofibrils. All the probability distributions could be fit by a single Gaussian y = a exp[−0.5(x−xo/b)2] (solid line). The goodness of fit was assessed by χ 2 in the Origin program. The FWHMs of contracting and relaxed myofibrils were 0.247 and 0.358, respectively
The dispersion could also be fitted by two Gaussians as reported in (Midde et al. 2011a), where it was suggested that one Gaussian represented cross-bridges in the pre-power stroke state and the second one cross-bridges in the post-power stroke state. For the purpose of the present review, however, in order to derive a single FWHM parameter from the distributions, we fitted all histograms by a single Gaussian.
The normalized FWHMs of 27 experiments are summarized in Table 1. The difference between the effective FWHMs of contracting and relaxed myofibrils was large enough to suggest that it was not due to the difference in random sampling (t = -−8.532 with 53 degrees of freedom, P = <0.001).
Table 1.
Effective FWHM values and their SD in all 27 experiments
| State | Effective FWHM | SD of effective FWHM | PF of the peak |
|---|---|---|---|
| Contracting | 0.121 | 0.06 | 0.2884 ± 0.0552 |
| Relaxing | 0.230 | 0.043 | 0.2861 ± 0.0255 |
We conclude that cross-bridges are more organized during contraction than during relaxation.
In principle, it is possible to translate polarization of fluorescence values to the absolute orientation of transition dipoles with respect to the myofibrillar axis (Dale et al. 1999). However, such translation is critically dependent on the model of arrangement of cross-bridges. For example, using the model of Tregear and Mendelson (Tregear and Mendelson 1975), which assumes that a fraction α of the cross-bridges are arranged helically along the long axis of muscle and the rest are arranged randomly and that the cross-bridges execute only polar motions (and not, more realistically, combination of polar and azimuthal motions; Burghardt and Ajtai 1994), the range of angles corresponding to a given range of polarizations can be very broad even if α is very small.
Cardiac muscle
We suspected that kinetics and the degree of order of cross-bridges was disturbed by the cardiac myopathy. The power of the mesoscopic approach is well demonstrated in our investigation of the effect of cardiac myopathy causing mutations of cardiac troponin I (cTnI) on dynamics and probability distribution of a mesoscopic number of cross-bridges in the left ventricle of transgenic mouse. cTnI inhibits actomyosin interactions in the absence of Ca2+ (Gordon et al. 2000). The R145W mutation in human cTnI is associated with restrictive cardiomyopathy (RCM). It is the least common of cardiomyopathies, but is associated with the greatest morbidity and mortality (Artz and Wynne 2000). RCM is characterized by near normal myocardial wall thickness and cavity size (Wen et al. 2009), but has impaired physiological functions including restrictive ventricular filling and reduced diastolic volume. Even though the systolic function is normal, RCM presents increased end diastolic pressure. Several mutations (L144Q, R145W, A171T, K178E, D190G, and R192H) in the highly conserved region of human cTnI I correlate with RCM (Mogensen et al. 2003). We investigated one of those mutations (R145W). The physiological measurements in skinned fibers reconstituted with this mutant showed an increase in the Ca2+ sensitivity of force development, in the basal force levels in the absence of Ca2+, and a decrease in the ability to inhibit actomyosin ATPase activity in the actin-Tm-activated myosin-ATPase assay in the presence of 1.0 mM EGTA (Wen et al. 2009; Gomes and Potter 2004; Gomes et al. 2005). In the present study, we asked what molecular changes in myosin are associated with these impaired physiological functions caused by R145W mutation.
The actual number of SeTau-LC1 molecules in the DV centered on the A-band of contracting cardiac myofibril was estimated, like in the case of skeletal muscle, from the photon rate collected in a typical experiment. Comparison of experimental and calibration curves showed that in this case we observed 6 molecules. Again, like in the case of skeletal muscle that as long as the number of cross-bridges is mesoscopic, the exact number does not matter, i.e. 10 molecule should give the same result as 100 molecules, etc.
Kinetics of conformational changes of cross-bridges during the contraction of cardiac muscle
In some cases, it was possible to distinguish peaks and valleys in the original trace. A few such experiments are shown in Fig. 7. In one experiment, data were smoothed and the peaks are indicated by red arrows. It’s possible that each arrow represents conformational changes of the lever arm. Such clustering of high polarizations also occurs in contracting MUT muscle but not in rigor, consistent with the fact that clustering reflects orientational changes. The average time to complete a cycle of orientational change of lever arm in all 34 WT contraction experiments (time between the peaks) was 2.28 ± 0.55 s.
Fig. 7.
Examples of conformational changes of the lever arm of WT myofibrils during contraction. Bottom panel shows a trace which was fitted by a 3rd degree polynomial. Each arrow corresponds to a peak of a polynomial and perhaps corresponds to one cross-bridge cycle
In order to find out whether the kinetics of cross-bridges was affected by myopathic mutations, we compared ACFs of contracting WT and R145W MUT cardiac muscle using the same approach as in skeletal muscle. Representative autocorrelation functions of WT and MUT myofibrils are shown in Fig. 8. Taking a1 = 0 and a2 = 1, where k1, and k2 are forward and reverse rate constants of cross-bridge binding and a1 and a2 are fluorescence intensities associated with conformational change, the solution of eq. 1 yields k1 = 4.07 s−1 and k2 = 0.48 s−1 for WT muscles and k1 = 0.76 s−1 and k2 = 0.06 s−1 for MUT muscles. In this particular case, the rate of myosin dissociation from actin during contraction was 8 times slower for MUT than for WT muscles. The same was true for A57G mutation. The difference in k1s of all experiments had t = 2.621, P = 0.013 with 36 degrees of freedom. The difference in k2s of all experiments had t = 2.721, P = 0.010 with 36 degrees of freedom. We conclude that the difference in the mean values of the two groups is greater than would be expected by chance, i.e. cross-bridges in MUT myofibrils bind and dissociate from actin statistically significant slower than cross-bridges in WT myofibrils.
Fig. 8.
Kinetics of orientation change of rigor lever arms of WT (a) and MUT (b) R145W myofibrils. The black lines are best fits for WT and MUT muscles. The fluctuations are caused by change of orientation of lever arms only. The other possible source of fluctuations, i.e., fluctuations in the number of fluorophores in the DV (Magde et al. 1974; Elson and Magde 1974) are not significant because PF is only sensitive to rotations and myofibrils were not twisting
To summarize contraction experiments: MUT myofibrils were unbinding from thin filaments relatively slowly. This reinforces the notion that diastolic dysfunction is caused by inability of cross-bridges to dissociate from actin in a timely manner.
Distribution of cross-bridge orientations during rigor of cardiac muscle
In order to find out whether cross-bridge orientations were affected by myopathic mutations, we compared the distribution of orientations of cross-bridges in WT and R145W MUT cardiac muscle in rigor using the same approach as in skeletal muscle. As mentioned before, when comparing distributions it is important to recognize that the half-sarcomeres of contracting, relaxing, and rigor myofibrils each give rise to fluorescent signals of different intensities. This is because the dye has different quantum yield, absorption, and fluorescent lifetime in different environments. The differences in environments occur because of possible solvent extrusion from myofilament space during the contraction of demembrenated myofibril (Szent-Gyorgyi 1974), and also because of inhomogeneities of label penetration of different sarcomeres and because of differences in solvent composition (e.g., ATP, Ca2+, EGTA). (Bracewell 1965; Elson 2004). Like in the case of skeletal muscle, to make a statistically valid comparison between many half-sarcomeres examined in the present study, the signals have to be normalized with respect to the total fluorescence intensity, i.e. the "effective variance” (FWHM/mean), not the absolute FWHMs, have to be compared. Figure 9 compares probability distributions obtained during rigor of Tg-WT and Tg-MUT muscle.
Fig. 9.
Probability distribution of orientations of cross-bridge lever arms during rigor of WT (top, red) and MUT (bottom, blue) of myofibril. All the probability distributions could be fit by a single Gaussian y = a exp[−0.5(x−xo/b)2]. The goodness of fit was assessed by χ 2 in the Origin program
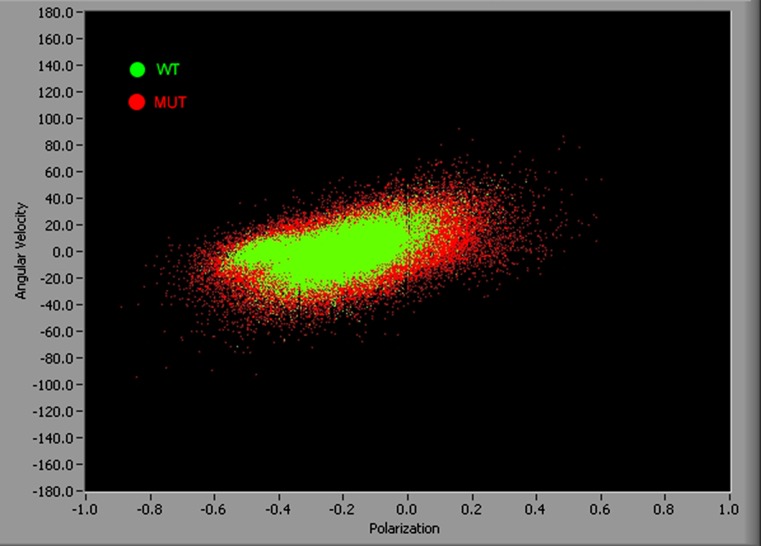
The other way to emphasize differences in probability distributions between WT and MUT myofibrils in rigor are "velocity" plots where the "angular velocity" is defined as the rate of change of PF. The average velocity in rigor is 0, but introducing a second dimension here allows presenting information contained in all 21 experiments in one plot. One experiment contains 2,000 measurements of PF, i.e. a velocity plot contains 42,000 points. It is impossible to show so many data points in 1D plots. Figure 10 shows the differences between rigor PF in WT and MUT myofibrils. It is clear that the distribution of polarizations is more compact in WT myofibrils (green) than in MUT myofibrils (red).
Fig. 10.
Polarized fluorescence plotted against angular velocity of 42,000 measurements of WT (green) and MUT (red) rigor myofibrils to emphasize that the distribution of polarizations is more compact in WT than in MUT myofibrils
To summarize: the cardiac muscle data showed that the rate of dissociation of cross-bridges from thin filaments was decreased in MUT muscle as compared to WT, while the probability distributions during rigor indicated a substantial loss of order. This was true not only in TnI R145W mutation but also in ELC-A57G, RLC-R58Q (Mettikolla et al. 2011), and TnT (Midde et al. 2011b) mutations. This suggests that the diastolic dysfunction (decreased ability to relax) may be related to the increased Ca2+ affinity to TnC (slower off rate of Ca2+ from TnC needed for relaxation). Since rigor bridges are known to increase the Ca2+ affinity of TnC (Guth and Potter 1987), it is possible that altered rigor bridge binding increases Ca2+ affinity and therefore contributes to diastolic dysfunction. Thus, it is likely that a decrease in the rate of cross-bridge dissociation from thin filaments and an increase in rigor disorder are general phenomena, i.e. the stereo-specific rigor attachment of force generating myosin cross-bridges is necessary for the normal working of the heart. Any alteration of this important energetic state of the myosin motor could be a triggering factor of cardiomyopathy.
Conclusion
The examples thus far have demonstrated that the novel technique of SMD is a viable alternative to more conventional methods. First, in the mesoscopic regime the averaging of the signal is minimal and the dynamic and steady-state behavior can be examined in great detail. Second, the method provides information from a few cross-bridges confined to one half-sarcomere, avoiding muscle heterogeneity. Third, it is well known that certain mutations in sarcomeric proteins of cardiac muscle cause severe cardiomyopathy. The mesoscopic method offers a possibility of examining mutations which are expressed at low levels. Often, the mutations can be generated in transgenic mouse with only ∼10 % incorporation of the mutated protein into a sarcomere. Such a small fraction may not lead to a significant change in the conventional contractile properties of muscle. The situation is different when examining few cross-bridges. They belong either to wild-type (WT) or mutated (MUT) sub-population. If the means of sub-populations differ by 1 standard deviation, there is a 34 % chance that, by doing 10 independent experiments, the existence of the MUT sub-population that is expressed at 10 % level will not be revealed. But this number goes rapidly to zero as the number of experiments increases. The chance that MUT sub-population will not be revealed in 100 experiments is virtually zero. Fourth, the method provides a possibility of studying the distribution of orientations of sarcomeric proteins. A good example is the question of the degree of order of myosin and the role of phosphorylation of the regulatory light chain (RLC) of myosin in the regulation of skeletal muscle contraction. This has recently become central to understanding of regulation. It has been demonstrated by electron microscopy (EM) that the helically ordered arrangement of the myosin heads characteristic of the relaxed state is lost upon phosphorylation of the RLC in the thick filaments isolated from striated muscles of tarantula (Craig et al. 1987), Limulus (Levine et al. 1991), and rabbit psoas muscle (Levine et al. 1995). Recent X-ray diffraction work showed that phosphorylation of RLC caused a change in cross-bridge mass distribution as they moved farther from the surface of thick filaments to become closer to the thin filament (Colson et al. 2010). CryoEM work of Craig et al. using the three-dimensional reconstruction of tarantula myosin filaments demonstrated the mechanism by which phosphorylation can regulate myosin activity (Alamo et al. 2008). Electron paramagnetic resonance work of Cooke and his collaborators using nucleotide-analog spin label probes showed that the myosin heads are highly ordered in the relaxed fibers, when all the heads are dephosphorylated, and that this perfectly ordered structure of myosin cross-bridges disappears with phosphorylation of RLC (Naber et al. 2011). A new super-relaxed state (SRX) in muscle has been identified in skinned skeletal (Stewart et al. 2010) and cardiac muscle fibers (Hooijman et al. 2011), where myosin has a very low ATP turnover rate. This SRX state corresponds to the highly order array of myosin heads in the absence of RLC phosphorylation that can be switched to a disordered array of myosin heads by phosphorylation of RLC (Cooke 2011). The use of the current technique to address this question may finally help to resolve the long-standing problem of the role of phosphorylation of RLC in regulation of muscle contraction.
Acknowledgements
Supported by NIH R01AR048622 and R01HL090786 grants and by Predoctoral Fellowship 12PRE8730003 from AHA. The transgenic and WT frozen hearts were kindly donated by Dr. James D. Potter (University of Miami, Miller School of Medicine) and Dr J. Pinto (Florida State University). We thank Amy Li for comments on the manuscript.
Conflict of interest
No conflict of interest to declare.
Abbreviations
- ACF
Autocorrelation function
- APD
Avalanche photodiode
- DIC
Differential interference contrast
- DV
Detection volume
- ECV
Elliptical confocal volume
- EDC
`1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide
- FCS
Fluorescence correlation spectroscopy
- FWHM
Full width at half maximum
- FLC
Fluorescence lifetime correlation
- HS
Half sarcomere
- LC1
Myosin alkaline light chain 1
- LMM
Light meromyosin
- PF
Polarization of fluorescence
- PSF
Point spread function
- QD
Quantum dots
- RCM
Restrictive cardiac myopathy
- RLC
Regulatory light chain
- S1
Myosin subfragment-1
- S2
Myosin subfragment-2
- SeTau
SeTau-647-mono-maleimide
- SeTau-LC1
LC1-myosin alkaline light chain 1 labeled with SeTau
- STED
Stimulated emission detection
- SMD
Single molecule detection
- TIRF
Total internal reflection fluorescence
References
- Alamo L, et al. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol. 2008;384(4):780–97. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson JF, Morales MF. Polarization of tryptophan fluorescence in muscle. Biochemistry. 1969;8:4517–4522. doi: 10.1021/bi00839a044. [DOI] [PubMed] [Google Scholar]
- Artz G, Wynne J. Restrictive Cardiomyopathy. Curr Treat Options Cardiovasc Med. 2000;2(5):431–438. doi: 10.1007/s11936-000-0038-6. [DOI] [PubMed] [Google Scholar]
- Bagshaw CR. Muscle Contraction. London: Chapman & Hall; 1982. [Google Scholar]
- Berger CL, et al. Fluorescence polarization from isomers of tetramethylrhodamine at SH-1 in rabbit psoas muscle fibers. Biophys J. 1995;68:78S–80S. [PMC free article] [PubMed] [Google Scholar]
- Berger CL, et al. Fluorescence polarization of skeletal muscle fibers labeled with rhodamine isomers on the myosin heavy chain. Biophys J. 1996;71:3330–3343. doi: 10.1016/S0006-3495(96)79526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J, Putnam S, Morales MF. Fluctuations in polarized fluorescence: evidence that muscle cross-bridges rotate repetitively during contraction. Proc Natl Acad Sci USA. 1979;76:6345–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J, et al. Cross-bridge orientation in skeletal muscle measured by linear dichroism of an extrinsic chromophore. J Mol Biol. 1982;158:391–414. doi: 10.1016/0022-2836(82)90205-4. [DOI] [PubMed] [Google Scholar]
- Borejdo J, et al. Rotation of the lever-arm of myosin in contracting skeletal muscle fiber measured by two-photon anisotropy. Biophys J. 2004;87:3912–3921. doi: 10.1529/biophysj.104.045450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J, et al. Rotations of a few cross-bridges in muscle by confocal total internal reflection microscopy. Biochim Biophys Acta. 2006;1763:137–140. doi: 10.1016/j.bbamcr.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Borejdo J, et al. Fluorescence correlation spectroscopy in surface plasmon coupled emission microscope. Opt Express. 2006;14(17):7878–7888. doi: 10.1364/OE.14.007878. [DOI] [PubMed] [Google Scholar]
- Borejdo J, et al. Application of Surface Plasmon Coupled Emission to Study of Muscle. Biophys J. 2006;91:2626–2635. doi: 10.1529/biophysj.106.088369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracewell R. The Fourier Transform and Its Applications. New York: McGraw-Hill; 1965. [Google Scholar]
- Bratton CB, Hopkins AL, Weinberg JW. Nuclear Magnetic Resonance Studies of Living Muscle. Science. 1965;147:738–9. doi: 10.1126/science.147.3659.738. [DOI] [PubMed] [Google Scholar]
- Brown JH, et al. Visualizing key hinges and a potential major source of compliance in the lever arm of myosin. Proc Natl Acad Sci USA. 2011;108(1):114–9. doi: 10.1073/pnas.1016288107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt TP, Ajtai K. Following the rotational trajectory of the principal hydrodynamic frame of a protein using multiple probes. Biochemistry. 1994;33:5376–5381. doi: 10.1021/bi00184a004. [DOI] [PubMed] [Google Scholar]
- Burghardt TP, Ajtai K, Borejdo J. In situ single-molecule imaging with attoliter detection using objective total internal reflection confocal microscopy. Biochemistry. 2006;45(13):4058–68. doi: 10.1021/bi052097d. [DOI] [PubMed] [Google Scholar]
- Burghardt TP, et al. In Situ Fluorescent Protein Imaging with Metal Film Enhanced Total Internal Reflection Microscopy. Biophys J. 2006;24:24. doi: 10.1529/biophysj.105.079442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt TP, Hipp AD, Ajtai K. Around-the-objective total internal reflection fluorescence microscopy. Appl Opt. 2009;48(32):6120–31. doi: 10.1364/AO.48.006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann V, Kramer B, Koberlink (2009) Quantitive FCS: Determination of confocal volume by FCS and bead scabnning with Micro Time 200. PicoQuant, Application note Quantitative FCS v. 1.1
- Cohen I, Tirosh R, Oplatka A. Active streaming in human thrombosthenin solutions. Pflugers Arch. 1974;352(1):81–5. doi: 10.1007/BF01061952. [DOI] [PubMed] [Google Scholar]
- Colson BA, et al. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol. 2010;588(Pt 6):981–93. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophys Rev. 2011;3(1):33–45. doi: 10.1007/s12551-011-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Crowder MS, Thomas DD. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cope FW. Nuclear magnetic resonance evidence using D2O for structured water in muscle and brain. Biophys J. 1969;9(3):303–19. doi: 10.1016/S0006-3495(69)86388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R, Padron R, Kendrick-Jones J. Structural changes accompanying phosphorylation of tarantula muscle myosin filaments. J Cell Biol. 1987;105(3):1319–27. doi: 10.1083/jcb.105.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RE, et al. Model-independent analysis of the orientation of fluorescent probes with restricted mobility in muscle fibers. Biophys J. 1999;76(3):1606–18. doi: 10.1016/S0006-3495(99)77320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Structured Water or Pumps? Science. 1976;193(4253):528–530. doi: 10.1126/science.193.4253.528-a. [DOI] [PubMed] [Google Scholar]
- Dos Remedios CG, Millikan RG, Morales MF. Polarization of tryptophan fluorescence from single striated muscle fibers. A molecular probe of contractile state. J Gen Physiol. 1972;59:103–120. doi: 10.1085/jgp.59.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios CG, Yount RG, Morales MF. Individual states in the cycle of muscle contraction. Proc Natl Acad Sci USA. 1972;69:2542–2546. doi: 10.1073/pnas.69.9.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson EL. Fluorescence correlataion spectroscopy and photobleaching recovery. Annu Rev Phys Chem. 1985;36:379–406. doi: 10.1146/annurev.pc.36.100185.002115. [DOI] [Google Scholar]
- Elson EL. Quick tour of fluorescence correlation spectroscopy from its inception. J Biomed Opt. 2004;9(5):857–64. doi: 10.1117/1.1779234. [DOI] [PubMed] [Google Scholar]
- Elson EL (2007) Introduction to FCS. Short Course on Cellular and Molecular Fluorescence. Gryczynski Z (ed), Vol 2. Fort Worth: UNT. 1–10
- Elson EL, Magde D. Fluorescence Correlation Spectroscopy: Coceptual Basis and Theory. Biopolymers. 1974;13:1–28. doi: 10.1002/bip.1974.360130102. [DOI] [PubMed] [Google Scholar]
- Forkey JN, et al. Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature. 2003;422(6930):399–404. doi: 10.1038/nature01529. [DOI] [PubMed] [Google Scholar]
- Goldman YE. Wag the tail: structural dynamics of actomyosin. Cell. 1998;93(1):1–4. doi: 10.1016/S0092-8674(00)81137-X. [DOI] [PubMed] [Google Scholar]
- Goldman YE, Hibberd MG, Trentham DR. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol (Lond) 1984;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman YE, Hibberd MG, Trentham DR. Initiation of active contraction by photogeneration of adenosine-5'-triphosphate in rabbit psoas muscle fibres. J Physiol (Lond) 1984;354:605–24. doi: 10.1113/jphysiol.1984.sp015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AV, Potter JD. Molecular and cellular aspects of troponin cardiomyopathies. Ann N Y Acad Sci. 2004;1015:214–24. doi: 10.1196/annals.1302.018. [DOI] [PubMed] [Google Scholar]
- Gomes AV, Liang J, Potter JD. Mutations in human cardiac troponin I that are associated with restrictive cardiomyopathy affect basal ATPase activity and the calcium sensitivity of force development. J Biol Chem. 2005;280(35):30909–15. doi: 10.1074/jbc.M500287200. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Gryczynski Z, et al. Minimization of Detection Volume by Surface Plasmon-Coupled Emission. Anal Biochem. 2006;356:125–131. doi: 10.1016/j.ab.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Guth K, Potter JD. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2 + -specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987;262(28):13627–35. [PubMed] [Google Scholar]
- Hazlewood CF, Nichols BL, Chamberlain NF. Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature. 1969;222(5195):747–50. doi: 10.1038/222747a0. [DOI] [PubMed] [Google Scholar]
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Optics Lett. 1994;19:780–782. doi: 10.1364/OL.19.000780. [DOI] [PubMed] [Google Scholar]
- Herrmann C, et al. A structural and kinetic study on myofibrils prevented from shortening by chemical cross-linking. Biochemistry. 1993;32(28):7255–63. doi: 10.1021/bi00079a023. [DOI] [PubMed] [Google Scholar]
- Hooijman P, Stewart MA, Cooke R. A new state of cardiac Myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J. 2011;100(8):1969–76. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SC, et al. Fluorescence polarization transients from rhodamine isomers on the myosin regulatory light chain in skeletal muscle fibers. Biophys J. 1998;74(6):3093–110. doi: 10.1016/S0006-3495(98)78016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SC, et al. Orientation changes of the myosin light chain domain during filament sliding in active and rigor muscle. J Mol Biol. 2002;318(5):1275–91. doi: 10.1016/S0022-2836(02)00189-4. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Jacobs DJ, et al. Kinetics and thermodynamics of the rate-limiting conformational change in the actomyosin V mechanochemical cycle. J Mol Biol. 2011;407(5):716–30. doi: 10.1016/j.jmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar TA, et al. Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. Proc Natl Acad Sci USA. 2000;97(15):8206–10. doi: 10.1073/pnas.97.15.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera N, et al. Video imaging of walking myosin V by high-speed atomic force microscopy. Nature. 2010;468(7320):72–6. doi: 10.1038/nature09450. [DOI] [PubMed] [Google Scholar]
- Lakowicz JR (2006) Principles of Fluorescence Spectroscopy. Springer
- Levine RJ, et al. Effects of phosphorylation by myosin light chain kinase on the structure of Limulus thick filaments. J Cell Biol. 1991;113(3):563–72. doi: 10.1083/jcb.113.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, et al. Myosin regulatory light chain phosphorylation and the production of functionally significant changes in myosin head arrangement on striated muscle thick filaments. Biophys J. 1995;68(4 Suppl):224S. [PMC free article] [PubMed] [Google Scholar]
- Lu H, et al. Diffusive movement of processive kinesin-1 on microtubules. Traffic. 2009;10(10):1429–38. doi: 10.1111/j.1600-0854.2009.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, et al. Simultaneous observation of tail and head movements of myosin V during processive motion. J Biol Chem. 2010;285(53):42068–74. doi: 10.1074/jbc.M110.180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magde D, Elson EL, Webb WW. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Mettikolla P, et al. Kinetics of a Single Cross-Bridge in a Familial Hypertrophic Cardiomyopathy Heart Muscle Measured by Reverse Kretschmann Fluorescence. J Biomed Optics. 2010;15(1):017011. doi: 10.1117/1.3324871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettikolla P, et al. Cross-bridge Kinetics in Myofibrils Containing Familial Hypertrophic Cardiomyopathy R58Q Mutation in the Regulatory Light Chain of Myosin. J Theor Biol. 2011;284:71–81. doi: 10.1016/j.jtbi.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde K, et al. Evidence for pre-and post-power stroke of cross-bridges of contracting skeletal myofibrils. Biophys J. 2011;100(4):1024–1033. doi: 10.1016/j.bpj.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde K, et al. Myosin Cross-Bridges Do Not Form Precise Rigor Bonds in Hypertrophic Heart Muscle Carrying Troponin T Mutations. J Mol Cell Cardiol. 2011;51(3):409–18. doi: 10.1016/j.yjmcc.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoda H, et al. Electron microscopic evidence for the myosin head lever arm mechanism in hydrated myosin filaments using the gas environmental chamber. Biochem Biophys Res Commun. 2011;405(4):651–6. doi: 10.1016/j.bbrc.2011.01.087. [DOI] [PubMed] [Google Scholar]
- Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem. 2001;276(14):10577–80. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- Mogensen J, et al. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest. 2003;111(2):209–16. doi: 10.1172/JCI16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales MF. A brief and subjective history of contractility. Protein Sci. 1995;4:130–2. doi: 10.1002/pro.5560040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu P, et al. Cross-bridge duty cycle in isometric contraction of skeletal myofibrils. Biochemistry. 2008;47:5657–5667. doi: 10.1021/bi7023223. [DOI] [PubMed] [Google Scholar]
- Naber N, Cooke R, Pate E (2011) EPR Spectroscopy Shows Oriented Myosin Heads in Relaxed Muscle Fibers. Biophysical Soc 55th Annual Meeting:26
- Oplatka A, Tirosh R. Active streaming in actomyosin solutions. Biochim Biophys Acta. 1973;305(3):684–8. doi: 10.1016/0005-2728(73)90093-5. [DOI] [PubMed] [Google Scholar]
- Oplatka A, et al. Demonstration of mechanochemical coupling in systems containing actin, atp and non-aggregating active myosin derivatives. J Mechanochem Cell Motil. 1974;2(4):295–306. [PubMed] [Google Scholar]
- Qian H, Saffarian S, Elson EL. Concentration fluctuations in a mesoscopic oscillating chemical reaction system. Proc Natl Acad Sci USA. 2002;99(16):10376–81. doi: 10.1073/pnas.152007599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan ME, Forkey JN, Goldman YE. Orientation of the myosin light chain region by single molecule total internal reflection fluorescence polarization microscopy. Biophys J. 2005;89(2):1132–42. doi: 10.1529/biophysj.104.053496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberger JG, et al. Myosin VI undergoes a 180 degrees power stroke implying an uncoupling of the front lever arm. Proc Natl Acad Sci USA. 2009;106(43):18255–60. doi: 10.1073/pnas.0900005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabido-David C, et al. Steady-state fluorescence polarization studies of the orientation of myosin regulatory light chains in single skeletal muscle fibers using pure isomers of iodoacetamidotetramethylrhodamine. Biophys J. 1998;74(6):3083–3092. doi: 10.1016/S0006-3495(98)78015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep J, Lewalle A, Smith D. Reconciling the working strokes of a single head of skeletal muscle myosin estimated from laser-trap experiments and crystal structures. Proc Natl Acad Sci USA. 2006;103(5):1278–82. doi: 10.1073/pnas.0506272103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JA. Molecular motors: a surprising twist in myosin VI translocation. Curr Biol. 2008;18(2):R68–70. doi: 10.1016/j.cub.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Stewart MA, et al. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA. 2010;107(1):430–5. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. Myosin VI walks "wiggly" on actin with large and variable tilting. Mol Cell. 2007;28(6):954–64. doi: 10.1016/j.molcel.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. Single-molecule stepping and structural dynamics of myosin X. Nat Struct Mol Biol. 2010;17(4):485–91. doi: 10.1038/nsmb.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Gyorgyi A. The mechanism of muscle contraction. Proc Natl Acad Sci USA. 1974;71(9):3343–4. doi: 10.1073/pnas.71.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980;32:891–905. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q et al (2011) Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. J Mol Cell Cardiol [DOI] [PubMed]
- Tirosh R, Oplatka A, Chet I. Motility in a "cell sap" of the slime mold physarum polycephalum. FEBS Lett. 1973;34(1):40–2. doi: 10.1016/0014-5793(73)80698-2. [DOI] [PubMed] [Google Scholar]
- Tregear RT, Mendelson RA. Polarization from a helix of fluorophores and its relation to that obtained from muscle. Biophys J. 1975;15:455–467. doi: 10.1016/S0006-3495(75)85830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaturyan AK, et al. Structural changes in the actin-myosin cross-bridges associated with force generation induced by temperature jump in permeabilized frog muscle fibers. Biophys J. 1999;77(1):354–72. doi: 10.1016/S0006-3495(99)76895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NT, et al. STED nanoscopy of actin dynamics in synapses deep inside living brain slices. Biophys J. 2011;101(5):1277–84. doi: 10.1016/j.bpj.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw DM, et al. Myosin conformational states determined by single fluorophore polarization. Proc Natl Acad Sci USA. 1998;95(14):8034–8039. doi: 10.1073/pnas.95.14.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, et al. Functional effects of a restrictive-cardiomyopathy-linked cardiac troponin I mutation (R145W) in transgenic mice. J Mol Biol. 2009;392(5):1158–67. doi: 10.1016/j.jmb.2009.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig KI, et al. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440(7086):935–9. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- Wu S et al (2010) Electron tomography of cryofixed, isometrically contracting insect flight muscle reveals novel actin-myosin interactions. PLoS One 5(9) [DOI] [PMC free article] [PubMed]
- Yamada T. 1H-NMR studies of the intracellular water of skeletal muscle fibers under various physiological conditions. Cell Mol Biol (Noisy-le-Grand) 2001;47(5):925–33. [PubMed] [Google Scholar]
- Yildiz A, et al. Kinesin walks hand-over-hand. Science. 2004;303(5658):676–8. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- Yildiz A, et al. Myosin VI steps via a hand-over-hand mechanism with its lever arm undergoing fluctuations when attached to actin. J Biol Chem. 2004;279(36):37223–6. doi: 10.1074/jbc.C400252200. [DOI] [PubMed] [Google Scholar]