Abstract
Striated muscle cells are characterised by a para-crystalline arrangement of their contractile proteins actin and myosin in sarcomeres, the basic unit of the myofibrils. A multitude of proteins is required to build and maintain the structure of this regular arrangement as well as to ensure regulation of contraction and to respond to alterations in demand. This review focuses on the actin filaments (also called thin filaments) of the sarcomere and will discuss how they are assembled during myofibrillogenesis and in hypertrophy and how their integrity is maintained in the working myocardium.
Keywords: Actin, Sarcomere, Myofibrillogenesis, Cardiomyopathy, Formin
Introduction
Cellular movement is brought about by the interaction of the motor protein myosin with actin filaments. In cross-striated heart and skeletal muscle (Fig. 1b), these two proteins are arranged in a para-crystalline fashion to myofibrils, ensuring maximal force output. The basic unit of a myofibril is called a sarcomere and is defined as the region between two neighbouring Z-discs. The Z-discs, which are structural and signalling hubs composed of a multitude of proteins (Frank et al. 2006), anchor the actin (thin) filaments. Muscle myosin is assembled as bipolar (thick) filaments that project laterally from the middle region of the sarcomere, along the region called the A-band. The thick filaments are integrated with the third filament system, which is composed of titin, in a structure in the centre of the sarcomere, the M-band (Lange et al. 2005; for schematic drawing of a sarcomere see Fig. 1c). The aim of this review is to focus on actin filaments in striated muscle cells.
Fig. 1.
Actin in vitro and in cyto. a The dynamics of actin filament assembly and disassembly in vitro are regulated by the kind of nucleotide that is bound and actin’s own nuclease activity. Therefore, a fast-growing end (plus end, barbed end) can be defined, where ATP-actin monomers associate and a slow growing end (minus end, pointed end) from which ADP-actin monomers dissociate. Barbed and pointed end refer to the decoration of actin filaments with S1 fragments of myosin (i.e. the myosin heads) in electron micrographs and the resulting arrow-like structures. b Cross-striated muscle cells share the organization of their contractile elements to para-crystalline myofibrils (represented by striations, which amount to 1 µm), but show different organisation at tissue level. Skeletal muscle is composed of multinucleated myofibres that can reach a length of a few millimetres/centimetres depending on the muscle size (only a central part of a skeletal myocyte is shown; indicated by the jagged ends), while cardiac tissue is composed of mono- or binucleated cells with a length of only a few hundred micrometres. c Schematic drawing of part of a myocyte (“hybrid” with characteristics of cardiac and skeletal muscle): the majority of the actin filaments (shown in red) are arranged to thin filaments in the sarcomeres, the basic contractile unit of the myofibril, where they interact with the myosin heads (purple) from the bipolar thick filaments (only one myosin head shown for reasons of simplicity). Sarcomeres are delineated by the Z-discs (alpha-actinin in turquoise). On the left the myofibrils are anchored in the adherens junctions (pink rectangles) at the intercalated disc (assuming the cardiomyocyte scenario), potentially mediated by actin filaments composed of cytoskeletal actins; the last Z-disc before the intercalated disc lacks proteins such as telethonin and is termed the transitional junction (Bennett et al. 2006). In addition, cytoskeletal actins (beta- and gamma-actin) appear to be involved in membrane anchorage around the costameres (green rectangles), together with dystrophin and the dystrophin associated protein complex (dark blue rectangle) and with different parts of the sarcoplasmic reticulum (light blue); T tubules in vertebrate heart muscle are situated above the Z-disc, in skeletal muscle they lie above the A/I-junction. Sarcomeric actin filaments: CapZ in green, nebulin in orange (stretching throughout the thin filament like in skeletal muscle sarcomeres), tropomyosin in grey, the troponin complex in yellow, tropomodulin in blue. A-band: titin in brown, myosin in purple, MyBP-C in turquoise. M-band: myomesin in green, M-protein in brown
Actin – a portrait
Actin is a ∼42-kDa protein that is ubiquitously expressed and highly conserved throughout eukaryotic evolution. In humans, it exists as six isoforms, which derive from the same ancestral gene and include alpha-skeletal muscle actin (gene name ACTA1), alpha-cardiac muscle actin (ACTC1), alpha-smooth muscle actin (ACTA2), gamma-smooth muscle actin (ACTG2), beta-cytoplasmic actin (ACTB), and gamma-cytoplasmic actin (ACTG1). Actin isoforms exhibit tissue and cell-type specific expression as well as being developmentally regulated (Tondeleir et al. 2009). Monomeric, or globular (G) actin is polymerised into helical, or filamentous (F) actin that resembles two entwined pearl-strings (Dominguez and Holmes 2011; Hanson and Lowy 1963). The growing end of an actin filament is known as the barbed (+) end and is characterised by ATP-bound actin, whereas ADP-containing monomers are lost at the pointed (−) end. This process is called treadmilling and is observed under specific conditions of actin monomer concentration in vitro (Fig. 1a). In the topology of the sarcomere, barbed end refers to the end of the actin filament at the Z-disc and pointed to the end stretching towards the M-band (Fig. 1c). However, the basic biochemistry of actin only allows a very simplistic view of its dynamics in cells, since there is an abundance of actin binding proteins present that exert a variety of effects on G- and F-actin (Dominguez and Holmes 2011; Pollard and Cooper 2009). In vitro experiments show that nucleation is the rate-limiting step of actin polymerisation (Chesarone and Goode 2009). To aid this process, there are a number of mechanisms that regulate actin dynamics at the level of filament nucleation, elongation and stabilisation. Together with accessory proteins, they determine which actin structures are formed and how stable they are (Chhabra and Higgs 2007). To simplify, basically two major ways to form an actin filament exist: (1) branched as seen in the lamellipodium of motile cells or (2) linear. Examples for linear actin filaments can be found in filopodia, microvilli, stress fibres and, last but not least, the sarcomere of muscle cells, but differ in the extent of cross-linking and whether they are linked in a parallel or anti-parallel fashion (Chhabra and Higgs 2007). While the Arp2/3 (actin related protein) complex plays a crucial role in the assembly of branched actin filaments, different members of the formin family have been shown to regulate the assembly of linear actin filaments, for example in filopodia.
Inspirations from cell migration for muscle research
While the regulation of actin filament formation in migrating fibroblasts and cancer cells has been studied intensively for decades, it is still unclear how this process is governed in developing muscle cells (Sparrow and Schöck 2009). Studies of the organisation of different thin filament proteins during myofibrillogenesis in the developing heart have shown that assembly starts in close proximity to the plasma membrane (Ehler et al. 2004) and therefore a role of integrins has been suggested (Sparrow and Schöck 2009). However, at the moment it is not known which actin nucleating proteins and which filament elongating proteins would be involved in the synthesis of those first membrane-associated actin filaments. It appears, at least in developing cardiomyocytes, that these filaments are already composed of muscle actin isoforms and that muscle troponin and tropomyosin are also there from early on (Ehler et al. 2004). There are tantalising data from non-mammalian systems that suggest that the assembly of thin filaments is a co-ordinated process that also involves interactions with the thick filaments. For example, knockdown of all troponin T isoforms in zebrafish leads to a failure to assemble sarcomeres, which can be rescued by inhibition of myosin, suggesting that lack of regulation of actin–myosin interaction is destructive (Ferrante et al. 2011). In C. elegans, a synergistic action of tropomodulin, ADF (actin depolymerising factor)/cofilin, AIP (actin interacting protein) and profilin was shown to be required for the assembly of muscle actin (Yamashiro et al. 2008). A close association of tropomodulin with the earliest actin filaments at the plasma membrane was also seen in mammalian embryonic hearts (Ehler et al. 2004). Knockout of tropomodulin in the mouse heart leads to a failure of the sarcomeres to mature properly, and to lethality at E10.5, a phenotype that can be rescued by expressing tropomodulin specifically in cardiomyocytes (Fritz-Six et al. 2003; McKeown et al. 2008; Ono et al. 2005).
While the regulation of initial myofibrillogenesis is still shrouded in mystery, recent studies have highlighted the role of proteins that are well known from cell motility in the assembly and maintenance of thin filaments in fully differentiated myocytes. For example, N-WASP (neural Wiskott Aldrich syndrome protein) is a prominent factor promoting actin nucleation (Padrick and Rosen 2010), and was shown to be involved in the synthesis of additional actin filaments downstream of IGF 1-signalling in skeletal muscle cells, i.e. in hypertrophic muscle growth (Takano et al. 2010). N-WASP is usually present in an autoinhibited form in cells and the binding of e.g. cdc42 is required to open up the molecule and to expose the domains that activate the Arp2/3 complex (Padrick and Rosen 2010). However, alternative mechanisms exist that increase N-WASP activity by leading to its oligomerisation via the interaction of an SH3 (Src homology 3)-domain with the proline-rich domain in N-WASP (Padrick and Rosen 2010). Interestingly, in muscle, a huge SH3-domain-containing protein seems to be the cause of N-WASP activation, namely nebulin. Nebulin is a giant (600–900 kDa) protein that extends along most of the length of the thin filament in skeletal muscle, and its SH3-domain is located at its C-terminus, which sits in the Z-disc region (Labeit et al. 2011; Pappas et al. 2011). The same SH3-domain has also been reported to bind to CapZ, which is currently considered the major barbed end capping protein in muscle (Witt et al. 2006; Schafer et al. 1995), so an interesting switch between actin capping and nucleation, depending on the interaction partner of nebulin’s SH3-domain, could be envisaged (Gautel and Ehler 2010). While it was shown that Arp2/3 does not seem to be involved in the actin filament polymerisation in skeletal myocytes (Takano et al. 2010), it is unclear at present whether an additional elongation factor has to be recruited for this process. The localisation and way of action of N-WASP also suggests that it causes actin subunit elongation from free barbed ends, whereas the few studies that have been able to resolve actin incorporation into sarcomeres so far tended to see actin elongation from free pointed ends in vertebrate myocytes as well as in indirect flight muscle in Drosophila (Littlefield et al. 2001; Mardahl-Dumesnil and Fowler 2001). Whether this phenomenon is specific for hypertrophic growth downstream of IGF-1 signalling or whether N-WASP activity can also be observed, for example in initial myofibrillogenesis during embryonic development, remains to be shown.
The importance of N-WASP was also clearly demonstrated for skeletal muscle in vivo (Takano et al. 2010), yet whether the same mechanism applies to cardiac muscle is not known. Cardiomyocytes express a relative of nebulin, nebulette (Moncman and Wang 1995), which is a much shorter protein (104 kDa), but would still fulfill the major requirement, i.e. the presence of an SH3-domain in the Z-disc region.
Our laboratory was able to demonstrate the role of a formin in the sarcomere: FHOD3, which is a member of the diaphanous-related formin family, is a formin with high expression levels in heart (Katoh and Katoh 2004). Formins are multidomain proteins found in all eukaryotes (Kovar 2006) and are typically over 1,000 amino acids in length (Higgs 2005). Their defining characteristics are the presence of formin homology (FH) domains, FH1 and FH2, with the FH1 domain binding to profilin and potentially recruiting G-actin, while the FH2 domains make up the business end as far as actin polymerisation is concerned. Formins tend to act as dimers through dimerisation via their FH2 domains and usually elongate filaments by remaining associated with them, allowing rapid addition of actin monomers (Goode and Eck 2007). We managed to clone a novel, striated muscle-specific isoform of the formin FHOD3, which is characterised by the possession of eight additional amino acids that make up a CK2 (casein kinase) phosphorylation site (Iskratsch et al. 2010). Phosphorylation by CK2, which is a ubiquitously expressed kinase, could be shown in vitro and in vivo and governs the subcellular localisation of muscle FHOD3 as well as the increased half-life of this isoform (Iskratsch et al. 2010). FHOD3 clearly plays a role in the maintenance of myofibrils in cultured cardiomyocytes, since its knockdown in culture leads to their fragmentation (Iskratsch et al. 2010; Taniguchi et al. 2009). In addition, we were able to demonstrate that FHOD3 also enhances the recovery of actin filaments in cardiomyocytes following latrunculin B treatment and that it does this in a much more efficient way than one of the classical formins, mDia1 (Iskratsch et al. 2010). Since the expression of FHOD3 is also downregulated in human heart failure, we proposed a crucial general role for this protein in the maintenance of myofibrils in a healthy heart. Interestingly, the subcellular localisation of FHOD3 depends on the maturity/developmental stage of the myofibrils. In freshly isolated rod-shaped adult cardiomyocytes and in heart tissue sections, FHOD3 is restricted to the Z-disc region, while in neonatal rat cardiomyocytes that adapt to cell culture conditions, both the endogenous as well as transfected, epitope-tagged, full-length FHOD3 distribute in a much broader pattern (Iskratsch and Ehler 2011; Iskratsch et al. 2010). Incidentally, a similar transition in localisation was also seen in the work on N-WASP (Takano et al. 2010). We propose that the Z-disc localisation may reflect a reserve state or even indicate a role in actin filament capping for FHOD3, while the broader localisation happens in situations of active sarcomere remodelling. As for N-WASP, no information is available at present whether FHOD3 also plays a role in the first stages of myofibrillogenesis.
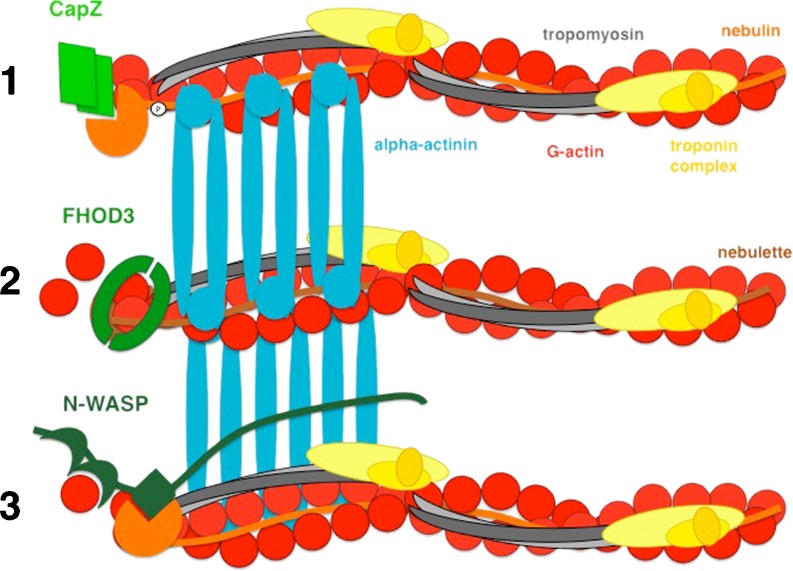
Based on all these observations, it is clear that it is unlikely that there is a “one fits all” regulator of actin filament synthesis at the barbed end (i.e. the Z-disc), but that there may be a co-existence of several factors downstream of different signaling pathways that help to elicit the crucial response to changes in demand (Fig. 2). CapZ appears to be the major capping protein of barbed ends in muscle (Schafer et al. 1995); however, at present, the data also point at least towards a transient role for FHOD3 in healthy muscle in this process. In skeletal muscle, there may be a switch between either CapZ capping or N-WASP activity, depending on the phosphorylation status of nebulin (Witt et al. 2006; Takano et al. 2010). However, since conflicting data exist on the exact CapZ interaction site on nebulin due to the identification of an alternative binding site outside of the SH3-domain further towards the N-terminus (Pappas et al. 2008), the exact molecular arrangement remains to be determined.
Fig. 2.
Three different models for the regulation of actin filament integrity and assembly at the barbed end at the Z-disc in myocytes. At resting state, actin filaments are capped by CapZ via its interaction with nebulin’s C-terminal SH3 domain (scenario 1) (Schafer et al. 1995; Witt et al. 2006), or potentially by FHOD3 (scenario 2) (Iskratsch and Ehler 2011). Upon activation e.g. while adapting to cell culture conditions or following actin filament depolymerisation, FHOD3 can switch to a mode that supports actin filament formation (Iskratsch et al. 2010). In skeletal muscle, nebulin’s SH3 domain can serve to activate N-WASP, which in turn stimulates actin filament formation via a hithertho not defined actin elongation factor (scenario 3) (Takano et al. 2010). Actin is represented in red, alpha-actinin in turquoise, CapZ in green, nebulin in orange, nebulette in orange, tropomyosin in grey and the troponin complex in yellow, CapZ, FHOD3 and N-WASP are represented in different shades of green. This is an extremely minimalistic view of the Z-disc, since the plethora of proteins that localises to that part of the sarcomere has been left out for reasons of simplification. For recent excellent reviews on the Z-disc, see Faulkner et al. (2001) and Frank et al. (2006); for a recent 3D model of the Z-disc, see Waardenberg et al. (2011)
How is thin filament length regulated?
Electron micrographs in textbooks give the very misleading impression that sarcomeres are static structures; however, this is far from the truth. Muscle has to continuously adapt to changes in demand, for example by changes in the expression of the contractile proteins (e.g. an upregulation of alpha-skeletal actin in cardiomyopathy; Suurmeijer et al. 2003) and additional synthesis in a hypertrophic response. In addition, there is a considerable amount of turnover occuring in sarcomeres. It was shown 30 years ago by isotope labelling of leucine that different components of the thin filaments (actin, tropomyosin, troponin C, T and I) are characterised by different half-lives in rat heart muscle, but that these are in the range of 3–10 days rather than months (Martin 1981). Indirect evidence for a high turnover rate was also obtained by the relative rapid incorporation of injected fluorescently labelled actins (LoRusso et al. 1992), or the distribution of epitope-tagged tropomyosin and troponin in rod-shaped adult rat cardiomyocytes following adenoviral expression (Michele et al. 1999). Since in these experiments it could also be shown that the overall stoichiometry of the thin filament composition remained unchanged despite the overexpression of tagged versions, this suggests that there is indeed incorporation rather than just decoration/non specific association. How exactly this impressive turnover would be managed is far from clear. It would appear that treadmilling does not occur in myofibrils (Littlefield and Fowler 2008), and at least for chick cardiomyocytes, it has been shown that fluorescent actin subunit change at the pointed end is 1.6 times greater than at the barbed end (Littlefield et al. 2001). Recent results have identified a second, more dynamic actin population in cultured neonatal cardiomyocytes (Skwarek-Maruszewska et al. 2009) and correlated the dynamics to differences in contractile activity. It remains to be shown whether a similar subpopulation is also existent in cardiomyocytes in situ or in isolated adult cardiomyocytes.
For decades, nebulin was assumed to fulfill a role as thin filament length regulator, since there is a nice correlation between its range of molecular weight (600–900 kDa) and the actual thin filament length in different muscles. However, several lines of evidence have made it rather unlikely that nebulin is directly involved in this process. Careful high resolution studies in a variety of rabbit skeletal muscles have shown that nebulin stops just short of the end of the actin filament since the N-terminus of nebulin and the pointed end capping protein tropomodulin are not colocalised (Castillo et al. 2009). Evidence from nebulin knockout mice has shown that nebulin is not necessary for initial myofibrillogenesis in skeletal muscle, but indispensable for myofibril maintenance (Bang et al. 2006; Witt et al. 2006). All taken together, it seems that nebulin’s major role is that as a stabiliser of the thin filament core (Littlefield and Fowler 2008) and therefore also for aiding myosin cross-bridge kinetics in skeletal muscle (Ottenheijm et al. 2009; Bang et al. 2009; Ochala et al. 2011). The stabilising hypothesis is nicely supported by the data from experiments with a synthetic mini-nebulin construct, which contains the N-and C-terminus of nebulin in addition to just four super-repeats. Mini-nebulin is sufficient to protect actin filaments from depolymerisation and interestingly can extend its stabilising effect on actin filaments even beyond the stretch that it actually covers (Pappas et al. 2010).
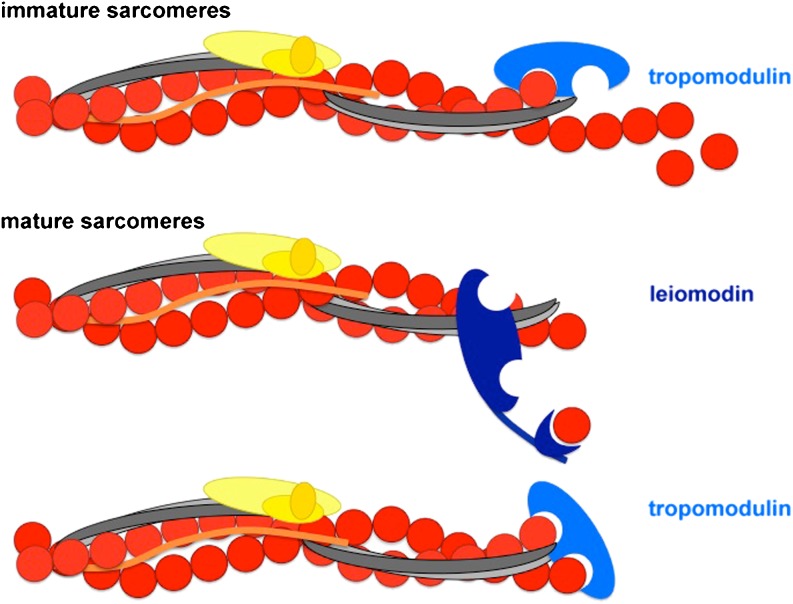
Leiomodin and tropomodulin are members of a family of actin pointed end capping proteins and seem to be crucial for different aspects of thin filament length regulation. While the role of tropomodulin in regulating thin filament length in cardiomyocytes has been established for a long time (Gregorio et al. 1995), and was also demonstrated by the severe cardiac phenotypes of tropomodulin gain of function and loss of function mice (Fritz-Six et al. 2003; Sussman et al. 1998), leiomodin was only described a few years ago as a protein that is required to maintain myofibrils (Chereau et al. 2008). Tropomodulin possesses two and leiomodin has three actin binding domains (ABD), with the third ABD in the case of leiomodin being of the WH2 type. Furthermore, tropomodulin and leiomodin share the ability to interact with tropomyosin. Recently, it has been shown that, despite their similarity as far as domain structure and interaction partners are concerned, tropomodulin and leiomodin display distinct characteristics in the developmental stage of expression as well as in their effects on F-actin (Skwarek-Maruszewska et al. 2010; Tsukada et al. 2010). While tropomodulin is expressed at the start of myofibrillogenesis and striations can be found as soon as the sarcomeres are established (Fritz-Six et al. 2003; Chu et al. 2003; Ehler et al. 2004), leiomodin expression can only be detected at later stages of heart development in the embryo (Tsukada et al. 2010). In cultured cardiomyocytes, the presence of leiomodin seems to be a marker of sarcomere maturity (Skwarek-Maruszewska et al. 2010). Co-localisation studies also seem to indicate that the two proteins associate with different populations of actin filaments, since, despite being in the same region of the sarcomere, the signals do not overlap (Skwarek-Maruszewska et al. 2010; Tsukada et al. 2010). It appears that the two play slightly different roles in the mature sarcomere, tropomodulin being the bona fide pointed end capper, while leiomodin, potentially due to its additional actin binding domain, can help to regulate thin filament length by addition of actin monomers, if required (Tsukada et al. 2010; Fig. 3). The existence of several different isoforms of tropomodulin, which play either a muscle cell type or a muscle cell compartment-specific role, makes the situation even more complex (Gokhin et al. 2010; Gokhin and Fowler 2011).
Fig. 3.
Regulation of actin filament integrity and assembly at the pointed end near the middle of the sarcomere. During myofibrillogenesis tropomodulin (light blue) could be a leaky capper, allowing actin filament synthesis; alternatively, several thin filament populations with different lengths could be capped by individual tropomodulins and account for the broader signal that is seen in immature sarcomeres (Ehler et al. 2004). No leiomodin (dark blue) is present at early stages of development (Tsukada et al. 2010) or at early stages of adaptation to cell culture conditions (Skwarek-Maruszewska et al. 2010). In mature sarcomeres, there may be a competition between tropomodulin and leiomodin, depending on the contractile demand on the cell, with tropomodulin acting primarily as capper, while leiomodin can stimulate thin filament extension. Nebulin molecules (orange) are involved in the stabilisation of the core of the actin filament, but end way short of the pointed end in skeletal muscle (which is the situation assumed here); nebulette molecules would only extend a little into the thin filament from the Z-disc. Actin is shown in red, the troponin complex in yellow and tropomyosin in grey
With the demise of the “nebulin acts as a ruler” hypothesis, it all seems to come back to a contractile activity driven length regulation of thin filaments, as proposed by Littlefield and Fowler (1998) more than a decade ago. This fits nicely with the correlation between thin filament length and sarcomere length (Castillo et al. 2009). It has been observed that thin filaments composed of the—ideally suited—alpha-cardiac actin isoform attain a defined length in the developing heart, while there is still a population of thin filaments composed of other actin isoforms that extends beyond (Ehler et al. 2004), which may be related to the more dynamic population seen in cultured cardiomyocytes (Skwarek-Maruszewska et al. 2009). Lack of efficient muscle contraction due to problems in A-band assembly also leads to a failure of the thin filaments to mature properly in dicky ticker mutants in Xenopus tropicalis (Geach and Zimmerman 2010), again supporting the importance of contractile work for thin filament length regulation.
Beyond muscle actins
Muscle actin isoforms are the predominant actin isoforms expressed in cardiac and skeletal muscle (Tondeleir et al. 2009); however, cytoplasmic actins, namely beta- and gamma-cytoplasmic actins, seem to play a minor but far from negligible role in certain subcellular domains in muscle cells (Kee et al. 2009). Recent data from knockout animals for beta- and gamma-cytoplasmic actin from the Ervasti laboratory have ruled out their role in myofibrillogenesis, but established their importance for at least skeletal muscle function (Prins et al. 2011; Sonnemann et al. 2006). Currently, there exist slightly divergent reports on the localisation and expression levels of cytoplasmic actin isoforms in muscle (Kee et al. 2009). Due to their extreme conservation, it has been a real challenge to generate antibodies that would be truly isoform specific, and there also seems to be a specimen fixation–protocol-dependent visibility of the actin N-termini, which are the sole regions of diversity between isoforms (Dugina et al. 2009). The knockout animals will be extremely helpful and the newly generated monoclonal antibodies (Dugina et al. 2009) will be invaluable to pinpoint the subcellular localisation of cytoplasmic actins in muscle. Already published data for the skeletal muscle specific knockouts for the cytoplasmic actins indicate that a lack of gamma-cytoplasmic actin, while resulting in overt muscle weakness with contractures, seems to have no dramatic effect on the organisation of the membrane cytoskeleton, which casts doubt on gamma-cytoplasmic actin’s proposed role in the costameres, the lateral membrane attachment sites of myofibrils (Sonnemann et al. 2006). Unfortunately, only the dystrophin glycoprotein complex was analysed in these studies and there no data are shown on, e.g., potential effects on vinculin localisation, a marker protein for costameres (Pardo et al. 1983). On the other hand, the lack of beta-cytoplasmic actin in skeletal muscle does have an effect on dystrophin stability, but also leaves the overall costamere structure relatively unperturbed (Prins et al. 2011). Several cytoplasmic actins, and also their associated proteins such as cytoskeletal tropomyosins and tropomodulin isoforms, have been localised to a compartment termed Z-LAC (Z-line adjacent cytoskeleton) in skeletal muscle (Kee et al. 2009). This cytoskeletal compartment may be involved in the organisation of the sarcoplasmic reticulum (SR). Gamma-cytoplasmic actin and tropomodulin 3 have been shown to contribute to the organisation of a second SR domain in the region of the M-band (Gokhin and Fowler 2011). Cardiac muscle seems to contain no gamma-cytoplasmic actin and very little beta-cytoplasmic actin (Tondeleir et al. 2009), despite the latter being often used as a loading control for heart tissue. Mice transgenic for a beta-cytoplasmic actin promoter driving eGFP expression show only very low levels of fluorescence in the heart (Okabe et al. 1997). The little beta-cytoplasmic actin there is in cardiomyocytes may be residing in the terminal actin filaments that join up the transitional junction to the intercalated disc (Bennett et al. 2006). However, this needs to be confirmed by the novel tools available to the field, i.e. truly isoform specific antibodies together with the tissue-specific knockout mice. In conclusion, cytoplasmic actins appear to be irrelevant during myofibril formation and maintenance, but seem to play an important role in the organisation of membranous subcompartments in muscle cells, at least in skeletal muscle.
Thin filament mutations in disease
Two major types of muscle diseases have been associated with mutations in thin filament proteins, nemaline myopathy (Clarkson et al. 2004) and cardiomyopathy (both hypertrophic and dilated; (Tardiff 2011). While the effects of specific point mutations on the functional characteristics of the proteins are beginning to be elucidated (e.g. Dyer et al. 2009; Song et al. 2011), and can in some cases be correlated to a certain type of disease (Chang et al. 2005; Debold et al. 2010; Lombardi et al. 2008), the complexity of thin filament assembly and regulation eluded to in this review would indicate that we are just at the beginning of understanding its mechanisms.
Acknowledgements
Work in the laboratory of Dr Elisabeth Ehler was supported by a Medical Research Council Career Establishment Grant and by the British Heart Foundation via a pump priming fellowship to Dr Iskratsch and currently a PhD fellowship to Mr Dwyer.
Conflict of interest
None
Abbreviations
- ABD
Actin binding domain
- ADF
Actin depolymerising factor
- AIP
Actin interacting protein
- Arp
Actin related proteins
- CK
Casein kinase
- FH
Formin homology
- N-WASP
Neural Wiskott Aldrich syndrome protein
- SH3
Src homology 3
- SR
Sarcoplasmic reticulum
- Z-LAC
Z-line adjacent cytoskeleton
References
- Bang ML, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol. 2006;173:905–16. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang ML, Caremani M, Brunello E, Littlefield R, Lieber RL, Chen J, Lombardi V, Linari M. Nebulin plays a direct role in promoting strong actin-myosin interactions. FASEB J. 2009;23:4117–25. doi: 10.1096/fj.09-137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett PM, Maggs AM, Baines AJ, Pinder JC. The transitional junction: a new functional subcellular domain at the intercalated disc. Mol Biol Cell. 2006;17:2091–100. doi: 10.1091/mbc.E05-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo A, Nowak R, Littlefield KP, Fowler VM, Littlefield RS. A nebulin ruler does not dictate thin filament lengths. Biophys J. 2009;96:1856–65. doi: 10.1016/j.bpj.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AN, Harada K, Ackerman MJ, Potter JD. Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in alpha-tropomyosin. J Biol Chem. 2005;280:34343–9. doi: 10.1074/jbc.M505014200. [DOI] [PubMed] [Google Scholar]
- Chereau D, Boczkowska M, Skwarek-Maruszewska A, Fujiwara I, Hayes DB, Rebowski G, Lappalainen P, Pollard TD, Dominguez R. Leiomodin is an actin filament nucleator in muscle cells. Science. 2008;320:239–43. doi: 10.1126/science.1155313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone MA, Goode BL. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol. 2009;21:28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–21. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- Chu X, Chen J, Reedy MC, Vera C, Sung KL, Sung LA. E-Tmod capping of actin filaments at the slow-growing end is required to establish mouse embryonic circulation. Am J Physiol Heart Circ Physiol. 2003;284:H1827–38. doi: 10.1152/ajpheart.00947.2002. [DOI] [PubMed] [Google Scholar]
- Clarkson E, Costa CF, Machesky LM. Congenital myopathies: diseases of the actin cytoskeleton. J Pathol. 2004;204:407–17. doi: 10.1002/path.1648. [DOI] [PubMed] [Google Scholar]
- Debold EP, Saber W, Cheema Y, Bookwalter CS, Trybus KM, Warshaw DM, Vanburen P. Human actin mutations associated with hypertrophic and dilated cardiomyopathies demonstrate distinct thin filament regulatory properties in vitro. J Mol Cell Cardiol. 2010;48:286–92. doi: 10.1016/j.yjmcc.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–86. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugina V, Zwaenepoel I, Gabbiani G, Clement S, Chaponnier C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci. 2009;122:2980–8. doi: 10.1242/jcs.041970. [DOI] [PubMed] [Google Scholar]
- Dyer EC, Jacques AM, Hoskins AC, Ward DG, Gallon CE, Messer AE, Kaski JP, Burch M, Kentish JC, Marston SB. Functional analysis of a unique troponin c mutation, GLY159ASP, that causes familial dilated cardiomyopathy, studied in explanted heart muscle. Circ Heart Fail. 2009;2:456–64. doi: 10.1161/CIRCHEARTFAILURE.108.818237. [DOI] [PubMed] [Google Scholar]
- Ehler E, Fowler VM, Perriard JC. Myofibrillogenesis in the developing chicken heart: role of actin isoforms and of the pointed end actin capping protein tropomodulin during thin filament assembly. Dev Dyn. 2004;229:745–55. doi: 10.1002/dvdy.10482. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Lanfranchi G, Valle G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life. 2001;51:275–82. doi: 10.1080/152165401317190761. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Kiff RM, Goulding DA, Stemple DL. Troponin T is essential for sarcomere assembly in zebrafish skeletal muscle. J Cell Sci. 2011;124:565–77. doi: 10.1242/jcs.071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med. 2006;84:446–68. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Fritz-Six KL, Cox PR, Fischer RS, Xu B, Gregorio CC, Zoghbi HY, Fowler VM. Aberrant myofibril assembly in tropomodulin1 null mice leads to aborted heart development and embryonic lethality. J Cell Biol. 2003;163:1033–44. doi: 10.1083/jcb.200308164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M, Ehler E. Cell biology. Gett'N-WASP stripes. Science. 2010;330:1491–2. doi: 10.1126/science.1199920. [DOI] [PubMed] [Google Scholar]
- Geach TJ, Zimmerman LB. Paralysis and delayed Z-disc formation in the Xenopus tropicalis unc45b mutant dicky ticker. BMC Dev Biol. 2010;10:75. doi: 10.1186/1471-213X-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Fowler VM. Cytoplasmic {gamma}-actin and tropomodulin isoforms link to the sarcoplasmic reticulum in skeletal muscle fibers. J Cell Biol. 2011;194:105–20. doi: 10.1083/jcb.201011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Lewis RA, McKeown CR, Nowak RB, Kim NE, Littlefield RS, Lieber RL, Fowler VM. Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology. J Cell Biol. 2010;189:95–109. doi: 10.1083/jcb.201001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995;377:83–6. doi: 10.1038/377083a0. [DOI] [PubMed] [Google Scholar]
- Hanson J, Lowy J. The structure of F-actin and of actin filaments isolated from muscle. J Mol Biol. 1963;6:46–60. doi: 10.1016/S0022-2836(63)80081-9. [DOI] [Google Scholar]
- Higgs HN. Formin proteins: a domain-based approach. Trends Biochem Sci. 2005;30:342–53. doi: 10.1016/j.tibs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Iskratsch T, Ehler E. Formin-g muscle cytoarchitecture. BioArchitecture. 2011;1:66–68. doi: 10.4161/bioa.1.2.15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskratsch T, Lange S, Dwyer J, Kho AL, dos Remedios C, Ehler E. Formin follows function: a muscle specific isoform of FHOD3 is regulated by CK2 phosphorylation and promotes myofibril maintenance. J Cell Biol. 2010;191:1159–1172. doi: 10.1083/jcb.201005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Identification and characterization of human FHOD3 gene in silico. Int J Mol Med. 2004;13:615–20. [PubMed] [Google Scholar]
- Kee AJ, Gunning PW, Hardeman EC. Diverse roles of the actin cytoskeleton in striated muscle. J Muscle Res Cell Motil. 2009;30:187–97. doi: 10.1007/s10974-009-9193-x. [DOI] [PubMed] [Google Scholar]
- Kovar DR. Molecular details of formin-mediated actin assembly. Curr Opin Cell Biol. 2006;18:11–7. doi: 10.1016/j.ceb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Labeit S, Ottenheijm CA, Granzier H. Nebulin, a major player in muscle health and disease. FASEB J. 2011;25:822–9. doi: 10.1096/fj.10-157412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Agarkova I, Perriard JC, Ehler E. The sarcomeric M-band during development and in disease. J Muscle Res Cell Motil. 2005;26:375–9. doi: 10.1007/s10974-005-9019-4. [DOI] [PubMed] [Google Scholar]
- Littlefield R, Fowler VM. Defining actin filament length in striated muscle: rulers and caps or dynamic stability? Annu Rev Cell Dev Biol. 1998;14:487–525. doi: 10.1146/annurev.cellbio.14.1.487. [DOI] [PubMed] [Google Scholar]
- Littlefield RS, Fowler VM. Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin Cell Dev Biol. 2008;19:511–9. doi: 10.1016/j.semcdb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol. 2001;3:544–51. doi: 10.1038/35078517. [DOI] [PubMed] [Google Scholar]
- Lombardi R, Bell A, Senthil V, Sidhu J, Noseda M, Roberts R, Marian AJ. Differential interactions of thin filament proteins in two cardiac troponin T mouse models of hypertrophic and dilated cardiomyopathies. Cardiovasc Res. 2008;79:109–17. doi: 10.1093/cvr/cvn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoRusso SM, Imanaka YK, Shuman H, Sanger JM, Sanger JW. Incorporation of fluorescently labeled contractile proteins into freshly isolated living adult cardiac myocytes. Cell Motil Cytoskeleton. 1992;21:111–122. doi: 10.1002/cm.970210204. [DOI] [PubMed] [Google Scholar]
- Mardahl-Dumesnil M, Fowler VM. Thin filaments elongate from their pointed ends during myofibril assembly in Drosophila indirect flight muscle. J Cell Biol. 2001;155:1043–53. doi: 10.1083/jcb.200108026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem. 1981;256:964–8. [PubMed] [Google Scholar]
- McKeown CR, Nowak RB, Moyer J, Sussman MA, Fowler VM. Tropomodulin1 is required in the heart but not the yolk sac for mouse embryonic development. Circ Res. 2008;103:1241–8. doi: 10.1161/CIRCRESAHA.108.178749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Albayya FP, Metzger JM. Thin filament protein dynamics in fully differentiated adult cardiac myocytes: toward a model of sarcomere maintenance. J Cell Biol. 1999;145:1483–95. doi: 10.1083/jcb.145.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncman CL, Wang K. Nebulette: A 107 kD nebulin-like protein in cardiac muscle. Cell Motil. Cytoskeleton. 1995;32:205–225. doi: 10.1002/cm.970320305. [DOI] [PubMed] [Google Scholar]
- Ochala J, Lehtokari VL, Iwamoto H, Li M, Feng HZ, Jin JP, Yagi N, Wallgren-Pettersson C, Penisson-Besnier I, Larsson L. Disrupted myosin cross-bridge cycling kinetics triggers muscle weakness in nebulin-related myopathy. FASEB J. 2011;25:1903–13. doi: 10.1096/fj.10-176727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9. doi: 10.1016/S0014-5793(97)00313-X. [DOI] [PubMed] [Google Scholar]
- Ono Y, Schwach C, Antin PB, Gregorio CC. Disruption in the tropomodulin1 (Tmod1) gene compromises cardiomyocyte development in murine embryonic stem cells by arresting myofibril maturation. Dev Biol. 2005;282:336–48. doi: 10.1016/j.ydbio.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Ottenheijm CA, Witt CC, Stienen GJ, Labeit S, Beggs AH, Granzier H. Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum Mol Genet. 2009;18:2359–69. doi: 10.1093/hmg/ddp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–35. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CT, Bhattacharya N, Cooper JA, Gregorio CC. Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol Biol Cell. 2008;19:1837–47. doi: 10.1091/mbc.E07-07-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CT, Bliss KT, Zieseniss A, Gregorio CC. The Nebulin family: an actin support group. Trends Cell Biol. 2011;21:29–37. doi: 10.1016/j.tcb.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CT, Krieg PA, Gregorio CC. Nebulin regulates actin filament lengths by a stabilization mechanism. J Cell Biol. 2010;189:859–70. doi: 10.1083/jcb.201001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, D'Angelo Siliciano J, Craig SW. Vinculin Is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol. 1983;97:1081–1088. doi: 10.1083/jcb.97.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–12. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins KW, Call JA, Lowe DA, Ervasti JM. Quadriceps myopathy caused by skeletal muscle-specific ablation of beta(cyto)-actin. J Cell Sci. 2011;124:951–7. doi: 10.1242/jcs.079848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Hug C, Cooper JA. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J Cell Biol. 1995;128:61–70. doi: 10.1083/jcb.128.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwarek-Maruszewska A, Boczkowska M, Zajac AL, Kremneva E, Svitkina T, Dominguez R, Lappalainen P. Different localizations and cellular behaviors of leiomodin and tropomodulin in mature cardiomyocyte sarcomeres. Mol Biol Cell. 2010;21:3352–61. doi: 10.1091/mbc.E10-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci. 2009;122:2119–26. doi: 10.1242/jcs.046805. [DOI] [PubMed] [Google Scholar]
- Song W, Dyer E, Stuckey DJ, Copeland O, Leung MC, Bayliss C, Messer A, Wilkinson R, Tremoleda JL, Schneider MD, Harding SE, Redwood CS, Clarke K, Nowak K, Monserrat L, Wells D, Marston SB. Molecular mechanism of the E99K mutation in cardiac actin (ACTC Gene) that causes apical hypertrophy in man and mouse. J Biol Chem. 2011;286:27582–93. doi: 10.1074/jbc.M111.252320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnemann KJ, Fitzsimons DP, Patel JR, Liu Y, Schneider MF, Moss RL, Ervasti JM. Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev Cell. 2006;11:387–97. doi: 10.1016/j.devcel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sparrow JC, Schöck F. The initial steps of myofibril assembly: integrins pave the way. Nat Rev Mol Cell Biol. 2009;10:293–8. doi: 10.1038/nrm2634. [DOI] [PubMed] [Google Scholar]
- Sussman MA, Welch S, Cambon N, Klevitsky R, Hewett TE, Price R, Witt SA, Kimball TR. Myofibril degeneration caused by tropomodulin overexpression leads to dilated cardiomyopathy in juvenile mice. J Clin Invest. 1998;101:51–61. doi: 10.1172/JCI1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurmeijer AJ, Clement S, Francesconi A, Bocchi L, Angelini A, Van Veldhuisen DJ, Spagnoli LG, Gabbiani G, Orlandi A. Alpha-actin isoform distribution in normal and failing human heart: a morphological, morphometric, and biochemical study. J Pathol. 2003;199:387–97. doi: 10.1002/path.1311. [DOI] [PubMed] [Google Scholar]
- Takano K, Watanabe-Takano H, Suetsugu S, Kurita S, Tsujita K, Kimura S, Karatsu T, Takenawa T, Endo T. Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science. 2010;330:1536–40. doi: 10.1126/science.1197767. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Takeya R, Suetsugu S, Kan OM, Narusawa M, Shiose A, Tominaga R, Sumimoto H. The mammalian formin Fhod3 regulates actin assembly and sarcomere organization in striated muscles. J Biol Chem. 2009;284:29873–29881. doi: 10.1074/jbc.M109.059303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff JC. Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res. 2011;108:765–82. doi: 10.1161/CIRCRESAHA.110.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondeleir D, Vandamme D, Vandekerckhove J, Ampe C, Lambrechts A. Actin isoform expression patterns during mammalian development and in pathology: insights from mouse models. Cell Motil Cytoskeleton. 2009;66:798–815. doi: 10.1002/cm.20350. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Pappas CT, Moroz N, Antin PB, Kostyukova AS, Gregorio CC. Leiomodin-2 is an antagonist of tropomodulin-1 at the pointed end of the thin filaments in cardiac muscle. J Cell Sci. 2010;123:3136–45. doi: 10.1242/jcs.071837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waardenberg AJ, Bernardo BC, Ng DC, Shepherd PR, Cemerlang N, Sbroggio M, Wells CA, Dalrymple BP, Brancaccio M, Lin RC, McMullen JR. Phosphoinositide 3-kinase (PI3K(p110alpha)) directly regulates key components of the Z-disc and cardiac structure. J Biol Chem. 2011;286:30837–46. doi: 10.1074/jbc.M111.271684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt CC, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 2006;25:3843–55. doi: 10.1038/sj.emboj.7601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Cox EA, Baillie DL, Hardin JD, Ono S. Sarcomeric actin organization is synergistically promoted by tropomodulin, ADF/cofilin, AIP1 and profilin in C. elegans. J Cell Sci. 2008;121:3867–77. doi: 10.1242/jcs.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]