Abstract
First coined by Alexander Sandow in 1952, the term excitation–contraction coupling (ECC) describes the rapid communication between electrical events occurring in the plasma membrane of skeletal muscle fibres and Ca2+ release from the SR, which leads to contraction. The sequence of events in twitch skeletal muscle involves: (1) initiation and propagation of an action potential along the plasma membrane, (2) spread of the potential throughout the transverse tubule system (T-tubule system), (3) dihydropyridine receptors (DHPR)-mediated detection of changes in membrane potential, (4) allosteric interaction between DHPR and sarcoplasmic reticulum (SR) ryanodine receptors (RyR), (5) release of Ca2+ from the SR and transient increase of Ca2+ concentration in the myoplasm, (6) activation of the myoplasmic Ca2+ buffering system and the contractile apparatus, followed by (7) Ca2+ disappearance from the myoplasm mediated mainly by its reuptake by the SR through the SR Ca2+ adenosine triphosphatase (SERCA), and under several conditions movement to the mitochondria and extrusion by the Na+/Ca2+ exchanger (NCX). In this text, we review the basics of ECC in skeletal muscle and the techniques used to study it. Moreover, we highlight some recent advances and point out gaps in knowledge on particular issues related to ECC such as (1) DHPR-RyR molecular interaction, (2) differences regarding fibre types, (3) its alteration during muscle fatigue, (4) the role of mitochondria and store-operated Ca2+ entry in the general ECC sequence, (5) contractile potentiators, and (6) Ca2+ sparks.
Keywords: Excitation–contraction coupling, Ca2+ transients, Skeletal muscle, Fibre types, Mitochondria
Introduction
The excitation–contraction coupling (ECC) phenomenon was defined by Alexander Sandow as the series of events occurring from the generation of the action potential (AP) in the skeletal muscle fibres to the beginning of muscle tension (Kahn and Sandow 1950; Sandow 1952). It has been more than 60 years since his early work on skeletal muscle, during which the temporal and spatial resolution of the techniques to study ECC have greatly improved, reaching a capacity for discrimination at a molecular level. Since then, a great amount of information on ECC morphological basis, physiological importance, and pharmacological modulation, initially in amphibians and more recently in mammalians, has been gathered.
Here, we review the basics of ECC, the techniques used to understand the phenomenon and the most recent advances in ECC knowledge, focused on the information gathered using fast Ca2+ dyes in mammalian preparations and on important issues still under research. These issues include the nature of the interaction among key molecules in ECC, the regulation of the ECC mechanism in different skeletal muscle fibre types, its role in phenomena such as fatigue, its drug modulation, the store-operated Ca2+ entry (SOCE) –mitochondria–ECC relationship and Ca2+ sparks.
The excitation–contraction coupling (ECC) mechanism in skeletal muscle
Ca2+ cell homeostasis and signalling result from dynamic interactions between mechanisms that provoke an increase of cytoplasmic free Ca2+ and those that reduce it. In the specific case of striated muscles, contraction and relaxation mechanisms are both regulated by rapid changes in myoplasmic free Ca2+ concentration. Even before knowing the molecular machinery involved in Ca2+ handling, it was shown that Ca2+ was the activator of the contractile mechanism (Heilbrunn and Wiercinsky 1947; Niedergerke 1955; Weber 1959), and that its concentration increase in the myoplasm elicited by electrical stimulation, precedes tension generation (Ridgway and Ashley 1967; Ebashi et al. 1969). It was also known that muscle contraction depends on Ca2+ released from the SR (Caputo and Giménez 1967; Armstrong et al. 1972) and that, after activation, most of the Ca2+ released goes back finally to the SR (Hasselbach and Makinose 1961; Hasselbach 1964; Winegrad 1968).
The ECC phenomenon represents a fast communication between electrical events occurring in the plasma membrane and Ca2+ release from the SR, which leads to muscle contraction. The sequence of events in skeletal twitch muscle fibres involves: (1) initiation and propagation of an AP along the plasma membrane, (2) radial spread of the potential along the transverse tubule system (T-tubule system), (3) dihydropyridine receptors (DHPR, L-type Ca2+ channel CaV1.1)-mediated detection of changes in membrane potential, (4) allosteric interaction of the DHPR with the sarcoplasmic reticulum (SR) ryanodine receptors (RyR), (5) release of Ca2+ from the SR and transient increase of Ca2+ concentration in the myoplasm, (6) transient activation of the myoplasmic Ca2+ buffering system and the contractile apparatus, followed by (7) disappearance of Ca2+ from the myoplasm mediated by its movement to the mitochondria, its transport by the Na+/Ca2+ exchanger (NCX) and its final reuptake by the SR through the SR Ca2+ adenosine triphosphatase (SERCA) (Sandow 1952; Caputo 1983; Fill and Copello 2002; Calderón-Vélez and Figueroa-Gordon 2009).
In twitch skeletal muscle cells, both the differential and selective conductance and the ion distribution across the membrane generate a resting potential of about –85 mV, with the interior of the cell negative compared to the exterior (Horowicz 1961; Luff and Atwood 1972). The acetylcholine neurotransmitter released into the motor plate by the inferior motor neuron acts as an initiator for the AP in muscle fibres causing the transmembrane potential change to reach values of up to 100 mV, through voltage-dependent ionic conductance changes (Horowicz 1961; Hodgkin and Huxley 1952; Luff and Atwood 1972). Since the AP is a regulator of ECC, its modifications (see below) may affect the kinetics of muscle contraction (Hodgkin and Horowicz 1960; Sandow et al. 1965). Experimentally, membrane depolarization can be achieved by replacing neurotransmitters with direct electrical stimulation or by increasing the extracellular K+ concentration (Hodgkin and Horowicz 1959). Additionally, contractile activation can be induced bypassing the membrane depolarization step, for instance with the help of caffeine (Axelsson and Thesleff 1958; Endo 1975).
The plasma membrane AP propagates longitudinally along the fibre and radially along the T-tubules, electrotonically (Adrian et al. 1969), or in a fast, homogeneous, regenerative Na+-dependent process (Huxley 1964; González-Serratos 1971; Bezanilla et al. 1972). T-tubules are invaginations of the plasma membrane that transversely extend into muscle fibres (they also have longitudinal components inside the fibre), and serve as a mean to rapidly carry electrical information from the surface to the interior of the muscle fibre, namely to the DHPR facing the SR membrane (Franzini-Armstrong and Porter 1964; Bezanilla et al. 1972; Edwards et al. 2012). The depolarization of the surface reaches the triadic region, where one T-tubule is surrounded by junctional SR segments from two different terminal cisternae (Porter and Palade 1957; Franzini-Armstrong and Porter 1964; Peachey 1965). In this region, arrays of molecules known as RyR and further identified as Ca2+ release channels face, vis à vis, but in alternate mode, the DHPR, located in the T-tubule membranes and arranged in groups of four, called tetrads (Block et al. 1988; Franzini-Armstrong and Jorgensen 1994; Franzini-Armstrong 1999). DHPR are heteropentamers formed by subunits α1, α2, β, γ, and δ, whose function is regulated by membrane potential; the S4 transmembrane domains of α1 subunit constitute the voltage sensors. The electrical manifestation of the voltage sensor operation is a non-linear intramembrane charge movement, characterized by S-shaped voltage dependence (Schneider and Chandler 1973; Ríos and Pizarro 1991). The voltage change induces a still unclear conformational change in the DHPR that gates the opening of the RyR in a cooperative manner (see below) (Schneider and Chandler 1973; Ríos and Brum 1987; Ríos and Pizarro 1991; Ríos et al. 1993; Bezanilla 2000). DHPR function and expression is also regulated by the RyR and the JP-45/CSQ complex (Ávila and Dirksen 2000; Treves et al. 2009; Mosca et al. 2013).
RyR are high molecular weight (~550 kDa per monomer) homotetramers whose shape has been classically associated to that of a mushroom: a bulky cytoplasmic domain which has regulatory sites, and a transmembrane domain which allow the protein to function as a large conductance Ca2+ channel that regulates Ca2+ outflow from the SR to the cytoplasm (see below) (Smith et al. 1988; Lai et al. 1988; Takeshima et al. 1989; Wagenknecht et al. 1989; Franzini-Armstrong and Jorgensen 1994; Franzini-Armstrong 1999; Serysheva et al. 2007). Regions for intraluminal regulation have also been described (Goonasekera et al. 2007). The electron microscopy appearance of the RyR proteins is known as "feet". RyR activity and expression are regulated by adenosine triphosphate (ATP), Mg2+, Ca2+, redox status, phosphorylation/dephosphorylation status, and several proteins including calsequestrin (CSQ), calmodulin, S100A1, FK 506 binding protein (FKBP), calumenin, triadin, junctin, and possibly by the SR protein-27 (SRP-27) and indirectly by SR protein 35 (SRP-35) (Lai et al. 1988; Meissner 1984; Coronado et al. 1994; Fill and Copello 2002; Wei et al. 2006; Jung et al. 2006; Goonasekera et al. 2007; Bleunven et al. 2008; Prosser et al. 2008; Treves et al. 2009, 2012; Wium et al. 2012).
In skeletal muscle, the RyR1 and the DHPR are in close apposition, constituting the Ca2+ release units (CRU), that are activated almost simultaneously as a response to an AP (Franzini-Armstrong and Jorgensen 1994; Franzini-Armstrong 1999). Due to the alternate disposition of RyR and DHPR arrays, for each RyR under control of a tetrad, there is one molecule that is not (Franzini-Armstrong and Jorgensen 1994). In amphibian muscle fibres, the sizeable presence in the parajunctional region of another isoform of the RyR (Felder and Franzini-Armstrong 2002) the RyR3, increases the fractional number of RyR that are not under DHPR control. The alternate arrangements of RyR and DHPR, and the presence of extrajunctional RyR3, constitute the structural basis for the proposal of a dual mechanism of Ca2+ release (O’Brien et al. 1995; Ríos and Pizarro 1988). According to this, RyR facing DHPR are under control of the membrane potential while RyR that do not face DHPR are activated by Ca2+ through a positive feedback mechanism, initially studied in frog muscles, known as Ca2+-induced Ca2+ release (CICR) (Endo et al. 1970; Fabiato 1984; Endo 2009). Then, the RyR activation in the triadic region provokes an outflow of Ca2+ into the myoplasm (Smith et al. 1988; Escobar et al. 1994). The CICR mechanism, however, may not participate in the massive Ca2+ release in mammalian muscle, as it does in frog muscle (Figueroa et al. 2012), since the voltage-activated Ca2+ release mechanism is responsible for the rapid, and highly synchronized, Ca2+ release in mammalian muscles.
The rate of Ca2+ release from the SR may be over 200 μmoles/ms in fast-twitch mammalian fibres and the cytoplasmic free Ca2+ concentration may increase up to 20 μM (Baylor and Hollingworth 2003), from a resting concentration close to 100 nM (Williams et al. 1990). Nonetheless, the peak values reported vary depending on the Ca2+ dye used, the calibration technique, the muscle type, whether it is amphibian or mammalian, and the muscle fibre type, slow or fast-twitch (Miledi et al. 1977; Klein et al. 1988; Konishi et al. 1991; Delbono and Stefani 1993; Shirokova et al. 1996; Baylor and Hollingworth 2003; Calderón et al. 2009, 2010, 2013). The amount of Ca2+ released during a single twitch in rat fast-twitch fibres seems to be constant over a range of SR Ca2+ content and equals about 20 % of the endogenous Ca2+ load (Posterino and Lamb 2003). For both slow- and fast-twitch fibres, total SR free Ca2+ content ranges from about 0.5 to 1 mM and can hardly be depleted (Fryer and Stephenson 1996; Wang et al. 2012). The high amount of Ca2+ inside the SR is buffered by CSQ, whose conformation and affinity changes determines its ability to also regulate RyR and then modulate Ca2+ release (Wei et al. 2006; Royer et al. 2010). Once released, the Ca2+ spreads in a matter of milliseconds, and interacts with troponin C. Ca2+-troponin interaction eliminates the inhibition imposed by troponin I and tropomyosin on the actin–myosin interaction, allowing the thin filaments to slide over the thick ones, thus producing tension (Huxley 1969; Ebashi 1974; Baylor and Hollingworth 2003; Craig and Padrón 2004).
Cytoplasmic Ca2+ removal rate is approximately 50 μmoles/ms and is initially buffered by soluble cytoplasmic proteins such as parvalbumin and is finally removed from the cytosol by the action of the SERCA, the mitochondria and the NCX, with fibre type-dependent kinetics (see “ECC and fibre types”) (Hasselbach 1964; Heizmann et al. 1982; Gillis et al. 1982; Balnave and Allen 1998; Baylor and Hollingworth 2003). Cytosolic proteins transiently buffer Ca2+ with moderate kinetics, while the SERCA takes the Ca2+ back to the SR with slower kinetics. Parvalbumin is a 12 kDa monomeric protein, that binds Ca2+ with high affinity and also binds Mg2+, playing a crucial role in fast muscles’ relaxation (see below) (Heizmann et al. 1982; Füchtbauer et al. 1991). Mitochondria and NCX are also slow Ca2+ handling mechanisms. Recently, Ca2+ uptake by mitochondria has been directly visualized in living cells, using genetically encoded chemiluminescent and fluorescent Ca2+ sensors, specifically targeted to the mitochondrial matrix (see below) (Rizzuto et al. 1992; Rudolf et al. 2004; Rogers et al. 2007) and NCX has been shown to activate under different conditions (Balnave and Allen 1998; Calderón et al. unpublished results). Most of these mechanisms finally deliver Ca2+ to the SERCA. SERCA is a 110 kDa, type P pump, mainly located in the SR's longitudinal region; factors regulating its expression and function include Ca2+, pH, thyroid hormones, and phospholamban and sarcolipin proteins (Odermatt et al. 1998; Hasselbach 1964, 1998; Hasselbach et al. 1975; Jorgensen and Jones 1986; James et al. 1989; Martonosi and Pikula 2003; Periasamy and Kalyanasundaram 2007). Three different genes encode 3 SERCA isoforms, but the number of isoforms produced by alternative splicing is higher (Martonosi and Pikula 2003; Periasamy and Kalyanasundaram 2007). This protein has 3 large cytoplasmic domains attached to a domain consisting of 10 hydrophobic trans-SR-membrane helices. Large movements, mainly of the cytoplasmic domains, mediate the Ca2+ pumping from the cytoplasm into the SR against its concentration gradient, reducing the cytoplasmic Ca2+ levels to nanomolar values in a matter of milliseconds, thus ensuring a fast relaxation of the skeletal muscles (Hasselbach 1964; MacLennan et al. 1985; Martonosi and Pikula 2003; Toyoshima and Mizutani 2004). Since some mechanisms (Ca2+ pumps, NCX and Ca2+ leak) may take Ca2+ out of the cells, with a yet not well known kinetics in skeletal muscle, a mechanism to ensure store Ca2+ replenishment exists (see below) (Parekh and Penner 1997; Kurebayashi and Ogawa 2001; Pan et al. 2002; Gonzalez-Narvaez and Castillo 2007; Bolaños et al. 2009).
The previous description gives a brief overview of most of the knowledge gathered on ECC coupling in skeletal muscle over the past 60 years, using a combination of experimental approximations, some of which will be discussed below.
Technical issues on the ECC study
Cell preparations used in ECC study
Enzymatic dissociation and hand dissection
Intact muscle fibres for physiological experiments can be obtained by means of enzymatic dissociation and manual isolation. In the first technique, described by Bekoff and Betz (Bekoff and Betz 1977) and modified by others (Caputo et al. 2004; Calderón et al. 2009; Calderón 2013), different rat or mouse muscles (mainly flexor digitorum brevis (FDB), extensor digitorum longus –EDL-, soleus and interossei) are subjected to an enzymatic dissociation with collagenase to digest the connective tissue surrounding the fibres, and subsequently subjected to mechanical dissociation through the use of glass-pipettes. The procedure yields complete, tendon-free muscle fibres. Once obtained, about 85 % of the fibres contract immediately and remain excitable for up to 24-36 hours when kept in Tyrode solution or culture medium (Calderón et al. 2009, 2010; Calderón 2013). One limitation with the use of dissociated fibres is their susceptibility to movement artifacts when recording Ca2+ transients. This drawback, however, has been overcome with the use of N-benzyl-p-toluene sulphonamide (BTS), butanedione monoxime (BDM) (Sun et al. 2001) and laminin. BTS is a small molecule which inhibits myosin type II and avoids shortening of the fibres (Cheung et al. 2002; Shaw et al. 2003; Calderón et al. 2009, 2010). Laminin works as a substrate to which muscle fibres adhere, limiting their movement and allowing the recording of movement artifacts-free Ca2+ transients with the advantage of working for all fibre types (Calderón et al. 2009, 2010).
Manual isolation appeals to the researcher's ability to dissect a muscle to obtain bundles with usually a few tens of fibres, or to obtain an isolated fibre still attached to its tendons. The fibre's integrity can be visually verified by observing its response to an electrical stimulus (Caputo and Giménez 1967; Lännergren and Westerblad 1987; Baylor and Hollingworth 2003; Bruton et al. 2003).
Although it has been suggested that fibres may be damaged during the enzymatic dissociation procedure (Hollingworth et al. 2012), the morphological evaluation and measurements of the levels of resting basal Ca2+, electrical properties of the sarcolemma, charge movement, amplitude of the AP and release of Ca2+ from the SR (Bekoff and Betz 1977; Williams et al. 1990; Szentesi et al. 1997; Woods et al. 2004; Wang et al. 2007) have shown that enzymatic dissociation of different muscles renders functionally intact fibres. Moreover, recent results showed that these fibres reproduce results previously described in manually isolated ones, such as the fatigue-induced increase in intramitochondrial Ca2+ and the tetanic Ca2+ transient’s amplitude reduction (Bruton et al. 2003; Calderón et al. 2011). Discrepancies between findings reported in different works (Calderón et al. 2010; Hollingworth et al. 2012) may have arisen from intrinsic differences between both preparations, such as the reduced sarcomere length found in dissociated fibres compared to manually isolated fibres mounted on transducers (Bolaños et al. 2008; Calderón et al. 2009).
Mechanical and chemical fibre skinning techniques
These techniques allow direct access to the interior of the muscle fibre, as both of them remove the sarcolemma, either mechanically (by microdissection) or with the use of glycerol or soft detergents such as Triton X-100 (Natori 1954; Wood et al. 1975; Lamb et al. 1995; Fryer et al. 1995; Knuth et al. 2006). The SR function can be preserved depending on the intensity of the treatment. It has been proven that in mechanically skinned fibres the T-tubules are resealed forming a closed compartment that allow the re-stablishment of ionic gradients and are capable of conducting AP. At the same time, the myoplasmic compartment remains open for experimental manipulation (Lamb et al. 1995; Fryer et al. 1995). One can hence determine, for instance, the effect of a change in pH or concentration of a metabolite like phosphate (Pi) or lactate on the myofibrils sensitivity to Ca2+, and the maximum strength generated with a saturating amount of cytosolic Ca2+. As a disadvantage, these techniques may remove cytosolic compounds like gluthathione, ATP, and parvalbumin, which can make the reading of certain results somewhat difficult.
Cut fibres preparation
This preparation allows control of the fibre membrane potential by double or triple vaseline gap voltage clamp techniques and also gives access to the myoplasm (Hille and Campbell 1976; Kovács and Schneider 1978; Kovács et al. 1983). The fibres, usually from frog or rat, are manually dissected and then cut, keeping or not part of the tendons. After that, the fibres are mounted on a chamber that allows the control of the composition of the intracellular medium through the cut fibre ends while the central portion of the fibre is electrically isolated from the cut extremes, using vaseline strips.
Cell cultures
Primary cultures and well-established myogenic cell lines, such as the mouse (C2C12) and rat (L6), or the dyspedic 1B5 (Yaffe and Saxel 1977; Rando and Blau 1994; Moore et al. 1998), have ideal characteristics for the in vitro study of differentiation, development, and signalling, on a functional, biochemical, and molecular level. In both cases (primary cultures and cell lines), the cultures must be kept in a growth medium with up to 20 % fetal bovine serum (FBS) until they reach ~60–90 % confluence to subsequently be induced to form myotubes, by reducing the amount of FBS in the culture medium or by its substitution for horse serum between 2 and 5 %. The functional results are limited to comparisons with early in vivo developmental stages, mainly because of the structural characteristics reached by myotubes formed in culture. When fibres isolated by enzymatic dissociation of FDB muscles from adult mice are kept in serum-free culture medium, they retain normal ECC properties for up to 7 days and are suitable for different physiological studies (Wang et al. 2007).
Experimental procedures
Wide-field quantitative fluorescence and measurements of Ca2+ transients
Over 45 years ago, Ridgway and Ashley (1967) were able to record global Ca2+ transients in electrically stimulated, intact muscle fibres. The authors injected the photoprotein aequorin, which emits light in presence of Ca2+. Due to the technique difficulty and stoichiometric problems, aequorin was substituted by metallochromic dyes, whose absorption spectra shift in the presence of Ca2+. However, these dyes were also substituted by the fluorescent ones due to the unsurpassed experimental advantages of the latter.
Usually, Ca2+ transients records obtained using fluorescent molecules have the following kinetic characteristics: (1) a rising phase, which reflects the Ca2+ outflow from the SR, and its free presence in the cytoplasm, (2) a peak, when Ca2+ outflow stops and Ca2+ removal mechanisms are already activated, and (3) a decay phase, which represents the sole operation of myoplasmic free Ca2+ removal mechanisms (Fig. 1). Ca2+ transients can be obtained in enzymatically dissociated or manually dissected fibres, in myotubes, and in cut fibres (Kovács et al. 1983; Westerblad and Allen 1991; Delbono and Stefani 1993; Caputo et al. 2004; Calderón et al. 2010). For these measurements, fluorescent indicators coupled to an acetoxymethyl (AM) ester moiety are commonly used, which allows them to diffuse into the cell. Once in the cytoplasm, endogenous esterases release the indicator molecule, which is now ready to bind Ca2+ and emit light (Tsien 1981). Fluorescent indicators in its salt form can also be injected into the cells, giving the possibility of determining the intracellular dye concentration and other dye-related data in a reliably way (Baylor and Hollingworth 1988; Konishi et al. 1991; Westerblad and Allen 1992).
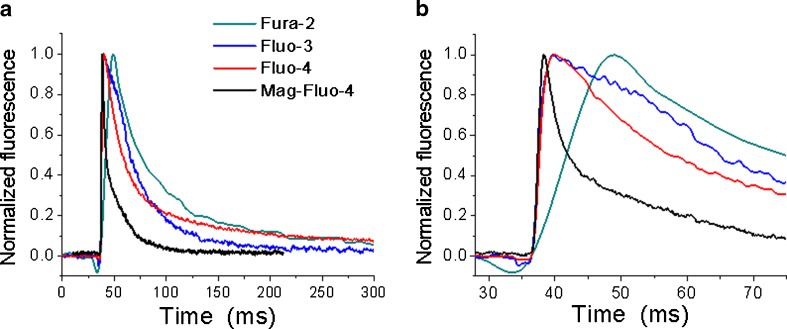
Fig. 1.
Comparison of single Ca2+ transients’ kinetics recorded in muscle fibres obtained by enzymatic dissociation of flexor digitorum brevis muscles from adult mice. Different cells were loaded with each of the Ca2+ dyes indicated in the figure and electrically stimulated. Ca2+ transients were recorded in an inverted fluorescence microscope using the appropriate set of filters, a photomultiplier and a Nikon amplifier. In (a), clear kinetic differences can be recognized, mostly derived from the different dissociation constants of the dyes used, being the fastest signal that obtained with Mag-Fluo-4 (black trace) and the slowest one that obtained with Fura-2 (green trace). In (b), the records are shown in an expanded time scale to better illustrate differences in the rising part of the signal
In general, fluorescent Ca2+ dyes can be classified as ratiometric or non-ratiometric (Grynkiewicz et al. 1985; Minta et al. 1989; Raju et al. 1989; Takahashi et al. 1999; Katerinopoulos and Foukaraki 2002; Kao et al. 2010; Bruton et al. 2012). Non-ratiometric dyes can be excited with visible light and, when bound to Ca2+, their fluorescence intensity increases without showing an important maximum excitation or emission wavelength shift. In this group, low and high affinity dyes can be found, having a dissociation constant in the micromolar or nanomolar range, respectively. The most commonly used dyes of this type are Mag-Fura-2 (first described as ratiometric, also giving the possibility of being used as non-ratiometric; Raju et al. 1989; Konishi et al. 1991; Baylor and Hollingworth 2003), Fluo-3 (Caputo et al. 1994), Fluo-4 (Prosser et al. 2010), Rhod-2 (Escobar et al. 1994; Caputo et al. 1999; Bruton et al. 2003) and Mag-Fluo-4 (Caputo et al. 2004; Calderón et al. 2009, 2010). The most suitable dyes to study ECC in skeletal muscle seem to be the low affinity Ca2+ dyes, Mag-Fura-2 and Mag-Fluo-4 (Fig. 1), since they are well known and can reliably track fast, large and brief Ca2+ transients such as those found in skeletal muscle (Hollingworth et al. 2009; Baylor and Hollingworth 2011; Calderón et al. 2010, 2013). Their disadvantages include the possible signal “contamination” with Mg2+-induced fluorescence, the need for taking measures to avoid the appearance of movement artifacts (see “Enzymatic dissociation and hand dissection”) in the Ca2+ transients and a more complex calibration procedure.
Ratiometric dyes, on the other hand, may show both intensity and spectrum changes when bound to Ca2+. The main examples of this group are: Fura-2 (Baylor and Hollingworth 1988; Westerblad and Allen 1991), Indo-1 (Chin and Allen 1998) and Mag-Fura-5 (Delbono and Stefani 1993; Szentesi et al. 1997). Their main disadvantages include the need for ultraviolet excitation and a more complex instrumentation. Also, with the exception of Mag-Fura-5, they may saturate and seem not to reliably track Ca2+ kinetics in skeletal muscle. One of the most important advantages of this group of dyes is the possibility of recording Ca2+ transients free of movement artifacts (Kao et al. 2010; Bruton et al. 2012) and their suitability for measuring myoplasmic basal Ca2+. Other advantages include their minimum Mg2+ affinity and the possibility of calibration disregarding the dye concentration.
Although for tracking Ca2+ changes focused on particular subcellular structures, some Ca2+ dyes may be used (Rhod-2, Mag-Fluo-4; Fluo-5N, CalciumOrange-5N; Bruton et al. 2003; Brochet et al. 2005; Bolaños et al. 2008; Kao et al. 2010), the genetically-encoded Ca2+ sensors seem to be now a better choice (Palmer and Tsien 2006; Rudolf et al. 2004; Tang et al. 2011; Wang et al. 2012).
As illustrated in Fig. 1, the kinetics of the single Ca2+ transients largely depend on the kinetic properties of the dye used. In this figure, Mag-Fluo-4 is the only low-affinity Ca2+ dye and shows the best kinetics for tracking skeletal muscle Ca2+ transients. In any case, the researcher should know how to deal with the limitations of a given dye and also how to exploit its advantages, since the ideal Ca2+ indicator is still missing.
Tension measurements
For tension measurements, small bundles of fibres and manually isolated fibres are used (Caputo and Giménez 1967; Lännergren and Westerblad 1987; Baylor and Hollingworth 2003; Bruton et al. 2003; Edman 2005). Therefore, muscle fibres remain attached to their tendons. Isolated fibres or bundles are horizontally mounted on the experimental chamber and attached with small aluminum clips, on one end to a tension transducer and on the other to a hook attached to the chamber. Contractions are produced by suprathreshold stimulation through electrodes. Simultaneous measurements of Ca2+ and tension can be obtained (Westerblad and Allen 1991; Bruton et al. 2003; Baylor and Hollingworth 2003; Calderón et al. 2011). This allows to calculate, for instance, myofibrils’ sensitivity to Ca2+ in intact fibres, or to follow the changes of both variables during muscle fatigue. Recently, a biological adhesive was successfully used to attach dissociated fibres to a tension transducer (Ward et al. 2011) ,opening up a large number of possibilities with this cellular preparation.
Electrophysiology
Membrane ionic current measurements, intramembrane charge movement recordings and studies of voltage dependence of Ca2+ release, through membrane voltage-clamp techniques are of great importance for the study of numerous aspects of physiology and physiopathology of skeletal muscle (Delbono and Stefani 1993; Szentesi et al. 1997; Hernández-Ochoa and Schneider 2012). Currently, the triple vaseline gap voltage clamp, the whole-cell patch-clamp and the silicone-clamp techniques are the most used for recording Ca2+ transients under controlled membrane potential conditions, in myotubes, intact or cut isolated fibres. In adult fibres, because of their length, it is convenient to use the vaseline-gap and silicone-clamp techniques to isolate areas of the fibres and thus restrict current measurements to small portions of the fibres. In the obtained records, the amplitude and voltage dependence are the most important variables to analyze. Depolarization occurs from a set voltage (holding potential) usually from –80 to –100 mV, to a variable voltage. Depolarization records from –80 to –10, 0, +10 and +30 mV are usually obtained (Delbono and Stefani 1993; Szentesi et al. 1997; Beam and Franzini-Armstrong 1997; Pouvreau et al. 2007a; Hernández-Ochoa and Schneider 2012).
In a different technique, by inserting purified proteins into artificial lipid bilayers and activating the passage of ions through them, it is possible to study their channel properties and to estimate their conductance, open probability, selectivity and drugs effects (Smith et al. 1988; Goonasekera et al. 2007).
Confocal laser scanning microscopy and super-resolution microscopy
Confocal laser scanning microscopy (CLSM), and, more recently, multiphoton microscopy, have allowed us to perform temporal and spatial precise analysis of ECC-related physiological phenomena at a subcellular level, besides the suitability of CLSM for structural studies (Cheng et al. 1993; Escobar et al. 1994; Rudolf et al. 2004; Brochet et al. 2005; Bolaños et al. 2008; Casas et al. 2010; Figueroa et al. 2012). Cells may be loaded with indicators such as di-8-ANEPPS and FM 1-43 to label sarcolemma and T-tubules and record transmembrane voltage; Rhod-2, Fluo-3 and Fluo-4 for cytoplasmic Ca2+; Endoplasmic Reticulum-Tracker Green (ERTG) for SR and MitoTracker Green, Rhod-2, CalciumOrange-5N, JC-1 and Tetramethyl rhodamine ethyl ester (TMRE) for different mitochondrial studies (Farkas et al. 1989; Escobar et al. 1994; Rudolf et al. 2004; Bolaños et al. 2008; Calderón et al. 2009; Casas et al. 2010; Manno et al. 2013). Fluorescent molecules-coupled antibodies and fluorescent proteins-coupled protein constructs are also visualized using CLSM. Confocal microscopy has the following advantages over optical microscopy and wide-field fluorescence: (1) higher z-resolution and better contrast, it allows us to obtain better structural (i.e. T tubules, SR or mitochondria) and functional information (Ca2+ and transmembrane potential imaging), (2) allows us to obtain images for further analysis (temporary changes, fluorescence intensity measurements, and morphometric measurements, among others), (3) allows us to simultaneously register different structures or events for further joint analysis, and (4) allows us to perform optical sections, which is especially important to determine the location of fluorescent compounds, and to perform three-dimensional reconstructions, in addition to producing multidimensional images according to time (xt or xyt modes). One limitation of CLSM has been its low temporal resolution when studying kinetics of Ca2+ transients with fast Ca2+ dyes. However, recently, some authors overcome this limitation, and were able to track Ca2+ transient’s propagation with fast scanning confocal microscopy in rat fibres (Edwards et al. 2012).
Additionally, the use of CLSM combined with new techniques such as FRET (Förster resonance energy transfer) and SEER (Shifted excitation and emission ratioing) have allowed, among other findings, to study conformational coupling between DHPR and RyR and to obtain images which allow to quantify the Ca2+ inside the SR or dynamically image transmembrane voltage (Papadopoulus et al. 2004; Launikonis et al. 2005; Manno et al. 2013).
Although the theoretical background for super-resolution microscopy has been developed during the last two decades (Hell and Wichmann 1994; Klar et al. 2000; Gustafsson 2000; Schermelleh et al. 2010), this technique became commercially available very recently and only a few works have used it to study skeletal muscle ECC (Rausch et al. 2013), besides cardiac ECC (Jayasinghe et al. 2012; Scriven et al. 2013; Wong et al. 2013). The main advantage of the technique is providing a xy-resolution between 30 and 120 nm, which recently allowed some authors to recognize a new pattern of RyR clustering in mouse FDB fibres (Rausch et al. 2013). The main disadvantages include the need for non-standard dyes in some devices, still limited temporal resolution and high costs.
Other procedures
Electron microscopy (standard, metal-shadowed, cryomicroscopy and three-dimensional reconstructions) has generated important information on the triad structure, membrane systems (T-tubules and SR), DHPR-RyR interaction and SR-mitochondria relationship (Block et al. 1988; Serysheva et al. 2007; Boncompagni et al. 2009; also see below). Because there are no X-ray diffraction patterns of the whole DHPR and RyR proteins, due to the difficulties of crystallizing them, cryomicroscopy of isolated particles and three-dimensional reconstructions have been used for structural and functional characterization. However, the resolution obtained does not allow a clear non-ambiguous secondary structural assignment for some proteins (see below).
Several molecular biology and genetic engineering techniques have provided valuable information on skeletal muscle in normal and pathological conditions, since they have allowed the functional evaluation of the expression (or absence) of proteins involved in ECC, i.e: (1) knockout mice for some proteins, including dysgenic mice lacking the subunit α-1 of DHPR, and dyspedic mice, lacking RyR1 (Buck et al. 1997; Beam and Franzini-Armstrong 1997; Prosser et al. 2008). Mice lacking CSQ or other minor ECC proteins have been under research during the past years (Paolini et al. 2007; Royer et al. 2010; Mosca et al. 2013); (2) gene silencing, as in the codifying gene for SR integral protein, JP-45, proving its importance for functional expression of DHPR (Anderson et al. 2006); and (3) production and extraction of recombinant proteins with high qualitative and quantitative performance in adult mammalian skeletal fibres, which has allowed researchers to evaluate the effect of mutated ion channel's expression and endogenous protein over-expression, such as DHPR, on ECC (DiFranco et al. 2006, 2011).
Particular issues on ECC
Dihydropyridine receptor-ryanodine receptor structure and coupling
There is evidence indicating a mechanical, bidirectional conformational coupling between DHPR and RyR in the skeletal muscle (Nakai et al. 1996; Ávila and Dirksen 2000; Fill and Copello 2002; Paolini et al. 2004), which would be mediated by regions of the internal loop joining DHPR’s II and III transmembrane domains and a specific short RyR region (Tanabe et al. 1990; el-Hayek et al. 1995; Leong and MacLennan 1998; Casarotto et al. 2006), although several studies suggest that there are multiple RyR1 regions interacting with DHPR (Protasi et al. 2002) and multiple DHPR subunits doing so with the RyR1 (Papadopoulus et al. 2004).
Two important and related developments opened the way for clarifying, at a molecular level, the roles of DHPR and RyR, and their relationship in ECC: (1) the cloning and sequencing of the complementary DNA (cDNA) that encoded for the α1 subunit of DHPR (Tanabe et al. 1987), and of the skeletal muscle RyR (Takeshima et al. 1989) and (2) the availability of mice with genetic alterations at level of the DHPR, or at level of the RyR. In the case of DHPR, it was shown that mice with a mutation in the α1 subunit of this molecule, dysgenic mice, lacked the L-type Ca2+ current and presented greatly reduced intramembrane charge movement (Beam et al. 1986). These results provided the first hint that DHPR could have a dual function, serving both as voltage-activated Ca2+ channels and as voltage sensors for ECC, an idea reinforced by the simultaneous evidence provided by Ríos and Brum (1987) that the DHPR were the molecules generating the intramembrane charge movement, which represents the electrical manifestation of the voltage sensors operation. Definitive evidence in favour of this idea was obtained by the fact that expressing skeletal muscle DHPR α1 subunit in dysgenic myotubes restored both the slow Ca2+ current (Tanabe et al. 1988) and the intramembrane charge movement (Adams et al. 1990).
An elegant experimental strategy was developed to determine the regions of the DHPR important for ECC, based on the differential sensibility of skeletal and cardiac ECC to extracellular Ca2+ and the use of myotubes of dysgenic animals that did not express DHPR (Tanabe et al. 1990). Injection of cDNA codifying for the cardiac isoform of the DHPR reproduced the cardiac type of ECC, that required the presence of external Ca2+ for contracting in response to electrical stimulation and was disrupted by Cd2+ poisoning. Injection of cDNA, codifying for skeletal type DHPR, reproduced the skeletal ECC with injected myotubes contracting in the absence of external Ca2+ and not sensitive to Cd2+. Further experiments using expression plasmids, in which only the loops between I-II and II-III domains of the DHPR were of skeletal origin, showed that the skeletal region between repeats II and III was a sufficient determinant for skeletal type ECC.
Most DHPR–RyR interaction studies have been based on site-directed mutagenesis of amino acids located in specific domains. However, the movements of different domains involved in such interactions are not completely clear, due to lack of information from crystallography or magnetic resonance studies of both channels under different circumstances. Although the three-dimensional structure of the isolated DHPR II-III loop is already known (Casarotto et al. 2006), it is still uncertain if it assumes a different structure when binding with RyR or if it remains unchanged in relation to the complete DHPR structure. The β1a subunit of the DHPR has also been shown to be important for ECC coupling, and ongoing work is devoted to clarify its role in interacting with and modulating RyR, as well as its role in the adecuate targeting (i.e. forming tetrads) of DHPR to the T-tubules (Pérez et al. 2013; Rebbeck et al. 2013).
So far, most structural information on DHPR and RyR structures comes basically from cryomicroscopy studies and three-dimensional reconstructions (Wagenknecht et al. 1989; Ludtke et al. 2005; Samsó et al. 2005, 2009; Serysheva et al. 2007). Despite the fact that several teams are solving the structure of both proteins, only recently have sub-nanometric resolution images been published (9.6 Å for the whole RyR (Ludtke et al. 2005) and 2.5 Å for the structure generated by the 559 aminoacids of the N-terminal region of the RyR (Tung et al. 2010). The authors have specially focused on the RyR’s pore structure at its closed state. Ludtke et al. (2005) identified 5 α helices per monomer in the transmembrane region and suggested that helices 1 of each subunit form the pore, and helices 2, the selectivity filter. Samsó et al. (2005) suggested the existence of at least 6 transmembrane helices per monomer, and described in some detail the structures known as columns (inner branches), which connect the transmembrane portion with the great cytosolic domain. It is possible that the pore's structure is similar to some already published K+ channel structures (Doyle et al. 1998; Jiang et al. 2002; Ludtke et al. 2005; Samsó et al. 2005, 2009). Recently, an image of the open channel (at 10.2 Å) was obtained and moderate conformational changes were recognized when compared to the closed state. The cytoplasmic domains move outward and the three constrictions identified by the authors in the ion pathway show a wider central passage (Samsó et al. 2009).
The highest resolution obtained (9,6 Å in RyR1 and 30 Å in DHPR) is not clear enough to show details such as: (1) the exact molecular mechanism involved in receptor's interactions, (2) presence, distribution and movement of α helices and β sheets to explain, for instance, how the signal is transmitted from the DHRP to the RyR and how the information from the RyR regulators is transmitted from somewhere in the protein to the pore, to allow SR Ca2+ release, (3) delimitation of the different subunits in each protein, and (4) the number of RyR helices going through the SR membrane, since the proposed number of transmembrane helices varies between 4 and 10–12 per monomer (Takeshima et al. 1989; Zorzato et al. 1990; Samsó et al. 2009).
The 2.5-Å resolved structure of the N-terminal domains of the RyR shows that it is located in the cytoplasmic portion of the protein, and the domains orientation likely represents the closed state of the channel (Tung et al. 2010). In this region, the positions of multiple disease-mutations and some regions of intersubunit interactions can be mapped, but it is likely that, in spite of the high resolution, no clear information on the above questions will be obtained by this structure. The answer to these and other issues relies on obtaining a complete structural image of both DHPR and RyR channels with at least a 3-6-Å resolution.
Isolated protein images do not necessarily reflect the structure of complexes such as the triad. Images of isolated rabbit triads (Wagenknecht et al. 2002) with an approximate resolution of 6 nm, obtained through several techniques, confirmed findings previously made by other authors (Franzini-Armstrong and Porter 1964; Block et al. 1988; Franzini-Armstrong and Jorgensen 1994; Franzini-Armstrong 1999) and drew attention to the existence of some new structural characteristics: (1) there could possibly be a 5-nm-wide structure under the SR's internal membrane, whose nature is still unknown, (2) CSQ particles seem to be interconnected within the SR, (3) inside the T-tubule's lumen, there appear to be structures whose molecular nature is still unknown but could play a structural role, and (4) the presence of structures covering the distance from the RyR's cytoplasmic region all the way to the T-tubule was found, which cannot be categorized in a clear protein domain, but could be DHPR loops. In spite of the advantages of this technique, the results obtained could be affected by artifacts during the sample processing, and by the low resolution of the images.
Aside from the above, we still need to know the three-dimensional structure of triadic major and minor proteins, including previously identified and some still unidentified ones. Although some models of the triadic complex have been recently put forward and the specific domains and aminoacids involved in intra- and interprotein interactions are being identified (Wagenknecht et al. 2002; Treves et al. 2009; Fourest-Lieuvin et al. 2012; Boncompagni et al. 2013), the huge amount of proteins and expected interactions among them that regulate ECC, and which may also function as molecular determinants of the shape of the membranous components of the triad, makes the work on these issues still far from finished. Nowadays, despite almost three decades of research on the DHPR-RyR coupling issue, two basic questions remain unanswered: how are different signals transmitted from the bulk of the channel to the pore, and how is the ion gating mechanism of RyR?
ECC and fibre types
In 1873, Ranvier stated that there were pale and red muscles, being different mainly regarding their contraction speed (Ranvier 1873). Decades later, a more complete profile of both types of fibres was generated and new fibre types were described (Dubowitz and Pearse 1960; Brooke and Kaiser 1970; Bär and Pette 1988; Schiaffino et al. 1989). Since four fibre types based on myosin heavy chain (MHC) presence have been described, I, IIA, IIX/D and IIB (there are also hybrid fibres), one important question that raises is whether ECC proteins and Ca2+ transient kinetics differ among all fibre types.
The molecular machinery involved in ECC is qualitatively (different isoforms) and quantitatively (different amount of proteins or different kinetics) different between slow and fast-twitch fibres (Table 1). The information presented in this table discriminates between slow- and fast-twitch fibre types, since the information is not available for each MHC-based fibre type. A great variability regarding the proteins involved in both Ca2+ release and Ca2+ removal from the myoplasm is evident. The most striking difference is found in parvalbumin concentration, ranging from almost zero in the slowest fibres up to 1 mM in the fastest fibres.
Table 1.
Biochemical and structural differences in ECC between slow and fast-twitch mammalian fibre types
| Proteins | Slow-twitch fibres | Fast-twitch fibres | References | ||
|---|---|---|---|---|---|
| Isoform | Content/kinetics | Isoform | Content/ kinetics | ||
| DHPR | α-1S | + | α-1S | +++ | Hollingworth and Marshall 1981; Lamb and Walsh 1987; Franzini-Armstrong et al. 1988. |
| RyR | RyR1 | + | RyR1 | +++ | Franzini-Armstrong et al. 1988; Appelt et al. 1989; Damiani and Margreth 1994. |
| PV | α-PV | – to + | α-PV | ++ to ++++ | Heizmann et al. 1982; Leberer and Pette 1986; Schmitt and Pette 1991; Füchtbauer et al. 1991. |
| SERCA | SERCA2a | + | SERCA1a | + to ++ | Leberer and Pette 1986; Dulhunty et al. 1987; Ferguson and Franzini-Armstrong 1988; Periamasy and Kalyanasundaram 2007. |
| Phospholamban | Phospholamban | + | Phospholamban | – | Jorgensen and Jones 1986. |
| Sarcolipin | Sarcolipin | + | Sarcolipin | +++ | Odermatt et al. 1998. |
| Calsequestrin | CSQ*fast and CSQ*cardiac | + | CSQ*fast | + | Leberer and Pette 1986; Damiani and Margreth 1994. |
| SRP-27 | SRP-27 | + | SRP-27 | +++ | Bleunven et al. 2008 |
| NCX | NCX1 | ++ | NCX1 and 3 | + | Fraysse et al. 2001; Hudecova et al. 2004 |
| TnC, TnI, TnT | TnC slow TnI slow TnT slow |
+ | TnC fast TnI fast TnT fast |
++ | Bottinelli and Reggiani 2000. |
| mATPase | Type I | + | Types IIA, IIX/D and IIB | ++ to +++ | Dubowitz and Pearse 1960; Brooke and Kaiser 1970; Bär and Pette 1988; Schiaffino et al. 1989; Bottinelli and Reggiani 2000. |
DHPR dihydropyridine receptors; RyR ryanodine receptors; PV parvalbumin; SERCA sarcoendoplasmic reticulum Ca2+ ATPase; CSQ calsequestrin; SRP-27 sarcoplasmic reticulum protein-27 kDa; NCX Na+/Ca2+ exchanger; TnC troponin C; TnI troponin I; TnT troponin T; mATPase myofibrillar A-band adenosine triphosphatase
– indicates absence of the protein; ++ indicates approximately twofold; +++ between twofold and tenfold; ++++ more than tenfold, in all cases compared to a reference of +
The differences mentioned in Table 1 constitute the biochemical and structural bases for the Ca2+ transients variability found among fibre types (Eusebi et al. 1980; Carroll et al. 1997; Bottinelli and Reggiani 2000; Baylor and Hollingworth 2003; Reggiani and te Kronnie 2006; Calderón et al. 2009, 2010).
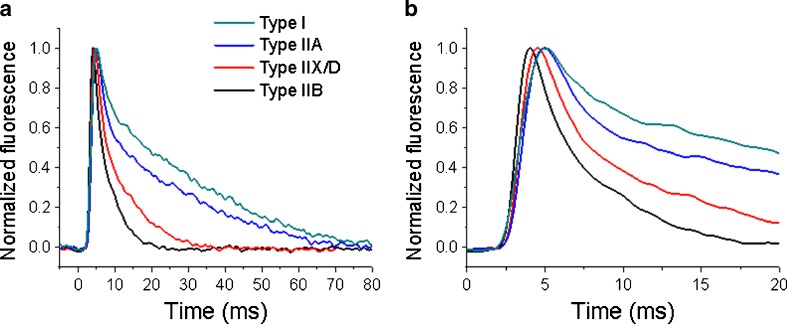
Single Ca2+ transients obtained using Mag-Fluo-4 can be classified according to their kinetics into two different morphologies (Calderón et al. 2009, 2010). One morphology, found in fibres type I and IIA, is slower, wider and of less amplitude and was called morphology type I (MT-I). The other morphology, found in fibre types IIX/D and IIB, is faster, narrower and of higher amplitude and was called morphology type II (MT-II). An example of the Ca2+ transients found in different fibre types from mice muscles is illustrated in Fig. 2. Since only minor differences can be recognized between fibres type I and IIA, for the sake of clarity, we have pooled in Table 2 the kinetics of a large number of MT-I fibres obtained from soleus muscles. The data are compared with the pooled values obtained in EDL fibres, since all of them are MT-II. Significant differences can be recognized in all parameters describing amplitude, rising and decay kinetics between soleus and EDL signals.
Fig. 2.
Time course of single Ca2+ transients of different fibre types obtained by enzymatic dissociation of extensor digitorum longus and soleus muscles from adult mice and typed by polyacrylamide gel electrophoresis. The cells were loaded with Mag-Fluo-4. A pattern can be recognized, with the pair I and IIA being the slowest and the pair IIX/D and IIB being the fastest both during decay (a) and rise (b)
Table 2.
Kinetic parameters of soleus and EDL single Ca2+ transients from adult mice
| Muscle | n | ∆F/F | 10-90 % Rise time (ms) | Half-width (ms) | Decay time (ms) | Time constants (ms) | A1 (%) | A2 (%) | ∆F/RT | |
|---|---|---|---|---|---|---|---|---|---|---|
| t1 | t2 | |||||||||
| Soleus* | 45 | 0.51 ± 0.03 | 1.56 ± 0.04 | 15.73 ± 0.95 | 61.35 ± 2.41 | 3.14 ± 0.11 | 43.63 ± 2.23 | 27.17 ± 1.32 | 72.83 ± 1.32 | 0.34 ± 002 |
| EDL | 26 | 0.65 ± 0.02 | 1.08 ± 0.03 | 4.07 ± 0.2 | 15.92 ± 0.88 | 1.58 ± 0.07 | 9.99 ± 0.67 | 39.45 ± 1.47 | 60.55 ± 1.47 | 0.61 ± 0.03 |
Values are mean ± SEM
EDL Extensor digitorum longus; A1 amplitude of fast component of decay; A2 amplitude of slow component of decay, ∆F/RT ratio of amplitude to rise time
*p < 0.05 for all comparisons between each one of the parameters (i.e. ∆F/F of soleus vs. EDL, 10–90 % rise time of soleus vs. EDL, etc.). From: Calderón 2013, with permission
Tetanic Ca2+ transients of the fibres type I and IIA show a staircase morphology (MT-I). In these transients, the decay can be fitted by a single exponential function. On the other hand, fibres type IIX/D and IIB have tetanic Ca2+ transients whose decay is better fitted with a biexponential function (MT-II) (Calderón et al. 2009, 2010).
In general, regarding Ca2+ transients and fibre types, it can be concluded that transient’s kinetics show a continuum from the slowest kinetics, obtained in the type I fibres, to the fastest ones, obtained in types IIX and IIB. Remarkably, type IIA fibres are fast regarding Ca2+ release and slow regarding Ca2+ clearance (Calderón et al. 2009, 2010). The differences in kinetics of the Ca2+ twitch and tetanic transients seem to underlie the different kinetics of contractile characteristics of slow and fast muscles (Calderón et al. 2010).
One application of the easily recognizable difference found between MT-I and MT-II records is the possibility of functionally recognizing fibre types while still alive during physiological experiments in which Ca2+ transients are recorded (Calderón et al. 2011, unpublished results).
Ongoing work has addressed the mechanisms responsible for the Ca2+ transient’s kinetics differences. The biochemical differences shown in Table 1 and new results suggest that higher DHPR and RyR content explain differences in the rising phase of the Ca2+ transients and that PV content and SERCA kinetics explain most of the decay differences (Calderón et al. 2009, 2010, unpublished results). Other mechanisms such as mitochondria and NCX only play a minor role in explaining the differences (Calderón et al., unpublished results).
ECC and muscle fatigue
The transient and progressive decrease in skeletal muscle performance during continuous stimulation is known as fatigue (Fitts 1994; Allen et al. 2008). This is a complex phenomenon with central and peripheral components (Bigland-Ritchie and Woods 1984; Abbiss and Laursen 2005; Allen et al. 2008). Central fatigue involves events occurring prior to the ECC phenomenon, while peripheral fatigue involves events including ECC and other phenomena occurring inside the muscle fibre as a consequence of its activation.
Although different models to study muscle fatigue show different complexity, it seems that data gathered with a relatively simple model such as the one of isolated fibres can be used to understand some fatigue mechanisms in more complex models (Place et al. 2010). The most important addressed questions on fatigue are: (1) what is the cause of skeletal muscle fatigue, and (2) what is the mechanism by which muscle fatigue develops?
In several preparations, fatigue has been shown to be mainly peripheral (Grabowski et al. 1972; Bigland-Ritchie and Woods 1984; Moussavi et al. 1989; Kent-Braun 1999; Place et al. 2008, 2010), explained in part by alterations in the Ca2+ release mechanism (Grabowski et al. 1972; Allen et al. 1989; Westerblad and Allen 1991). In the same way, alterations of the SERCA function and slowing of single-twitch and tetanic relaxation have been demonstrated as a result of fatigue (Gollnick et al. 1991; Westerblad and Lännergren 1991; Westerblad and Allen 1993; Green 1998; Leppik et al. 2004), but the slowing of relaxation cannot be totally explained by the decreased SERCA pumping rate (Westerblad andAllen 1993). Other phenomena, such as a decrease in the intra-SR Ca2+ content and the damage of membrane systems involved in ECC, may also be implicated (Takehura et al. 2001; Tupling 2004). An alteration of the inactivation of the Ca2+ release mechanism seems not to mediate Ca2+ release disturbances found in fatigue (Calderón et al. 2011).
Although a wealth of evidence supports the concepts according to which both a decrease in AP amplitude or ATP depletion can be ruled out as causes of fatigue (Luttgau 1965; Grabowski et al. 1972; Nassar-Gentina et al. 1978; Moussavi et al. 1989; Allen et al. 2002), recent findings, suggesting that localized ATP decrease close to critical cellular regions may be important for fatigue (Allen et al. 1997) or that an AP decrease in fatigued fibres can be measured by SEER (Manno et al. 2013), warrant further research on these topics.
ECC alterations during fatigue are strongly supported by the fact that, in a fatigued muscle, tension development can be reactivated by caffeine (Grabowski et al. 1972; Allen et al. 1989) and because it was actually proved that fatigued fibres have reduced tetanic Ca2+ transients (Westerblad and Allen 1991; Calderón et al. 2011). The ECC alteration, however, is more notorious in fast-twitch fibres compared to slow ones (Calderón et al. 2011), as expected by the known profile of fatigability of the different fibre types (Burke et al. 1973; Petrofsky and Lind 1979; Westerblad and Allen 1991; Bruton et al. 2003).
Metabolic factors, such as changes in Ca2+ itself, ATP, phosphocreatine (PCr), H+, Mg2+, Pi and reactive oxygen species (ROS) concentrations observed during fatigue may be involved in altering the ability of the SR to release and reuptake Ca2+ (Lamb 2002; Tupling 2004; Allen et al. 2008; Calderón-Vélez and Figueroa-Gordon 2009).
Lactate accumulation and acidosis were the most claimed factors in explaining the decrease in muscle performance; however, its role has been challenged, although not without some controversy (Hill and Kupalov 1929; Westerblad 1999; Westerblad et al. 2002; Pedersen et al. 2004; Bangsbo and Juel 2006), even when pH values as low as 6.5 and lactate increase have been documented in several works as related to fatigue (McCully et al. 1991; Lindinger and Heigenhauser 1991; Kent-Braun et al. 1993; Kent-Braun 1999). pH reduction seems to decrease fibre's maximum strength, myofibrillar Ca2+ sensitivity and SR ability to release Ca2+ (Rousseau and Pinkos 1990; Fitts 1994; Lamb 2002; Knuth et al. 2006).
Another possible mechanism involving ECC as a potential fatigue site could be that the increase in basal myoplasmic Ca2+ (Ca2+ myo) found during the development of fatigue (Westerblad and Allen 1991; Caputo et al. 1994) may disrupt DHPR–RyR interaction (Lamb 2002). This seems to be associated to an alteration of the fibre to produce tension although the SR's ability to deal with Ca2+ does not become altered (Lamb et al. 1995; Verburg et al. 2005). SERCA function alteration (Green 1998; Westerblad and Lännergren 1991; Westerblad and Allen 1993; Leppik et al. 2004; Tupling 2004) could explain the persisting increase of Ca2+ myo leading to the ECC disruption and subsequently to a decrease in Ca2+ release after each stimulus. Opposite to this, it was suggested that a more important factor for the production of uncoupling is the localized increase of Ca2+ near the triad, associated to each tetanus, instead of the increase of basal Ca2+ myo (Verburg et al. 2006).
Although free myoplasmic Mg2+ increases during repeated stimulation (Westerblad and Allen 1992) and it is an inhibitor of the SR Ca2+ release (Meissner 1984), Mg2+ does not have an important fatiguing effect unless reaching very high concentrations, which do not occur in a fatigued muscle fibre (Westerblad and Allen 1992; Lamb and Stephenson 1994).
Even though theoretical considerations could also support a role for ROS and free radicals in fatigue (Barclay and Hansel 1991; Sen 1995; Reid 2001; Darnley et al. 2001), by altering Ca2+ release from the SR (Brotto and Nosek 1996; Oba et al. 2002; Hidalgo 2005; Bruton et al. 2008), or by reducing myofibril's sensitivity to Ca2+ in mammalians (Moopanar and Allen 2005, 2006; Bruton et al. 2008; Reardon and Allen 2009), conflicting results are available on whether these species are actually produced during a repetitive stimulation protocol in different experimental preparations (Davies et al. 1982; Reid et al. 1992; Kanter et al. 1993; Bruton et al. 2008; van der Poel et al. 2008) and thus on whether their effect would be physiologically relevant to explain fatigue-induced ECC alterations in mammalians. The issue of the relationship among high temperature-ROS and fatigue seems to be illustrative. High temperature may increase ROS production in rodents, and ROS may induce fatigue (van der Poel et al. 2008; Reardon and Allen 2009; Michaelson et al. 2010; see also Place et al. 2009); however, high temperature did not alter fatigability in some reports (Place et al. 2009; Reardon and Allen 2009).
Nowadays, we are not certain about what are the exact physiological targets of free radicals and ROS in fatigue, what is the main source of ROS during repetitive contraction (see van der Poel et al. 2008; Michaelson et al. 2010), or if the lipid peroxidation may explain the alterations of the different ECC-related proteins affected in the fatigue state and which are the chemical species involved. Until now, the results depend on the animal species, the techniques used for detecting ROS and the experimental protocols applied (muscle preparation, stimulation duration and temperature). Some recently developed tools may help solve technical problems with the detection of intracellular ROS and their role in muscle fatigue (Pal et al. 2013).
During the last two decades, an increase in free myoplasmic Pi has emerged as an important cause of fatigue. Pi meets important requirements to be considered as cause of ECC fatigue. Firstly, its change has a time course similar to the time course of the fatigue-induced Ca2+ kinetic changes (Westerblad and Allen 1991; Calderón et al. 2011). Changes in Pi can start early during the fatigue development, although are more notorious during the second half of the stimulation periods (Bergström and Hultman 1988; Moussavi et al. 1989; Kent-Braun et al. 1993; Kent-Braun 1999); several studies have shown that Pi is important for the early changes in the kinetics of Ca2+ signals (Dahlstedt et al. 2001; Westerblad et al. 2002). Secondly, an increase in Pi can explain the most important fatigue-induced Ca2+ kinetic changes (Westerblad and Allen 1991; Calderón et al. 2011). Pi can regulate both the release of Ca2+ and the function of SERCA (Duke and Steele 2000; Dahlstedt et al. 2001; Westerblad et al. 2002) and it seems to favor Ca2+ deposit inside the SR, in the form of Ca2+ phosphate, which diminishes the amount of Ca2+ available for release (Fryer et al. 1995; Dutka et al. 2005). It can also decrease myofibrillar Ca2+ sensitivity (Fitts 1994; Westerblad et al. 2002). Thirdly, the differential change in Pi observed in slow and fast fibres during contraction gives support to the differential alteration seen in the rising and decay phase of tetanic Ca2+ transients demonstrated in different fibre types during fatigue (He et al. 2000; see Calderón et al. 2011). And, fourthly, Pi concentration manipulation modifies in the same way the fatiguing properties of different muscles (Dahlstedt et al. 2001; Westerblad et al. 2002).
The development and availability of Pi fluorescent indicators would be of great impact to prove a direct relationship between intracellular changes of the metabolite and the phenomena known to occur during fatigue. Also, to confirm the role of Pi on muscle fatigue, the management of and the sensitivity to Pi alterations by the different fibre types in different experimental models should be evaluated.
Other questions are still open, such as: (1) what are the kinetics and mechanisms of muscle fatigue in developing or ageing fibres, (2) which mechanisms explain the fatigue-resistant properties found in slow vs. fast-twitch fibres, and (3) what is the role of ECC regulators or minor proteins in muscle fatigue (see Prosser et al. 2010)?
Contractile potentiators
The early observation by Kahn and Sandow (1950) that nitrate augmented the twitch response of frog skeletal muscle led to the demonstration by Hodgkin and Horowicz (1960) that nitrate and other anions, of the lyotropic series SCN > I > NO3 > Br > Cl, shifted, in this order of potency, the relationship between contractile force and membrane potential, toward more negative potentials, thus reducing the contractile threshold, that in frog fibres is around –50 mV (Sandow 1964; Kao and Stanfield 1968). The lyotropic series describes the degree of adsorbability of these anions on the membrane external surface that cause a change in the density of fixed charges (Hodgkin and Horowicz 1960; McLaughlin et al. 1975), altering the surface potential and the potential gradient through the fibre membrane. Of particular importance is the chaotropic anion perchlorate, ClO4 -, that causes the largest change in the contractile threshold, potentiating manifold twitch tension (Foulks et al. 1973; Gomolla et al. 1983). A group of divalent heavy metals, such as Zn2+, Be2+, Pt4+ and others including uranil ions (UO2)2+, also act as twitch potentiators, by a selective block of the K+ conductance, that prolongs the duration of the AP, overcoming the non-specific effects due to their adsorbability on the surface membrane (Sandow and Isaacson 1966). Sandow et al. (1965) proposed that both types of compounds enhanced the twitch, by prolonging a parameter, called the mechanically effective period (MEP), defined as the period during which the AP causes the release of an amount of Ca2+, greatly exceeding that necessary for a twitch response (Sandow 1964). MEP is increased either by lowering the contractile threshold (type A potentiators, like the lyotropic anions), or by prolonging the AP (type B potentiators, like the divalent heavy metals Zn2+, Cd2+, etc.). Other compounds, like caffeine, quinine and quinidine, that also potentiated contraction, were initially classified as type C, since they seemed to act by a combination of both type A and type B mechanisms (Sandow 1964), but later, however, they were classified as type A, due to their sizeable effect on the contractile threshold. In all cases, prolongation of the active state could explain twitch potentiation, according to the classical view as type A potentiators (Hill 1949; Sandow 1965). While the prolongation of the AP remains the undiscussed mechanism of action for type B potentiators, the mechanism of action of type A potentiators requires some revision, in view of the increased knowledge about ECC. Nowadays, it is generally accepted that, in skeletal muscle, Ca2+ release from the SR is triggered by the mechanisms described in “The excitation–contraction coupling (ECC) mechanism in skeletal muscle”.
The demonstration that lyotropic anions had similar effects on intramembrane charge movement and ICa2+ supported the idea that the DHPR serve as voltage sensor for ECC (Ríos and Brum 1987; Delay et al. 1990). In contrast, ClO4 at concentrations lower than 10 mM selectively affected charge movement and contractile activation, without affecting activation of the AP (Ina) and activation of the delayed rectifier (Ik). SCN, the most potent of the lyotropic anion series, was found to be less selective than ClO4 -. In frog muscle fibres, NO3 and Zn2+ greatly increase the amplitude and duration of both Ca2+ transient and contractile responses while caffeine at 1 mM potentiates twitch amplitude, without affecting the response time course (Caputo and Bolaños, unpublished). In mammalian fibres, Ca2+ transients are potentiated in a similar way by lyotropic anions, while the effect of caffeine is much less robust.
With respect to caffeine, and other alkaloids, including ryanodine, it was known that, depending on the concentration, they could potentiate twitches or induce contractures by interacting directly with the RyR (Lüttgau and Oetliker 1968; Weber and Herz 1968). In amphibian skeletal muscle, two isoforms of RyR are expressed in similar amounts, RyR1 and RyR3. While RyR1 is strictly located in the triadic junctional region, RyR3 has also been found in the extrajunctional region. Thus, in frog muscle fibres, due to the alternate disposition of RyR and DHPR, half of the RyR located in the junctional region, plus the amount located extrajunctionally, are not coupled to DHPR, suggesting a different gating mechanism. Evidence has been obtained indicating that caffeine directly acts on the latter channels that serve as target for voltage-independent activation.
In mammalian muscle, RyR3 is only expressed during post-natal development, almost disappearing in adult animals, except for the case of few muscles, like diaphragm and soleus, possibly explaining why caffeine is much less effective than in amphibian muscles. In support of this possibility, it was shown that responses to caffeine decreased during mice post-natal development and in adult animals caffeine remained effective only in those muscles as soleus and diaphragm that contained RyR3 (Rossi et al. 2001).
In conclusion, contractile potentiators have provided valuable insight into the mechanisms pertaining ECC, such as the role of DHPR as the voltage sensors for ECC. More recently, they are helping in understanding the differential functional significance of RyR1 and RyR3.
Role of mitochondria in the ECC mechanism
Mitochondria are organelles present in most cell types, in variable number, depending on the metabolic function and energetic needs of the tissue. The development of new techniques such as electron microscopy 3D reconstructions, tomography, CLSM, targeted mitochondria fluorescent probes (i.e. GFP-fusion proteins), and other molecular biology techniques, have provided copious information on mitochondria structure, organization and relationship with other subcellular organelles such as the SR. In this section, besides presenting recent evidence about muscle mitochondria relationship with the SR, we have also aimed at covering mitochondrial channels and inward Ca2+ transport as part of a more complex ECC sequence.
The outer mitochondrial membrane (OMM), although traditionally considered freely permeable, is a critical determinant for the mitochondrial Ca2+ accumulation. Nowadays, it is known that Ca2+ import across the OMM occurs through a Voltage Dependent Anionic Channel (VDAC), first described by Schein et al. (1976), and later identified as a porin of 30-kDa (Zalman et al. 1980; Mannella et al. 1983; Rizzuto et al. 2009). Increased expression of VDAC enhances Ca2+ signal propagation into the mitochondria increasing the extent of mitochondrial Ca2+ uptake (Rapizzi et al. 2002).
The inner membrane (IMM) is semi-permeable and highly selective; besides containing all the machinery for oxidative phosphorylation, it also contains Ca2+ channels and transporters. The discovery of the electron chain transport and the chemiosmotic hypothesis formulation by Mitchell and Moyle (1967) led to the proposal that Ca2+ uptake was mediated by a Ca2+ channel, named Mitochondrial Calcium Uniporter (MCU), and driven by the large potential difference (–150 to –180 mV) established by the electrogenic extrusion of protons (Kirichok et al. 2004; Baughman et al. 2011; De Stefani et al. 2011).
MCU is a low affinity/high capacity (Kd of 10–20 μM) oligomeric complex, identified as a highly selective Ca2+ channel (Gunter and Pfeiffer 1990; Gunter et al. 1994; Bernardi 1999; Kirichok et al. 2004) formed by a 40-kDa protein with two transmembrane domains (Baughman et al. 2011; De Stefani et al. 2011). It was recently found that MCU of mitoplasts from skeletal muscle present higher conductance than from other tissues (Fieni et al. 2012). At rest, Ca2+ concentration in mitochondria and cytoplasm are similar, due to the activity of Ca2+ extruding systems and to the low activity of MCU under the normally low cytoplasmic Ca2+ conditions (Alonso et al. 2006). Because of its Kd, only when global Ca2+ rises above the micromolar level, the MCU is activated and a net uptake of Ca2+ by the mitochondria occurs, which is later slowly reversed, until reestablishment of the resting Ca2+ (Rizzuto and Pozzan 2006; Bolaños et al. 2009). The establishment of high Ca2+ microdomains around the sites of release by IP3R or RyR will favor the Ca2+ transport into mitochondria (Rizzuto et al. 1993; Rizzuto and Pozzan 2006; Drago et al. 2012).
A rapid mode of Ca2+ uptake (RaM) has been shown to occur in response to imposed Ca2+ signals in isolated liver and heart mitochondria (Sparagna et al. 1995; Bernardi 1999; Buntinas et al. 2001). The RaM activates transiently at the beginning of cytoplasmic Ca2+ pulses and is detectable above 200 nM in isolated heart mitochondria (Sparagna et al. 1995; Bernardi 1999; Buntinas et al. 2001). An additional Ca2+ uptake inhibited by ryanodine indicates the presence of mRyR within the IMM. This channel, which shares several similar biochemical, pharmacological, and physiological properties with both the RyR and RaM (Beutner et al. 2001) have been identified as RyR1 (Beutner et al. 2005). Both uptake modes (RaM and mRyR) exhibit kinetics, Ca2+ dependence, and pharmacology that allow them to be distinguished from the MCU (Ryu et al. 2010, 2011).
Two different antiporter systems responsible for exporting or importing Ca2+ have been described in mitochondria, the NCX (mNCX) which shares properties with the classical NCX (Jung et al. 1995; Smets et al. 2004) and is expressed in excitable cells, and the H/Ca2+ (mHCX) exchanger, present in non-excitable cells (Carafoli et al. 1974; Bernardi 1999; Jiang et al. 2009) and whose molecular identity is the Leucine zipper EF-hand transmembrane protein (Letm1). This protein allows mitochondrial Ca2+ uptake at nanomolar concentrations (Jiang et al. 2009).
Finally, the co-existence of low (MCU) and high-affinity (RaM, RyR and Letm1) modes of Ca2+ uptake into mitochondria, would allow different mitochondrial populations to take up different amounts of Ca2+ during cell activation, thus modulating Ca2+ signalling, depending on their location relative to Ca2+ stores and channels (Santo-Domingo and Demaurex 2010).
The permeability transient pore (PTP) (Hunter and Haworth 1979) is a large pore whose identity had remained elusive, and spans both IMM and OMM and is activated by Ca2+ overloading (Kinnally et al. 1989; Petronilli et al. 1989; Bernardi 1992, 1999; Saris and Carafoli 2005; Zoratti et al. 2005; Bernardi and von Stockum 2012). It can also operate as a Ca2+ release channel under physiological conditions (Bernardi and von Stockum 2012). Recently, it has been found that reconstituted dimers of the FoF1 ATP synthase form a channel with properties identical to those of the PTP (Giorgio et al. 2013).
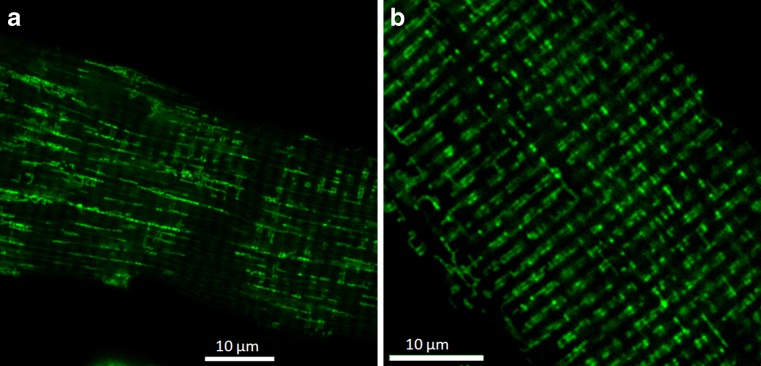
In skeletal muscle, mitochondria occupy 10–15 % of the fibre volume and are mainly located either peripherically in subsarcolemmal clusters or between myofibrils (Fig. 3), largely within the I-bands, surrounding the SR network (Gauthier and Padykula 1966; Eisenberg 1983). This structural arrangement is developmentally regulated, with mitochondria arranged in non-ordered longitudinal fashion in newborn mice (Boncompagni et al. 2009; Rossi et al. 2011). These intermyofibrillar mitochondria are highly organized in pairs at the I-band level, close to the CRU on either side of the Z-line, between T-Tubules, and contacting the SR near sites of Ca2+ uptake by the SERCA (Ogata and Yamasaki 1985; Ramesh et al. 1998; Vendelin et al. 2005; Franzini-Armstrong 2007; Bolaños et al. 2008). This arrangement favours fast ATP delivery to support Ca2+ transport into SR and to participate in Ca2+ homeostasis capturing Ca2+ from high Ca2+ microdomains near the sites of release (Rizzuto and Pozzan 2006; Shkryl and Shirokova 2006; Franzini-Armstrong 2007; Rossi et al. 2011; Yi et al. 2011; Drago et al. 2012). Subsarcolemmal mitochondria are organized in clusters, densely packed and less regularly arranged as compared with intermyofibrillar ones (Ogata and Yamasaki 1985; Kuznetsov et al. 2006). Skeletal muscle fibre types show differences in their mitochondria content being higher in slow-twitch fibres (Gauthier and Padykula 1966; Ogata and Yamasaki 1985), as well as morphological and functional diversity, existing specialisation in function between mitochondria from both slow-oxidative and fast-glycolitic fibre types (Kuznetsov et al. 2006; Picard et al. 2012).
Fig. 3.
Subsarcolemmal (left) and inner (right) differential mitochondrial distribution in flexor digitorum brevis muscle fibres stained with Mitotracker Green. The images were acquired with a Nikon C1 confocal microscope. A pattern of paired columns of mitochondria, parallel to the short axis of the cell, can be identified in the inner or intermyofibrillar location, while single, longer rows of mitochondria, parallel to the long axis of the cell, can be identified in the subsarcolemmal region of the cell
The close proximity of mitochondria to the SR and calcium release units has been studied in many preparations using many techniques (Franke and Kartenbeck 1971; Morre et al. 1971; Lewis and Tata 1973; Shore and Tata 1977; Meier et al. 1981; Mannella et al. 1998; Franzini-Armstrong and Boncompagni 2011). It was shown that as much as 20 % of the mitochondrial surface is in direct contact with the ER (Rizzuto et al. 1998) and that the maintenance of a proper spacing between ER and mitochondria seems to guarantee cell function and survival in some tissues (Csordás et al. 2006). In mouse FDB muscle fibres, strands of 3–4 nm diameter and 9–10 nm length (Franzini-Armstrong 2007; Boncompagni et al. 2009) appear to anchor mitochondria to the SR. The number of these tethers increases during postnatal development and might restrict mitochondrial movement away from sites of SR Ca2+ release, especially during contractile movements and shortening (Boncompagni et al. 2009; Rossi et al. 2011), providing a structural framework for bidirectional SR-mitochondrial signalling (Dirksen 2009b). In adult skeletal muscle, since mitochondria are anchored on the far side of the triads a diffusional distance of about ~130 nm separates the release sites from mitochondria (Franzini-Armstrong 2007), and a dissipation of Ca2+ microdomains could occur (Stern 1992). However, in developing muscle, this distance can be less than 100 nm and mitochondria could experience a significant Ca2+ release microdomain (Rossi et al. 2011).
Although the exact nature of tethers remains unresolved, it has been proposed that they are formed by a complex termed the “ER–mitochondria encounter complex” (ERMES), involving several proteins present in the OMM and in the ER that appear essential to keep the contact points between the two organelles in yeast (Kornmann et al. 2009). In mammals, silencing mitofusin disrupts ER morphology and loosens ER–mitochondria interactions, thereby reducing the efficiency of mitochondrial Ca2+ uptake in response to stimuli (de Brito and Scorrano 2008; see Eisner et al. 2013).
Mitochondrial Ca2+ uptake has been shown to occur during electrically elicited contractile responses in mouse muscle fibres, with a relatively short delay, 10 ms, between the Ca2+ signal in the myoplasm and that in the mitochondria (Rudolf et al. 2004; Yi et al. 2011). Transgenic mice expressing the Ca2+-sensitive bioluminescent reporter GFP-aequorin targeted to the mitochondrial matrix were studied in mice in vivo and showed a readily detected rapid Ca2+ rise inside the mitochondrial matrix during single-twitch muscle contractions (Rogers et al. 2007). In both slow- and fast-twitch fibres from rat, mitochondria are capable of accumulating Ca2+ in the presence of BAPTA that suppress Ca2+ transients but not mitochondrial Ca2+ increases, suggesting some kind of Ca2+ tunnelling from SR to mitochondria (Shkryl and Shirokova 2006).
In muscle cell lines and myotubes, mitochondria can pick up Ca2+ mobilised from a single RyR release unit (sparks) leading to the generation of single mitochondrial miniature Camyt signals (Ca2+ marks) and to feedback control on the Ca2+ release (Pacher et al. 2002; Isaeva et al. 2005).
Inhibition of mitochondrial Ca2+ uptake, either by blocking MCU with ruthenium red or by dissipating the mitochondrial membrane potential with protonophores like Carbonyl cyanide 4-trifluoromethoxy phenylhydrazone (FCCP), results in a large cytoplasmic Ca2+ increase with a slow rate of recovery (Rizzuto et al. 2000; Caputo and Bolaños 2008). In mice FDB muscle fibres, poisoning the mitochondria with FCCP (Fig. 4) causes a sizeable and rapid increase in the basal Ca2+ concentration followed by a marked decrease of Mag-Fluo-4 Ca2+ transients, and variable effects on the time course of Ca2+ transient decay, probably related to the composition of fibre types in FDB fibres (Caputo and Bolaños 2008; Calderón et al. 2009). Ca2+ uptake by mitochondria has also been observed in mice FDB fibres loaded with Rhod-2 and subjected to a SR depletion procedure to study SOCE (Fig. 5) or Ca2+ release in FDB fibres loaded with CaOrange-5N and exposed to the protonophore FCCP (Bolaños et al. 2008, 2009).
Fig. 4.
Time course of fluorescence decay after FCCP poisoning (right), in one flexor digitorum brevis fibre stained with TMRE to visualize mitochondrial potential (Ψm). On the left, confocal images of the cell indicating regions of interest (ROI, numbered squares) and time of acquisition after starting the experiment are shown. On the right, the black squares represent the mean±SEM of the measurements carried out at level of the ROI as a function of time. A rapid decrease in fluorescence, suggesting a dissipation of Ψm, is seen after the application of FCCP
Fig. 5.
Increase in intramitochondrial Ca2+ during a sarcoplasmic reticulum (SR) depletion protocol in a flexor digitorum brevis fibre imaged in a confocal microscope. a A pseudocolor image of mitochondria loaded with Rhod-2. b Summarizes the time course of the mitochondrial mean Rhod-2 fluorescence variation in the regions of interest (white squares) marked in (a). The black circles represent the mean fluorescence in the small white squares in (a), and the black squares the mean fluorescence in the big white square in (a). The mitochondrial fluorescence increases during the protocol in which the cell is in absence of external Ca2+ and the SR Ca2+ ATPase is blocked by cyclopiazonic acid (CPA). When 5 mM Ca2+ external solution reaches the cell the mitochondria uptake part of the Ca2+ entering the fibre via the store operated Ca2+ entry mechanism. K indicates the moments in which an external solution with high K+ concentration was applied to elicit SR Ca2+ release in order to deplete this Ca2+ store
Ca2+ mitochondria uptake after contractile activation during repetitive tetanic stimulation in toad and mouse fibres is reduced by FCCP poisoning (Lännergren et al. 2001; Bruton et al. 2003). In PV knockout mouse, the muscle fibres content of mitochondria doubles and the fibres appear to have an increased resistance to fatigue. The increased mitochondrial content results in a faster than expected removal of Ca2+ following brief tetanic stimulation (Racay et al. 2006).
Even when it is clear that mitochondria have a role in Ca2+ regulation during ECC and during contractile activity, their physiological relevance for different fibre types is not clear. Some have claimed that mitochondria may be relevant mainly in slow-twitch fibres, but others have not seen differential effect of blocking mitochondrial Ca2+ uptake in slow- and fast-twitch fibres during a tetani (Gillis 1997; Sembrowich et al. 1985; Lännergren et al. 2001; Calderón et al., unpublished results).
Future work may address the details of the SR-mitocondrial communication in skeletal muscle, and the tethers nature, considering also different fibre types, differences between subsets of mitochondria in skeletal muscle and different conditions such as fatigue, ageing and diseases.
SOCE-mitochondria-ECC relationship
SOCE was originally described in non-excitable cells, where it constitutes a major pathway for Ca2+ influx. It was denominated capacitative calcium influx (ICRAC) and characterised as a very small but highly Ca2+ selective current that insures the replenishment of the ER (Putney 1986; Hoth and Penner 1992; Parekh and Putney 2005).
SOCE has also been demonstrated in excitable cells (Bernardi 1999; Rizzuto et al. 2000; Parekh and Putney 2005) as adult skeletal muscle fibres (Kurebayashi and Ogawa 2001; Ma and Pan 2003; Ducret et al. 2006; Gonzalez Narvaez and Castillo 2007; Launikonis and Ríos 2007; Bolaños et al. 2009; Dirksen 2009a), though there is less information on the regulatory mechanisms involved in SOCE activation in excitable cells, in particular adult skeletal muscle fibres, than in non-excitable cells.
The molecules involved in the SOCE complex have been identified. The first one, the stromal interacting molecule STIM1, present in the ER/SR membrane, senses the degree of filling of Ca2+ in the ER/SR (Liou et al. 2005; Roos et al. 2005; Zhang et al. 2005). The second, Orai1, has been identified as the conductive pore sub-unit of the ICRAC channel (Feske et al. 2006; Prakriya et al. 2006; Vig et al. 2006). In mammals, two STIM genes and three Orai genes have been identified (Zhang et al. 2005; Feske et al. 2006; Vig et al. 2006). When Ca2+ levels are low, STIM1 clusters in regions of the ER/plasma membrane junctions denominated punctae, where it interacts with Orai1 activating Ca2+-influx (Luik et al. 2006; Smyth et al. 2006; Soboloff et al. 2006; Muik et al. 2009). In endothelial cells exposed to FCCP and/or oligomicin, STIM1–Orai1-dependent SOCE is completely prevented, thus pointing to an essential contribution of mitochondrial Ca2+ handling to STIM1–Orai1-dependent SOCE. These finding suggest that a Ca2+-dependent process in the mitochondria unlike (or in addition to) local Ca2+ buffering is essential and specific for the activity of the STIM1–Orai1-dependent SOCE (Naghdi et al. 2010).
In skeletal muscle, STIM1 and Orai1 are highly expressed (Stiber et al. 2008; Vig et al. 2008) and are localized at triadic level and in the SR terminal cisternae. In this tissue, SOCE activation and deactivation is in the order of milliseconds indicating that STIM1 and Orai1 must be evenly distributed throughout the junctional membrane and can activate rapidly (Launikonis and Ríos 2007; Stiber et al. 2008; Edwards et al. 2010). Two models that allow for rapid SOCE activation upon SR Ca2+-store depletion were proposed by Dirksen (2009a). The first one states that STIM1 monomers are prelocalized at SR terminal cisternae in the vicinity of inactive Orai1 channels at the triadic junctions. When Ca2+ dissociates from STIM1, in response to SR depletion, their conformational change and oligomerization will permit the activation of Orai1 also prelocalized in the T-tubule membrane. The second model proposes that STIM1/Orai1 complex exists preformed but inactive until SR Ca2+ depletion reaches a certain level, triggering direct activation of Orai1-mediated Ca2+ influx. This last possibility would allow an ultrafast, very efficient and controlled activation of Ca2+ influx through Orai1 (Launikonis and Ríos 2007; Dirksen 2009a; Edwards et al. 2010).
Though it seems that SOCE has no physiological role in skeletal muscle short-term activation, and it is not required to refill SR (Cully and Launikonis 2013) Ca2+ entry through SOC is crucial for long-term Ca2+ homeostasis, such that reduced SOC activity exaggerates muscle fatigue under conditions of intensive exercise (Pan et al. 2002).
In FDB adult mouse skeletal muscle fibres, FCCP mitochondria poisoning increases the Ca2+ myoplasmic levels, reduces the amplitude of Ca2+ transients and reduces SOCE (Fig. 6) (Bolaños et al. 2008, 2009; Caputo and Bolaños 2008). This effect has been explained in terms of different mechanisms: (1) reduction of Ca2+ uptake by mitochondria increases cytoplasmic Ca2+, thus favouring Ca2+ dependent inactivation of the SOC channels (Hoth et al. 2000); (2) removal of mitochondrial competition with SERCA pumps that favors store replenishment (Parekh 2003); and (3) inhibition of some factor produced by functional mitochondria necessary for SOCE activation (Glitsch et al. 2002; Naghdi et al. 2010). The close functional relationship between SR and mitochondria (Isaeva and Shirokova 2003; Shkryl and Shirokova 2006; Bolaños et al. 2008; Boncompagni et al. 2009; Rossi et al. 2011) facilitates the establishment of microdomains (Rizzuto et al. 1993; Berridge 2006; Rizzuto and Pozzan 2006) and the uptake of Ca2+ by mitochondria immediately after its release from the SR or the influx through SOCs, before it can diffuse out the restricted space of the SR–mitochondrial junctions.
Fig. 6.
Activation of store-operated Ca2+ entry (SOCE) in a flexor digitorum brevis fibre loaded with Fura-2 after sarcoplasmic reticulum (SR) depletion by high K+ exposures in the absence of external Ca2+ and in the presence of thapsigargin (TG) 5-10 μM. a Once depleted as shown by the absence of response to high K+, Ca2+ reintroduction in the external medium activates SOCE. The Ca2+ entrance can be reversibly blocked by 80 μM 2-APB. b When a similar protocol is applied to another fibre, but exposed to FCCP, SOCE cannot be activated again. It can be partially recovered after a long washout of the drug. This suggests that the mitochondrial depolarization affects the Ca2+ entry induced by SR depletion
In spite of recent studies claiming that SOCE is not relevant in skeletal muscle for SR Ca2+ replenishment (Cully and Launikonis 2013), all the machinery needed to activate it is present at the triad. On the other hand, it is known that SOCE activation requires fully energized mitochondria in excitable and non-excitable tissues (Glitsch et al. 2002; Bolaños et al. 2009; Naghdi et al. 2010). Future work must be addressed to clarify not only the physiological relevance of SOCE but also its modulation by mitochondria and/or other components involved in ECC in skeletal muscle.
Ca2+ sparks
Ca2+ sparks were first described as minute, spontaneous fluorescence signals originating from highly restricted zones of cardiac myocytes (Cheng et al. 1993). They could also be elicited, at a much higher frequency, by cell membrane depolarization. The occurrence of the events did not depend on external Ca2+ or on Ca2+ entry through the L-type Ca2+ channels. The properties of the sparks, and the localization of their origin, at the level of the junctional region between SR and T-tubules, led to the conclusion that they derived from point Ca2+ sources, and constituted the elementary Ca2+ release events for ECC, resulting from the transient openings of Ca2+ release channels. In the heart, these channels were identified as the RyR2, activated via a CICR mechanism, by Ca2+ entering the myocytes through the DHPR (Cheng and Lederer 2008).
Ca2+ sparks were also demonstrated in amphibian striated muscle fibres (Tsugorka et al. 1995), where they could occur spontaneously or be evoked by membrane depolarization; in both cases, their spatio-temporal characteristics were similar and were not affected by the level of the membrane potential in the range from –80 and +40 mV (LaCampagne et al. 1996). The frequency of spark generation was potential-dependent and could be modulated by Ca2+ that increased it and Mg2+ that had the opposite effect (Zhou et al. 2004).
Contrary to the initial idea that one spark corresponded to the opening of a single release channel, Shtifman et al. (2000) estimated the number of release channels contributing to the generation of one event to be between 2 and 4. Moreover, the idea that one spark represented one quantum of Ca2+ release was also challenged by the demonstration of diffused Ca2+ release, not associated with defined sparks, both in cardiac (Lipp and Niggli 1996) and in skeletal (Shirokova and Ríos 1997) muscle fibres.
Also in skeletal muscle, Ca2+ sparks occur at level of the triadic junctional region (see “The excitation–contraction coupling (ECC) mechanism in skeletal muscle”). Due to the alternate disposition of the RyR and DHPR arrays, for each tetrameric molecule facing, and under control of, a tetrad, there is one molecule that is not. Furthermore, the presence of another RyR isoform, RyR3, that forms arrays in the extra-junctional regions of amphibian skeletal muscle fibres, increases the fraction of Ca2+ release channels that are not under control of the DHPR (Felder and Franzini-Armstrong 2002).
Opposite to the case of frog muscles, Ca2+ sparks are rarely observed in mammalian muscle under physiological conditions. Instead, release events not composed of Ca2+ sparks were observed in response to membrane depolarization or exposure to caffeine leading to the proposal that in rats this release was the unique response to voltage activation, while in frog the initial release events served as a trigger for CICR responses visualised as sparks. The idea that RyR3 was associated with spark generation in amphibian was proved correct by the demonstration that exogenous expression of RyR3 in adult mouse muscle caused an abundance of sparks in response to depolarisation, changing a spark-free mammalian response, into a frog one (Pouvreau et al. 2007b).
On the other hand, in mammalian muscle, Ca2+ sparks could be observed under special conditions, such as permeabilization by saponine, osmotic stress, membrane damage and mitochondrial metabolic uncoupling (Apostol et al. 2009; Weisleder 2012; Isaeva and Shirokova 2003), demonstrating that the machinery and capacity for spark generation are present in mammalian muscle, but somehow repressed. In favour of this view, it has been shown that, during myotubes differentiation, the presence of T-tubules determine the absence of Ca2+ sparks and of RyR1 and RyR3, that abound where T-tubules are not present (Zhou et al. 2006).
Although the specific mechanisms by which RyR1 and RyR3 operate have not yet been clarified, there is strong evidence pointing to the importance of RyR3 in determining the mode of Ca2+ release.
Perspectives
Some tasks still remain to gain full knowledge on ECC. The search for new minor ECC proteins (Treves et al. 2009) and to study their role in fatigue, ageing and diseases, as well as their variability according to fibre types, should continue. The application of ECC knowledge is of paramount importance to fight muscle diseases and help in conditions such as ageing (Wang et al. 2012; Mosca et al. 2013). To develop a mathematical model on ECC that integrates most of the recent information gathered on ECC under different conditions will allow us to better understand and manipulate Ca2+ kinetics. Also, a better understanding of the relationsip between ECC machinery and muscle biochemical and metabolic functions (besides the production of ATP) is an important endeavor that deserves full attention in the coming years. Considering that many RyR are not directly coupled to DHPR, and that CICR is not operational in mammalian muscle under physiological conditions, the puzzle that remains to be solved is whether and how the DHPR-coupled RyR signal the uncoupled ones to synchronize Ca2+ release. In addressing many of these questions it is likely that the use of super-resolution microscopy and the improvement of its time-resolution will allow for a new era of physiological studies.
Conclusions
In conclusion, the ECC mechanism represents a rapid communication between electrical events occurring in the plasma membrane and the Ca2+ release from the SR, which leads to skeletal muscle contraction. A large body of knowledge on the concerted function of the main macromolecules and some minor molecules involved in ECC mechanism has been gathered as a result of six decades of research. Additionally, ECC understanding will benefit from the continuous gain in temporal and spatial resolution of most of the techniques currently used for ECC study. The application of the knowledge gathered on ECC will likely help understand the pathophysiology of some muscle diseases and develop tools to fight them.
Acknowledgments
The financial support comes from University of Antioquia, Medellín, Colombia (J.C.C.) and Venezuelan Institute for Scientific Research, Venezuela (P.B. and C.C.). We want to acknowledge Alis Guillén for help in obtaining some experimental results presented in this review and Carolina Figueroa por sharing some information with us.
Conflict of interest
Juan C. Calderón, Pura Bolaños and Carlo Caputo declare that they have no conflict of interest.
Human and animal studies
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Special Issue Advances in Biophysics in Latin America
References
- Abbiss C, Laursen P. Models to explain fatigue during prolonged endurance cycling. Sports Med. 2005;35:865–898. doi: 10.2165/00007256-200535100-00004. [DOI] [PubMed] [Google Scholar]
- Adams B, Tanabe T, Mikami A, Numa S, Beam K. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Adrian R, Costantin L, Peachey L. Radial spread and contraction in frog muscle fibres. J Physiol. 1969;204:231–257. doi: 10.1113/jphysiol.1969.sp008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D, Lee J, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D, Lännergren J, Westerblad H. The role of ATP in the regulation of intracellular Ca2+ release in single fibres of mouse skeletal muscle. J Physiol. 1997;498:587–600. doi: 10.1113/jphysiol.1997.sp021885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D, Lännergren J, Westerblad H. Intracellular ATP measured with luciferin/luciferase in isolated single mouse skeletal muscle fibres. Pflugers Arch. 2002;443(5–6):836–842. doi: 10.1007/s00424-001-0756-y. [DOI] [PubMed] [Google Scholar]
- Allen D, Lamb G, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Alonso M, Villalobos C, Chamero P, Alvarez J, Garcia-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006;40:513–525. doi: 10.1016/j.ceca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Anderson A, Altafaj X, Zheng Z, Wang Z, Delbono O, Ronjat M, et al. The junctional SR protein JP-45 affects the functional expression of the voltage-dependent Ca2+ channel Cav1.1. J Cell Sci. 2006;119:2145–2155. doi: 10.1242/jcs.02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol S, Ursu D, Lehmann-Horn F, Melzer W. Local calcium signals induced by hyper-osmotic stress in mammalian skeletal muscle cells. J Muscle Res Cell Motil. 2009;30:97–109. doi: 10.1007/s10974-009-9179-8. [DOI] [PubMed] [Google Scholar]
- Appelt D, Buenviaje B, Champ C, Franzini-Armstrong C. Quantitation of “junctional feet” content in two types of muscle fiber from hind limb muscles of the rat. Tissue Cell. 1989;21:783–794. doi: 10.1016/0040-8166(89)90087-6. [DOI] [PubMed] [Google Scholar]
- Armstrong C, Bezanilla F, Horowitz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N, N′-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Ávila G, Dirksen R. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J Gen Physiol. 2000;114:467–480. doi: 10.1085/jgp.115.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J, Thesleff S. Activation of the contractile mechanism in striated muscle. Acta Physiol Scand. 1958;44:55–66. doi: 10.1111/j.1748-1716.1958.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Balnave C, Allen D. Evidence for Na+/Ca2+ Exchange in intact single skeletal muscle fibers from the mouse. Am J Physiol Cell Physiol. 1998;274:940–946. doi: 10.1152/ajpcell.1998.274.4.C940. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Juel C. Lactic acid accumulation is a disadvantage during muscle activity. J Appl Physiol. 2006;100:1412–1413. doi: 10.1152/japplphysiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- Bär A, Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett. 1988;235:153–155. doi: 10.1016/0014-5793(88)81253-5. [DOI] [PubMed] [Google Scholar]
- Barclay J, Hansel M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can J Physiol Pharmacol. 1991;69:279–284. doi: 10.1139/y91-043. [DOI] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S, Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S, Hollingworth S. Calcium indicators and calcium signalling in skeletal muscle fibres during excitation-contraction coupling. Prog Biophys Mol Biol. 2011;105:162–179. doi: 10.1016/j.pbiomolbio.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K, Franzini-Armstong C. Functional and structural approaches to the study of excitation-contraction coupling. Methods Cell Biol. 1997;52:283–306. doi: 10.1016/s0091-679x(08)60384-2. [DOI] [PubMed] [Google Scholar]
- Beam K, Knudson C, Powell J. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986;320:168–170. doi: 10.1038/320168a0. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Betz W. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977;271:25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström M, Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol. 1988;65:1500–1505. doi: 10.1152/jappl.1988.65.4.1500. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–8839. [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bernardi P, von Stockum S. The permeability transition pore as a Ca(2+) release channel: new answers to an old question. Cell Calcium. 2012;52:22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Beutner G, Sharma V, Giovannucci D, Yule D, Sheu S. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- Beutner G, Sharma V, Lin L, Ryu S, Dirksen R, Sheu S. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005;1717:1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Caputo C, González-Serratos H, Venosa R. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. J Physiol. 1972;223:507–523. doi: 10.1113/jphysiol.1972.sp009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Woods J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7:691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- Bleunven C, Treves S, Jinyu X, Leo E, Ronjat M, De Waard M, et al. SRP-27 is a novel component of the supramolecular signaling complex involved in skeletal muscle excitation-contraction coupling. Biochem J. 2008;411:343–349. doi: 10.1042/BJ20070906. [DOI] [PubMed] [Google Scholar]
- Block B, Imagawa T, Campbell K, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños P, Guillén A, Rojas H, Boncompagni S, Caputo C. The use of CalciumOrange-5N as a specific marker of mitochondrial Ca2+ in mouse skeletal muscle fibers. Pflugers Arch. 2008;455:721–731. doi: 10.1007/s00424-007-0312-5. [DOI] [PubMed] [Google Scholar]
- Bolaños P, Guillén A, DiPolo R, Caputo C. Factors affecting SOCE activation in mammalian skeletal muscle fibers. J Physiol Sci. 2009;59:317–328. doi: 10.1007/s12576-009-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompagni S, Rossi A, Micaroni M, Beznoussenko G, Polishchuk R, Dirksen R, Protasi F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompagni S, Thomas M, Lopez J, Allen P, Yuan Q, Kranias E, Franzini-Armstrong C, Perez C. Triadin/Junctin double null mouse reveals a differential role for Triadin and Junctin in anchoring CASQ to the jSR and regulating Ca(2+) homeostasis. PLoS ONE. 2013;7:e39962. doi: 10.1371/journal.pone.0039962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- Brochet D, Yang D, Di Maio A, Lederer W, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci U S A. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M, Kaiser K. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Brotto M, Nosek T. Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. J Appl Physiol. 1996;81:731–737. doi: 10.1152/jappl.1996.81.2.731. [DOI] [PubMed] [Google Scholar]
- Bruton J, Tavi P, Aydin J, Wasterblad H, Lanergren J. Mitochondrial and myoplasmic [Ca2+] in single fibers from Mouse limb muscles during repeated tetanic contraction. J Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Place N, Yamada T, Silva J, Andrade F, Dahlstedt A, Zhang S, Katz A, Larsson N, Westerblad H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol. 2008;586:175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Cheng A, Westerblad H. Methods to detect Ca2+ in living cells. Adv Exp Med Biol. 2012;740:27–43. doi: 10.1007/978-94-007-2888-2_2. [DOI] [PubMed] [Google Scholar]
- Buck E, Nguyen H, Pessah I, Allen P. Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function. J Biol Chem. 1997;272:7360–7367. doi: 10.1074/jbc.272.11.7360. [DOI] [PubMed] [Google Scholar]
- Buntinas L, Gunter K, Sparagna G, Gunter T. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta. 2001;1504:248–261. doi: 10.1016/s0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- Burke R, Levine D, Tsairis P, Zajac F. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón J. Enzymatic dissociation of long muscles from mouse: a model for the study of fiber types in skeletal muscle. Iatreia. 2013;26:117–126. [Google Scholar]
- Calderón JC, Bolaños P, Torres SH, Rodriguez-Arroyo G, Caputo C. Different fibre populations distinguished by their calcium transient characteristics in enzymatically dissociated murine flexor digitorum brevis and soleus muscles. J Muscle Res Cell Motil. 2009;30:125–137. doi: 10.1007/s10974-009-9181-1. [DOI] [PubMed] [Google Scholar]
- Calderón JC, Bolaños P, Caputo C. Myosin heavy chain isoform composition and Ca2+ transients in fibres from enzymatically dissociated murine soleus and extensor digitorum longus muscles. J Physiol. 2010;588(1):267–279. doi: 10.1113/jphysiol.2009.180893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón JC, Bolaños P, Caputo C. Kinetic changes in tetanic calcium transients in enzymatically dissociated muscle fibres under repetitive stimulation. J Physiol. 2011;589(21):5269–5283. doi: 10.1113/jphysiol.2011.213314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón J, Raigosa D, Giraldo M, Bolaños P, Caputo C. Calibration of Ca2+ transients obtained with the fast Ca2+ and Mg2+ dye Magfluo-4. Biophys J. 2013;104(2–S1):293a. [Google Scholar]
- Calderón-Vélez J, Figueroa-Gordon C. El acoplamiento exitación-contracción en el músculo esquelético: preguntas por responder a pesar de 50 años de studio. Biomedica. 2009;29:140–160. [PubMed] [Google Scholar]
- Caputo C. Pharmacological investigations of excitation-contraction coupling. Chapter 14. In: Peachey L, Adrian R, editors. Handbook of physiology. Bethesda: American Physiological Society; 1983. [Google Scholar]
- Caputo C, Bolaños P. Effect of mitochondria poisoning by FCCP on Ca2+ signaling in mouse skeletal muscle fibers. Pflugers Arch. 2008;455:733–743. doi: 10.1007/s00424-007-0317-0. [DOI] [PubMed] [Google Scholar]
- Caputo C, Giménez M. Effects of external Ca2+ deprivation on single muscle fibres. J Gen Physiol. 1967;50:2177–2195. doi: 10.1085/jgp.50.9.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Edman K, Lou F, Sun Y. Variation in myoplasmic Ca concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. J Physiol. 1994;478:137–148. doi: 10.1113/jphysiol.1994.sp020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Bolaños P, Escobar A. Fast calcium removal during single twitches in amphibian skeletal muscle fibres. J Muscle Res Cell Motil. 1999;20:555–567. doi: 10.1023/a:1005526202747. [DOI] [PubMed] [Google Scholar]
- Caputo C, Bolaños P, González A. Inactivation of Ca2+ transients in amphibian and mammalian muscle fibres. J Muscle Res Cell Motil. 2004;25:315–328. doi: 10.1007/s10974-004-4071-z. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Tiozzo R, Lugli G, Crovetti F, Kratzing C. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol. 1974;6:361–371. doi: 10.1016/0022-2828(74)90077-7. [DOI] [PubMed] [Google Scholar]
- Carroll S, Klein M, Schneider M. Decay of calcium transients after electrical stimulation in rat fast- and slow-twitch skeletal muscle fibres. J Physiol. 1997;501:573–588. doi: 10.1111/j.1469-7793.1997.573bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto M, Cui Y, Karunasekara Y, Harvey P, Norris N, Borrad P, et al. Structural and functional characterization of interactions between the dihydropyridine receptor II-III loop and the ryanodine receptor. Clin Exp Pharmacol Physiol. 2006;33:1114–1117. doi: 10.1111/j.1440-1681.2006.04501.x. [DOI] [PubMed] [Google Scholar]
- Casas M, Figueroa R, Jorquera G, Escobar M, Molgó J, Jaimovich E. IP(3)-dependent, post-tetanic calcium transients induced by electrostimulation of adult skeletal muscle fibers. J Gen Physiol. 2010;136:455–467. doi: 10.1085/jgp.200910397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer W. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer W, Cannell M. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheung A, Dantzig J, Hollingworth S, Baylor S, Goldman Y, Mitchison T, Straight A. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–89. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Chin E, Allen D. The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. J Physiol. 1998;512:831–840. doi: 10.1111/j.1469-7793.1998.831bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R, Morrissette J, Sukhareva, Vaughan D. Structure and function of ryanodine receptors. Am J Physiol. 1994;266:C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Craig R, Padrón R. Molecular structure of the sarcomere. Chapter 7. In: Engel A, Franzini-Armstrong C, editors. Myology. 3. New York: McGrawHill; 2004. pp. 129–166. [Google Scholar]
- Csordás G, Renken C, Várnai P, Walter L, Weaver D, Buttle K, Balla T, Mannella C, Hajnóczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully T, Launikonis B. Store-operated Ca2+ entry is not required for store refilling in skeletal muscle. Clin Exp Pharmacol Physiol. 2013;40:338–344. doi: 10.1111/1440-1681.12078. [DOI] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Westerblad H. Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. J Physiol. 2001;533:379–388. doi: 10.1111/j.1469-7793.2001.0379a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Margreth A. Characterization study of the ryanodine receptor and of calsequestrin isoforms of mammalian skeletal muscles in relation to fibre types. J Muscle Res Cell Motil. 1994;15:86–101. doi: 10.1007/BF00130421. [DOI] [PubMed] [Google Scholar]
- Darnley G, Duke A, Steele D, MacFarlane N. Effects of reactive oxygen species on aspects of excitation-contraction coupling in chemically skinned rabbit diaphragm muscle fibres. Exp Physiol. 2001;86:161–168. doi: 10.1113/eph8602109. [DOI] [PubMed] [Google Scholar]
- Davies K, Quintanilha A, Brooks G, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- de Brito O, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay M, Garcia D, Sanchez J. The effects of lyotropic anion on charge movement, calcium currents and calcium signals in frog skeletal muscle fibres. J Physiol. 1990;425:449–469. doi: 10.1113/jphysiol.1990.sp018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O, Stefani E. Calcium transients in single mammalian skeletal muscle fibres. J Physiol. 1993;463:689–707. doi: 10.1113/jphysiol.1993.sp019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco M, Neco P, Capote J, Meera P, Vergara J. Quantitative evaluation of mammalian skeletal muscle as a heterologous protein expression system. Protein Expr Purif. 2006;47:281–288. doi: 10.1016/j.pep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- DiFranco M, Tran P, Quiñonez M, Vergara J. Functional expression of transgenic 1sDHPR channels in adult mammalian skeletal muscle fibres. J Physiol. 2011;589:1421–1442. doi: 10.1113/jphysiol.2010.202804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen R. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol. 2009;587:3139–3147. doi: 10.1113/jphysiol.2009.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen R. Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:389–395. doi: 10.1139/h09-044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D, Morais Cabral J, Pfuetzner R, Kuo A, Gulbis J, Cohen S, et al. The structure of potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci U S A. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V, Pearse A. A comparative histochemical study of oxidative enzyme and phophorylase activity in skeletal muscle. Histochemie. 1960;2:105–117. doi: 10.1007/BF00744575. [DOI] [PubMed] [Google Scholar]
- Ducret T, Vandebrouck C, Cao M, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol. 2006;575:913–924. doi: 10.1113/jphysiol.2006.115154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke A, Steele D. Characteristics of phosphate-induced Ca(2+) efflux from the SR in mechanically skinned rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;278:C126–C135. doi: 10.1152/ajpcell.2000.278.1.C126. [DOI] [PubMed] [Google Scholar]
- Dulhunty A, Banyard M, Medveczky C. Distribution of calcium ATPase in the sarcoplasmic reticulum of fast- and slow-twitch muscles determined with monoclonal antibodies. J Membr Biol. 1987;99:79–92. doi: 10.1007/BF01871228. [DOI] [PubMed] [Google Scholar]
- Dutka T, Cole L, Lamb G. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Ebashi S. Regulatory mechanism of muscle contraction with special reference to the Ca-troponin-tropomyosin system. Essays Biochem. 1974;10:1–36. [PubMed] [Google Scholar]
- Ebashi S, Endo M, Ohtsuki I. Control of muscle contraction. Q Rev Biophys. 1969;2:351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Edman K. Contratile properties of mouse single muscle fibers, a comparison with amphibian muscle fibers. J Exp Biol. 2005;208:1905–1913. doi: 10.1242/jeb.01573. [DOI] [PubMed] [Google Scholar]
- Edwards J, Murphy R, Cully T, von Wegner F, Friedrich O, Launikonis B. Ultra-rapid activation and deactivation of store-operated Ca(2+) entry in skeletal muscle. Cell Calcium. 2010;47:458–467. doi: 10.1016/j.ceca.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Edwards J, Cully T, Shannon T, Stephenson D, Launikonis B. Longitudinal and transversal propagation of excitation along the tubular system of rat fast-twitch muscle fibres studied by high speed confocal microscopy. J Physiol. 2012;590:475–492. doi: 10.1113/jphysiol.2011.221796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, editor. Handbook of Physiology Skeletal Muscle. Bethesda: American Physiological Society; 1983. p. 95. [Google Scholar]
- Eisner V, Csordás G, Hajnóczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca2+ and reactive oxygen species signaling. J Cell Sci. 2013;126:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Hayek R, Antoniu B, Wang J, Hamilton SL, Ikemoto N. Identification of calcium release-triggering and blocking regions of the II-III loop of the skeletal muscle dihydropyridine receptor. J Biol Chem. 1995;270:22116–22118. doi: 10.1074/jbc.270.38.22116. [DOI] [PubMed] [Google Scholar]
- Endo M. Mechanism of action of caffeine on the sarcoplasmic reticulum of skeletal muscle. Proc Jpn Acad. 1975;51:479–484. [Google Scholar]
- Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev. 2009;89:1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Escobar A, Monck J, Fernandez J, Vergara J. Localization of the site of Ca2+ release at the level of a single sarcomere in skeletal muscle fibres. Nature. 1994;367:739–741. doi: 10.1038/367739a0. [DOI] [PubMed] [Google Scholar]
- Eusebi F, Miledi R, Takahashi T. Calcium transients in mammalian muscles. Nature. 1980;284:560–561. doi: 10.1038/284560a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Dependence of the Ca2+-induced release from the sarcoplasmic reticulum of skinned skeletal muscle fibres from the frog semitendinosus on the rate of change of free Ca2+ concentration at the outer surface of the sarcoplasmic reticulum. J Physiol. 1984;353:56P. [Google Scholar]
- Farkas D, Wei M, Febbroriello P, Carson J, Loew L. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1989;56:1053–1069. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder E, Franzini-Armstrong C. Type 3 ryanodine receptors of skeletal muscle are segregated in a parajunctional position. Proc Natl Acad Sci U S A. 2002;99:1695–1700. doi: 10.1073/pnas.032657599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D, Franzini-Armstrong C. The Ca2+ ATPase content of slow and fast twitch fibers of guinea pig. Muscle Nerve. 1988;11:561–570. doi: 10.1002/mus.880110607. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S, Tanasa B, Hogan P, Lewis R, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun. 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa L, Shkryl VM, Zhou J, Manno C, Momotake A, Brum G, Blatter LA, Ellis-Davies GC, Ríos E. Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle. J Physiol. 2012;590:1389–1411. doi: 10.1113/jphysiol.2011.225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M, Copello J. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Fitts R. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Foulks J, Miller J, Perry F. Repolarization-induced reactivation ofcontracture tension in frog skeletal muscle. Can J Physiol Pharmacol. 1973;51:324–334. doi: 10.1139/y73-049. [DOI] [PubMed] [Google Scholar]
- Fourest-Lieuvin A, Rendu J, Osseni A, Pernet-Gallay K, Rossi D, Oddoux S, Brocard J, Sorrentino V, Marty I, Fauré J. Role of triadin in the organization of reticulum membrane at the muscle triad. J Cell Sci. 2012;125:3443–3453. doi: 10.1242/jcs.100958. [DOI] [PubMed] [Google Scholar]
- Franke W, Kartenbeck J. Outer mitochondrial membrane continuous with endoplasmic reticulum. Protoplasma. 1971;73:35–41. doi: 10.1007/BF01286409. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. The sarcoplasmic reticulum and the control of muscle contraction. FASEB J. 1999;13:S266–S270. doi: 10.1096/fasebj.13.9002.s266. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. ER-mitochondria communication. How privileged? Physiology (Bethesda) 2007;22:261–268. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Boncompagni S. The evolution of the mitochondria-to-calcium release units relationship in vertebrate skeletal muscles. J Biomed Biotechnol. 2011;2011:830573. doi: 10.1155/2011/830573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Jorgensen A. Structure and development of e-c coupling units in skeletal muscle. Annu Rev Physiol. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Porter K. Sarcolemmal invaginations constituting the T system in fish muscle fibres. J Cell Biol. 1964;22:675–696. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Ferguson D, Champ C. Discrimination between fast- and slow-twitch fibres of guinea pig skeletal muscle using the relative surface density of junctional transverse tubule membrane. J Muscle Res Cell Motil. 1988;9:403–414. doi: 10.1007/BF01774067. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Rouaud T, Millour M, Fontaine-Pérus J, Gardahaut M, Levitsky D. Expression of the Na+/Ca2+ exchanger in skeletal muscle. Am J Physiol. 2001;280:C146–C154. doi: 10.1152/ajpcell.2001.280.1.C146. [DOI] [PubMed] [Google Scholar]
- Fryer M, Stephenson G. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer M, Owen V, Lamb G, Stephenson G. Effects of creatine phosphate and Pi on Ca movements and tension development in rat skinned skeletal muscle fibres. J Physiol. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füchtbauer E, Rowlerson A, Gotz K, Friedrich G, Mabuchi K, Gergely J, Jockusch H. Direct correlation of parvalbumin levels with myosin isoforms and succinate dehydrogenase activity on frozen sections of rodent muscle. J Histochem Cytochem. 1991;39:355–361. doi: 10.1177/39.3.1825216. [DOI] [PubMed] [Google Scholar]
- Gauthier G, Padykula H. Cytological studies of fiber types in skeletal muscle. J Cell Biol. 1966;28:333–354. doi: 10.1083/jcb.28.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J. Inhibition of mitochondrial calcium uptake slows down relaxation in mitochondria-rich skeletal muscles. J Muscle Res Cell Motil. 1997;18:473–483. doi: 10.1023/a:1018603032590. [DOI] [PubMed] [Google Scholar]
- Gillis J, Thomason D, Lefévre J, Kretsinger R. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982;3:377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick G, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M, Bakowski D, Parekh A. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;21:6744–6754. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P, Korge P, Karpakka J, Saltin B. Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man. Acta Physiol Scand. 1991;142:135–136. doi: 10.1111/j.1748-1716.1991.tb09139.x. [DOI] [PubMed] [Google Scholar]
- Gomolla M, Gottschalk G, Lüttgau H. Perchlorate-induced alterations in electrical and mechanical parameters of frog skeletal muscle fibres. J Physiol. 1983;343:197–214. doi: 10.1113/jphysiol.1983.sp014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Narvaez A, Castillo A. Ca2+ store determines gating of store operated calcium entry in mammalian skeletal muscle. J Muscle Res Cell Motil. 2007;28:105–113. doi: 10.1007/s10974-007-9105-x. [DOI] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971;212:777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonasekera S, Beard N, Groom L, Kimura T, Lyfenko A, Rosenfeld A, Marty I, Dulhunty A, Dirksen R. Triadin binding to the C-terminal luminal loop of the ryanodine receptor is important for skeletal muscle excitation-contraction coupling. J Gen Physiol. 2007;130:365–378. doi: 10.1085/jgp.200709790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski W, Lobsiger E, Luttgau H. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflugers Arch. 1972;334:222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- Green H. Cation pumps in skeletal muscle: potential role in muscle fatigue. Acta Physiol Scand. 1998;162:201–213. doi: 10.1046/j.1365-201X.1998.0300f.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien R. A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gunter T, Pfeiffer D. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Gunter T, Gunter K, Sheu S, Gavin C. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Gustafsson M. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J Microsc. 2000;198:82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- Hasselbach W. Relaxing factor and the relaxation of muscle. Prog Biophys Mol Biol. 1964;14:167–222. [Google Scholar]
- Hasselbach W. The Ca2+-ATPase of the sarcoplasmic reticulum in skeletal and cardiac muscle. Ann NY Acad Sci. 1998;853:1–8. doi: 10.1111/j.1749-6632.1998.tb08251.x. [DOI] [PubMed] [Google Scholar]
- Hasselbach W, Makinose M. The calcium pump of the “relaxing granules” of muscle and its dependence on ATP splitting. Biochem Z. 1961;333:518–528. [PubMed] [Google Scholar]
- Hasselbach W, Suko J, Stromer M, The R. Mechanism of calcium transport in sarcoplasmic reticulum. Ann NY Acad Sci. 1975;264:335–349. doi: 10.1111/j.1749-6632.1975.tb31494.x. [DOI] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbrunn L, Wiercinsky F. The action of various cations on muscle protoplasm. J Cell Comp Physiol. 1947;29:15–32. doi: 10.1002/jcp.1030290103. [DOI] [PubMed] [Google Scholar]
- Heizmann C, Berchtold M, Rowlerson A. Correlation of parvalbumin concentration with relaxation speed in mammalian muscle. Proc Natl Acad Sci U S A. 1982;79:7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19:780–782. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- Hernández-Ochoa E, Schneider M. Voltage clamp methods for the study of membrane currents and SR Ca2+ release in adult skeletal muscle fibres. Prog Biophys Mol Biol. 2012;108:98–118. doi: 10.1016/j.pbiomolbio.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C. Cross talk between Ca2+ and redox signaling cascades in muscle and neurons through the combined activation of ryanodine receptors/Ca2+ release channels. Phil Trans R Soc B. 2005;360:2237–2246. doi: 10.1098/rstb.2005.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. The abrupt transition from rest to activity in muscle. Proc R Soc B. 1949;136:399–420. doi: 10.1098/rspb.1949.0033. [DOI] [PubMed] [Google Scholar]
- Hill A, Kupalov P. Anaerobic and aerobic activity in isolated muscle. Proc R Soc London B. 1929;105:313–322. [Google Scholar]
- Hille B, Campbell T. An improved Vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976;67:265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A, Horowicz P. The effect of nitrate and other anions on the mechanical response of single muscle fibers. J Physiol. 1960;153:404–412. doi: 10.1113/jphysiol.1960.sp006542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A, Huxley A. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S, Marshall M. A comparative study of charge movement in rat and frog skeletal muscle fibres. J Physiol. 1981;321:583–602. doi: 10.1113/jphysiol.1981.sp014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S, Gee K, Baylor S. Low-affinity Ca2+ indicators compared in measurements of skeletal muscle Ca2+ transients. Biophys J. 2009;97:1864–1872. doi: 10.1016/j.bpj.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S, Kim M, Baylor S. Measurement and simulation of myoplasmic calcium transients in mouse slow-twitch muscle fibres. J Physiol. 2012;590:575–594. doi: 10.1113/jphysiol.2011.220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P. Influence of ions on the membrane potential of muscle fibres. In: Shanes A, editor. Biophysics of physiological and pharmacological actions. American Association for the Advancement of Science: Washington; 1961. pp. 217–234. [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hoth M, Button D, Lewis R. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci U S A. 2000;97:10607–10612. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudecova S, Vadaszova A, Soukup T, Krizanova O. Effect of thyroid hormones on the gene expression of calcium transport systems in rat muscles. Life Sci. 2004;75:923–931. doi: 10.1016/j.lfs.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Hunter D, Haworth R. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- Huxley H. Evidence for continuity between the central elements of the triads and extracellular space in frog sartorius muscle. Nature. 1964;202:1067. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- Huxley H. The mechanism of muscular contraction. Science. 1969;164:1356–1366. [PubMed] [Google Scholar]
- Isaeva E, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J Physiol. 2003;547:453–462. doi: 10.1113/jphysiol.2002.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva E, Shkryl V, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J Physiol. 2005;565:855–872. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Inui M, Tada M, Chiesi M, Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989;342:90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Jayasinghe I, Baddeley D, Kong C, Wehrens X, Cannell M, Soeller C. Nanoscale organization of junctophilin-2 and ryanodine receptors within peripheral couplings of rat ventricular cardiomyocytes. Biophys J. 2012;102(5):L19–L21. doi: 10.1016/j.bpj.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chalt B, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham D. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A, Jones L. Localization of phospholamban in slow but not fast canine skeletal muscle fibers. J Biol Chem. 1986;261:3775–3781. [PubMed] [Google Scholar]
- Jung D, Baysal K, Brierley G. The sodium-calcium antiport of heart mitochondria is not electroneutral. J Biol Chem. 1995;270:672–678. doi: 10.1074/jbc.270.2.672. [DOI] [PubMed] [Google Scholar]
- Jung D, Mo S, Kim D. Calumenin, a multiple EF-hands Ca2+-binding protein, interacts with ryanodine receptor-1 in rabbit skeletal sarcoplasmic reticulum. Biochem Biophys Res Commun. 2006;34:34–42. doi: 10.1016/j.bbrc.2006.02.115. [DOI] [PubMed] [Google Scholar]
- Kahn A, Sandow A. The potentiation of muscular contraction by the nitrate-ion. Science. 1950;112:647–649. doi: 10.1126/science.112.2918.647. [DOI] [PubMed] [Google Scholar]
- Kanter M, Nolte L, Holloszy J. Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J Appl Physiol. 1993;74:965–969. doi: 10.1152/jappl.1993.74.2.965. [DOI] [PubMed] [Google Scholar]
- Kao C, Stanfield P. Action of some ions on the electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968;198:291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J, Li G, Auston D. Practical aspects of measuring intracellular calcium signals with fluorescent indicators. Methods Cell Biol. 2010;99:113–152. doi: 10.1016/B978-0-12-374841-6.00005-0. [DOI] [PubMed] [Google Scholar]
- Katerinopoulos H, Foukaraki E. Polycarboxylate fluorescent indicators as ion concentration probes in biological systems. Curr Med Chem. 2002;9:275–306. doi: 10.2174/0929867023371193. [DOI] [PubMed] [Google Scholar]
- Kent-Braun J. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol. 1999;80:57–63. doi: 10.1007/s004210050558. [DOI] [PubMed] [Google Scholar]
- Kent-Braun J, Miller R, Weiner M. Phases of metabolism during progressive exercise to fatigue in human skeletal muscle. J Appl Physiol. 1993;75:573–580. doi: 10.1152/jappl.1993.75.2.573. [DOI] [PubMed] [Google Scholar]
- Kinnally K, Campo M, Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr. 1989;21:497–506. doi: 10.1007/BF00762521. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham D. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Klar T, Jakobs S, Dyba M, Egner A, Hell S. Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. Proc Natl Acad Sci U S A. 2000;97:8206–8210. doi: 10.1073/pnas.97.15.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Simon B, Szucs G, Schneider M. Simultaneous recording of calcium transients in skeletal muscle using high and low-affinity calcium indicators. Biophys J. 1988;53:971–988. doi: 10.1016/S0006-3495(88)83178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuth S, Dave H, Peters J, Fitts R. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 °C and 15 °C: implications for muscle fatigue. J Physiol. 2006;575:887–899. doi: 10.1113/jphysiol.2006.106732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Hollingworth S, Harkins A, Baylor S. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991;97:271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman J, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L, Schneider M. Contractile activation by voltage clamp depolarization of cut skeletal muscle fibres. J Physiol. 1978;277:483–506. doi: 10.1113/jphysiol.1978.sp012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L, Ríos E, Schneider M. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov A, Troppmair J, Sucher R, Hermann M, Saks V, Margreiter R. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: possible physiological role? Biochim Biophys Acta. 2006;1757:686–691. doi: 10.1016/j.bbabio.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Lacampagne A, Lederer W, Schneider M, Klein M. Repriming and activation alter the frequency of stereotyped discrete Ca2+ release events in frog skeletal muscle. J Physiol. 1996;497:581–588. doi: 10.1113/jphysiol.1996.sp021791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Erickson H, Rousseau E, Liu Q, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331:315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lamb G. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mecanically skinned fibres. J Muscle Res Cell Motil. 2002;23:81–91. doi: 10.1023/a:1019932730457. [DOI] [PubMed] [Google Scholar]
- Lamb G, Stephenson D. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G, Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G, Junankar P, Stephenson D. Raised intracellular Ca2+ abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H, Bruton J. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J Muscle Res Cell Motil. 2001;22:265–275. doi: 10.1023/a:1012227009544. [DOI] [PubMed] [Google Scholar]
- Launikonis B, Ríos E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Zhou J, Royer L, Shannon T, Brum G, Ríos E. Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing of fluorescence. J Physiol. 2005;567:523–543. doi: 10.1113/jphysiol.2005.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Pette D. Immunochemical quantification of sarcoplasmic reticulum Ca-ATPase, of calsequestrin and of parvalbumin in rabbit skeletal muscles. Eur J Biochem. 1986;156:489–496. doi: 10.1111/j.1432-1033.1986.tb09607.x. [DOI] [PubMed] [Google Scholar]
- Leong P, MacLennan D. A 37-amino acid sequence in the skeletal muscle ryanodine receptor interacts with the cytoplasmic loop between domains II and III in the skeletal muscle dihydropyridine receptor. J Biol Chem. 1998;273:7791–7794. doi: 10.1074/jbc.273.14.7791. [DOI] [PubMed] [Google Scholar]
- Leppik J, Aughey R, Medved I, Fairweather I, Carey M, McKenna M. Prolongued exercise to fatigue in humans impairs skeletal muscle Na-K ATPase activity, sarcoplasmic reticulum Ca release and Ca uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Lewis J, Tata J. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973;13:447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Lindinger M, Heigenhauser G. The roles of ion fluxes in skeletal muscle fatigue. Can J Physiol Pharmacol. 1991;69:246–253. doi: 10.1139/y91-038. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim M, Heo W, Jones J, Myers J, Ferrell J, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes. J Physiol. 1996;492:31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke S, Serysheva I, Hamilton S, Chiu W. The pore structure of the closed RyR1 channel. Structure. 2005;13:1203–1211. doi: 10.1016/j.str.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff A, Atwood H. Membrane properties and contraction of single muscle fibers in the mouse. Am J Physiol. 1972;222:1435–1440. doi: 10.1152/ajplegacy.1972.222.6.1435. [DOI] [PubMed] [Google Scholar]
- Luik R, Wu M, Buchanan J, Lewis R. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttgau H. The effect of metabolic inhibitors on the fatigue of the action potential in single muscle fibres. J Physiol. 1965;178:45–67. doi: 10.1113/jphysiol.1965.sp007613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H, Oetliker H. The action of caffeine on the activation of the contractile mechanism in striated muscle fibres. J Physiol. 1968;194:51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Pan Z. Junctional membrane structure and store operated calcium entry in muscle cells. Front Biosci. 2003;8:d242–d255. doi: 10.2741/977. [DOI] [PubMed] [Google Scholar]
- MacLennan D, Brandl C, Korczak B, Green N. Amino-acid sequence of a Ca2++Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985;316:696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Mannella C, Colombini M, Frank J. Structural and functional evidence for multiple channel complexes in the outer membrane of Neurospora crassa mitochondria. Proc Natl Acad Sci U S A. 1983;80:2243–2247. doi: 10.1073/pnas.80.8.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C, Buttle K, Rath B, Marko M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors. 1998;8:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- Manno C, Figueroa L, Fitts R, Ríos E. Confocal imaging of transmembrane voltage by SEER of di-8-ANEPPS. J Gen Physiol. 2013;141(3):371–387. doi: 10.1085/jgp.201210936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martonosi A, Pikula S. The structure of the Ca2+-ATPase of sarcoplasmic reticulum. Acta Biochim Pol. 2003;50:337–365. [PubMed] [Google Scholar]
- McCully K, Clark B, Kent J, Wilson J, Chance B. Biochemical adaptations to training: implications for resisting muscle fatigue. Can J Physiol Pharmacol. 1991;69:274–278. doi: 10.1139/y91-042. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Bruder A, Chen S, Moser C. Chaotropic anions and the surface potential of bilayer membranes. Biochim Biophys Acta. 1975;394:304–313. doi: 10.1016/0005-2736(75)90267-9. [DOI] [PubMed] [Google Scholar]
- Meier P, Spycher M, Meyer U. Isolation and characterization of rough endoplasmic reticulum associated with mitochondria from normal rat liver. Biochim Biophys Acta. 1981;646:283–297. doi: 10.1016/0005-2736(81)90335-7. [DOI] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984;259:2365–2374. [PubMed] [Google Scholar]
- Michaelson L, Shi G, Ward C, Rodney G. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve. 2010;42:522–529. doi: 10.1002/mus.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Parker I, Schalow G. Calcium transients in frog slow muscle fibres. Nature. 1977;268:750–752. doi: 10.1038/268750a0. [DOI] [PubMed] [Google Scholar]
- Minta A, Kao J, Tsien R. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- Mitchell P, Moyle J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature. 1967;213:137–139. doi: 10.1038/213137a0. [DOI] [PubMed] [Google Scholar]
- Moopanar T, Allen D. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 °C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moopanar T, Allen D. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol. 2006;571:191–200. doi: 10.1113/jphysiol.2005.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R, Nguyen H, Galceran J, Pessah I, Allen P. A transgenic myogenic cell line lacking ryanodine receptor protein for homologous expression studies: reconstitution of Ry1R protein and function. J Cell Biol. 1998;140:843–851. doi: 10.1083/jcb.140.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morre D, Merritt W, Lembi C. Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma. 1971;73:43–49. doi: 10.1007/BF01286410. [DOI] [PubMed] [Google Scholar]
- Mosca B, Delbono O, Messi M, Bergamelli L, Wang Z, Vukcevic M, Lopez R, Treves S, Nishi M, Takeshima H, Paolini C, Martini M, Rispoli G, Protasi F, Zorzato F. Enhanced dihydropyridine receptor calcium channel activity restores muscle strength in JP45/CASQ1 double knockout mice. Nat Commun. 2013;4:1541. doi: 10.1038/ncomms2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi R, Carson P, Boska M, Weiner M, Miller R. Nonmetabolic fatigue in exercising human muscle. Neurology. 1989;39:1222–1226. doi: 10.1212/wnl.39.9.1222. [DOI] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghdi S, Waldeck-Weiermair M, Fertschai I, Poteser M, Graier W, Malli R. Mitochondrial Ca2+ uptake and not mitochondrial motility is required for STIM1-Orai1-dependent store-operated Ca2+ entry. J Cell Sci. 2010;123:2553–2564. doi: 10.1242/jcs.070151. [DOI] [PubMed] [Google Scholar]
- Nakai J, Dirksen R, Nguyen H, Pessah I, Beam K, Allen P. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Nassar-Gentina V, Passonneau J, Vergara J, Rapoport S. Metabolic correlates of fatigue and recovery from fatigue in single frog muscle fibers. J Gen Physiol. 1978;72:593–606. doi: 10.1085/jgp.72.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori R. The property and contraction process of isolated myofibrils. Jikeikai Med J. 1954;1:119–126. [Google Scholar]
- Niedergerke R. Local muscular shortening by intracellularly applied calcium. J Physiol. 1955;128:12P–13P. [Google Scholar]
- Oba T, Kurono C, Nakajima R, Takaishi T, Ishida K, Fuller G, Klomkleaw W, Yamaguchi M. H2O2 activates ryanodine receptor but has little effect on recovery of release Ca2+ content after fatigue. J Appl Physiol. 2002;93:1999–2008. doi: 10.1152/japplphysiol.00097.2002. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Valdivia H, Block B. Physiological differences between the alpha and beta ryanodine receptors of fish skeletal muscle. Biophys J. 1995;68:471–482. doi: 10.1016/S0006-3495(95)80208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A, Becker S, Khanna V, Kurzydlowski K, Leisner E, Pette D, MacLennan D. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 1998;273:12360–12369. doi: 10.1074/jbc.273.20.12360. [DOI] [PubMed] [Google Scholar]
- Ogata T, Yamasaki Y. Scanning electron-microscopic studies on the three-dimensional structure of sarcoplasmic reticulum in the mammalian red, white and intermediate muscle fibers. Cell Tissue Res. 1985;242:461–467. doi: 10.1007/BF00225410. [DOI] [PubMed] [Google Scholar]
- Pacher P, Thomas A, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Li S, Thakur P, Rodney G. Real-time imaging of NADPH oxidase activity in living cell by using novel bio-sensor. Biophys J. 2013;104(2–S1):530a. doi: 10.1371/journal.pone.0063989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, Tsien R. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172. [DOI] [PubMed] [Google Scholar]
- Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4:379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- Paolini C, Fessenden J, Pessah I, Franzini-Armstrong C. Evidence for conformational coupling between two calcium channels. Proc Natl Acad Sci U S A. 2004;101:12748–12752. doi: 10.1073/pnas.0404836101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen PD, Reggiani C, Protasi F. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol. 2007;583:767–784. doi: 10.1113/jphysiol.2007.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulus S, Leuranguer V, Bannister R, Beam K. Mapping sites of potential proximity between the DHPR and RyR1 in muscle using a cyan fluorescent protein-yellow fluorescent protein tandem as a fluorescent resonance energy transfer probe. J Biol Chem. 2004;279:44046–44056. doi: 10.1074/jbc.M405317200. [DOI] [PubMed] [Google Scholar]
- Parekh A. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547:333–348. doi: 10.1113/jphysiol.2002.034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Parekh A, Putney J., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Peachey L. The sarcoplasmic reticulum and transverse tubules of the frog’s Sartorius. J Cell Biol. 1965;25:209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Nielsen O, Lamb G, Stephenson D. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Perez C, Thomas M, Franzini-Armstrong C. Carboxyl-terminal domain of DHPR β1A is essential for DHPR tetrad formation. Biophys J. 2013;104(2–S1):104a. [Google Scholar]
- Periasamy M, Kalyanasundaram A. Serca pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Petrofsky J, Lind A. Isometric endurance in fast and slow muscles in the cat. Am J Physiol. 1979;236:C185–C191. doi: 10.1152/ajpcell.1979.236.5.C185. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989;259:137–143. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- Picard M, Hepple R, Burelle Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol. 2012;302:C629–C641. doi: 10.1152/ajpcell.00368.2011. [DOI] [PubMed] [Google Scholar]
- Place N, Yamada T, Bruton JD, Westerblad H. Interpolated twitches in fatiguing single mouse muscle fibres: implications for the assessment of central fatigue. J Physiol. 2008;586:2799–2805. doi: 10.1113/jphysiol.2008.151910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place N, Yamada T, Zhang S, Westerblad H, Bruton J. High temperature does not alter fatigability in intact mouse skeletal muscle fibres. J Physiol. 2009;587:4717–4724. doi: 10.1113/jphysiol.2009.176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place N, Yamada T, Bruton J, Westerblad H. Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol. 2010;110:1–15. doi: 10.1007/s00421-010-1480-0. [DOI] [PubMed] [Google Scholar]
- Porter K, Palade G. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino G, Lamb G. Effect of sarcoplasmic reticulum Ca2+ content on action-potential induced Ca2+ release in rat skeletal muscle fibres. J Physiol. 2003;551:219–237. doi: 10.1113/jphysiol.2003.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau S, Collet C, Allard B, Jacquemond V. Whole-cell voltage clamp on skeletal muscle fibers with silicone-clamp technique. Methods Mol Biol. 2007;403:185–194. doi: 10.1007/978-1-59745-529-9_12. [DOI] [PubMed] [Google Scholar]
- Pouvreau S, Royer L, Yi J, Brum G, Meissner G, Ríos E, Zhou J. Ca(2+) sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle. Proc Natl Acad Sci U S A. 2007;104:5235–5240. doi: 10.1073/pnas.0700748104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan P. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Prosser B, Wright N, Hernandez-Ochoa E, Varney K, Liu Y, Olojo R, Zimmer D, Weber D, Schneider M. S100A1 binds to the calmodulin binding site of ryanodine receptor and modulates skeletal muscle coupling. J Biol Chem. 2008;283:5046–5057. doi: 10.1074/jbc.M709231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser B, Hernández-Ochoa E, Lovering R, Andronache Z, Zimmer D, Melzer W, Schneider M. S100A1 promotes action potential-initiated calcium release flux and force production in skeletal muscle. Am J Physiol Cell Physiol. 2010;299:C891–C902. doi: 10.1152/ajpcell.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protasi F, Paolini C, Nakai J, Beam K, Franzini-Armstrong C, Allen P. Multiple regions of RyR1 mediate functional and structural interactions with α1s-dihidropyridine receptors in skeletal muscle. Biophys J. 2002;83:3220–3244. doi: 10.1016/S0006-3495(02)75325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Racay P, Gregory P, Schwaller B. Parvalbumin deficiency in fast-twitch muscles leads to increased 'slow-twitch type' mitochondria, but does not affect the expression of fiber specific proteins. FEBS J. 2006;273:96–108. doi: 10.1111/j.1742-4658.2005.05046.x. [DOI] [PubMed] [Google Scholar]
- Raju B, Murphy E, Levy L, Hall R, London R. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989;256:C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Sharma V, Sheu S, Franzini-Armstrong C. Structural proximity of mitochondria to calcium release units in rat ventricular myocardium may suggest a role in Ca2+ sequestration. Ann N Y Acad Sci. 1998;853:341–344. doi: 10.1111/j.1749-6632.1998.tb08295.x. [DOI] [PubMed] [Google Scholar]
- Rando T, Blau H. Primary mouse myoblast purification, characterization and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranvier L. Propriétés et structures différentes des muscles rouges et des muscles blancs, chez les Lapins et chez les Raies. Compt Rendus. 1873;77:1030–1034. [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski M, Vandecasteele G, Baird G, Tuft R, Fogarty K, Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch M, Treves S, Zorzato F. 3D Structural illumination microscopy of the skeletal muscle excitation-contraction coupling macromolecular complex. Biophys J. 2013;104(2–S1):105a. [Google Scholar]
- Reardon T, Allen D. Time to fatigue is increased in mouse muscle at 37 °C; the role of iron and reactive oxygen species. J Physiol. 2009;587:4705–4716. doi: 10.1113/jphysiol.2009.173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck R, Willemse H, Groom L, Dirksen R, Dulhunty A. Interactions between dihydropyridine β1A subunit and ryanodine receptor isoforms. Biophys J. 2013;104(2–S1):105a. [Google Scholar]
- Reggiani C, te Kronnie T. RyR isoforms and fibre-type specific expression of proteins controlling intracellular calcium concentration in skeletal muscles. J Muscle Res Cell Motil. 2006;27:327–335. doi: 10.1007/s10974-006-9076-3. [DOI] [PubMed] [Google Scholar]
- Reid M. Plasticity in Skeletal, Cardiac, and Smooth Muscle. Invited Review: Redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Reid M, Haack K, Kathleen F, Valberg P, Kobzik L, West S. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- Ridgway E, Ashley C. Calcium transients in single muscle fibres. Biochem Biophys Res Commun. 1967;29:229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- Ríos E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Ríos E, Pizarro G. Voltage sensors and calcium channels of excitation-contraction coupling. News Physiol Sci. 1988;3:223–227. [Google Scholar]
- Ríos E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991;71:849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Ríos E, Karhanek M, Ma J, González A. An Allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J Gen Physiol. 1993;102:449–481. doi: 10.1085/jgp.102.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft R, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K, Picaud S, Roncali E, Boisgard R, Colasante C, Stinnakre J, Tavitian B, Brulet P. Non-invasive in vivo imaging of calcium signaling in mice. PLoS One. 2007;2:e974. doi: 10.1371/journal.pone.0000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman K. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Bottinelli R, Sorrentino V, Reggiani C. Response to caffeine and ryanodine receptor isoforms in mouse skeletal muscle. Am J Physiol Cell Physiol. 2001;281:C585–C594. doi: 10.1152/ajpcell.2001.281.2.C585. [DOI] [PubMed] [Google Scholar]
- Rossi A, Boncompagni S, Wei L, Protasi F, Dirksen R. Differential impact of mitochondrial positioning on mitochondrial Ca(2+) uptake and Ca(2+) spark suppression in skeletal muscle. Am J Physiol Cell Physiol. 2011;301:C1128–C1139. doi: 10.1152/ajpcell.00194.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Pinkos J. pH modulates conducting and gating behaviour of single calcium release channels. Pflugers Arch. 1990;415:645–657. doi: 10.1007/BF02583520. [DOI] [PubMed] [Google Scholar]
- Royer L, Sztretye M, Manno C, Pouvreau S, Zhou J, Knollmann B, Protasi F, Allen P, Rios E. Paradoxical buffering of calcium by calsequestrin demonstrated for the calcium store of skeletal muscle. J Gen Physiol. 2010;136:325–338. doi: 10.1085/jgp.201010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Mongillo M, Magalhaes P, Pozzan T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166:527–536. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Beutner G, Dirksen R, Kinnally K, Sheu S. Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 2010;584:1948–1955. doi: 10.1016/j.febslet.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Beutner G, Kinnally K, Dirksen R, Sheu S. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J Biol Chem. 2011;286:21324–21329. doi: 10.1074/jbc.C111.245597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsó M, Wagenknecht T, Allen D. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat Struct Mol Biol. 2005;12:539–544. doi: 10.1038/nsmb938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsó M, Feng W, Pessah I, Allen P. Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 2009;7:e85. doi: 10.1371/journal.pbio.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952;XXV:176–201. [PMC free article] [PubMed] [Google Scholar]
- Sandow A. Potentiation of muscular contraction. Arch Phys Med Rehabil. 1964;45:62–81. [PubMed] [Google Scholar]
- Sandow A. Excitation-Contraction Coupling in skeletal muscle. Pharmacol Rev. 1965;17:265–320. [PubMed] [Google Scholar]
- Sandow A, Isaacson A. Topochemical factors in potentiation of contraction by heavy metal cations. J Gen Physiol. 1966;49:937–961. doi: 10.1085/jgp.49.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A, Taylor S, Preiser H. Role of the action potential in excitation-contraction coupling. Fed Proc. 1965;24:1116–1123. [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta. 2010;1797:907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Saris N, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Mosc) 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- Schein S, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Schermelleh L, Heintzmann R, Leonhardt H. A guide to super-resolution fluorescence microscopy. J Cell Biol. 2010;190:165–175. doi: 10.1083/jcb.201002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Schmitt T, Pette D. Fiber type-specific distribution of parvalbumin in rabbit skeletal muscle. Histochemistry. 1991;96:459–465. doi: 10.1007/BF00267071. [DOI] [PubMed] [Google Scholar]
- Schneider M, Chandler W. Voltage dependent charge movement in skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Scriven D, Tafteh R, Chou K, Moore E. Super-resolution localization and distribution of proteins within the mammalian couplon. Biophys J. 2013;104(2–S1):105a. [Google Scholar]
- Sembrowich W, Quintinskie J, Li G. Calcium uptake in mitochondria from different skeletal muscle types. J Appl Physiol. 1985;59:137–141. doi: 10.1152/jappl.1985.59.1.137. [DOI] [PubMed] [Google Scholar]
- Sen C. Oxidants and antioxidants in exercise. J Appl Physiol. 1995;79:675–686. doi: 10.1152/jappl.1995.79.3.675. [DOI] [PubMed] [Google Scholar]
- Serysheva I, Chiu W, Ludtke S. Single-particle electron cryomicroscopy of the ion channels in the excitation-contraction coupling junction. Methods Cell Biol. 2007;79:407–435. doi: 10.1016/S0091-679X(06)79016-1. [DOI] [PubMed] [Google Scholar]
- Shaw M, Ostap E, Goldman Y. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluene sulphonamide. Biochemistry. 2003;42:6128–6135. doi: 10.1021/bi026964f. [DOI] [PubMed] [Google Scholar]
- Shirokova N, Ríos E. Small event Ca2+ release: a probable precursor of Ca2+ sparks in frog skeletal muscle. J Physiol. 1997;502:3–11. doi: 10.1111/j.1469-7793.1997.003bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, García J, Pizarro G, Ríos E. Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J Gen Physiol. 1996;107:1–18. doi: 10.1085/jgp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkryl V, Shirokova N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J Biol Chem. 2006;281:1547–1554. doi: 10.1074/jbc.M505024200. [DOI] [PubMed] [Google Scholar]
- Shore G, Tata J. Two fractions of rough endoplasmic reticulum from rat liver. I. Recovery of rapidly sedimenting endoplasmic reticulum in association with mitochondria. J Cell Biol. 1977;72:714–725. doi: 10.1083/jcb.72.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtifman A, Ward C, Wang J, Valdivia H, Schneider M. Effects of imperatoxin A on local sarcoplasmic reticulum Ca(2+) release in frog skeletal muscle. Biophys J. 2000;79:814–827. doi: 10.1016/S0006-3495(00)76338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets I, Caplanusi A, Despa S, Molnar Z, Radu M, VandeVen M, Ameloot M, Steels P. Ca2+ uptake in mitochondria occurs via the reverse action of the Na+/Ca2+ exchanger in metabolically inhibited MDCK cells. Am J Physiol Ren Physiol. 2004;286:F784–F794. doi: 10.1152/ajprenal.00284.2003. [DOI] [PubMed] [Google Scholar]
- Smith J, Imagawa T, Ma J, Fill M, Campbell K, Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the Ca2+release channel of the SR. J Gen Physiol. 1988;92:1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J, Dehaven W, Jones B, Mercer J, Trebak M, Vazquez G, Putney J., Jr Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Dziadek M, Gill D. Calcium signals mediated by STIM and Orai proteins–a new paradigm in inter-organelle communication. Biochim Biophys Acta. 2006;1763:1161–1168. doi: 10.1016/j.bbamcr.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Sparagna G, Gunter K, Sheu S, Gunter T. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Stern M. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992;13:183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Stiber J, Hawkins A, Zhang Z, Wang S, Burch J, Graham V, Ward C, Seth M, Finch E, Malouf N, Williams R, Eu J, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Lou F, Edman K. 2,3-Butanedione monoxime increases speed of relaxation in single muscle fibres of frog. Acta Physiol Scand. 2001;172:53–61. doi: 10.1046/j.1365-201X.2001.00818.x. [DOI] [PubMed] [Google Scholar]
- Szentesi P, Jacquemond V, Kovács L, Csernoch L. Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibres. J Physiol. 1997;502:371–384. doi: 10.1111/j.1469-7793.1997.371bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Camacho P, Lechleiter J, Herman B. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–1125. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- Takehura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol. 2001;533:571–583. doi: 10.1111/j.1469-7793.2001.0571a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Beam K, Powell J, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Beam K, Adams B, Niidome T, Numa S. Regions of the skeletal dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- Tang S, Wong H, Wang Z, Huang Y, Zhuo Y, Pennati A, Gadda G, Delbono O, Yang J. Design and application of a class of sensors to monitor Ca2+ dynamics in high Ca2+ concentration cellular compartments. Proc Natl Acad Sci U S A. 2011;108:16265–16270. doi: 10.1073/pnas.1103015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima H, Mizutani T. Crystal structure of the calcium pump with a bound ATP analogue. Nature. 2004;430:529–535. doi: 10.1038/nature02680. [DOI] [PubMed] [Google Scholar]
- Treves S, Vukcevic M, Maj M, Thurnheer R, Mosca B, Zorzato F. Minor sarcoplasmic reticulum membrane components that modulate excitation-contraction coupling in striated muscles. J Physiol. 2009;587:3071–3079. doi: 10.1113/jphysiol.2009.171876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S, Thurnheer R, Mosca B, Vukcevic M, Bergamelli L, Voltan R, Oberhauser V, Ronjat M, Csernoch L, Szentesi P, Zorzato F. SRP-35, a newly identified protein of the skeletal muscle sarcoplasmic reticulum, is a retinol dehydrogenase. Biochem J. 2012;441:731–741. doi: 10.1042/BJ20111457. [DOI] [PubMed] [Google Scholar]
- Tsien R. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsugorka A, Ríos E, Blatter L. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995;269:1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- Tung C, Lobo P, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468:585–58. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- Tupling R. The sarcoplasmic reticulum in muscle fatigue and disease: role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can J Appl Physiol. 2004;29:308–329. doi: 10.1139/h04-021. [DOI] [PubMed] [Google Scholar]
- van der Poel C, Edwards J, Macdonald W, Stephenson D. Effect of temperature-induced reactive oxygen species production on excitation-contraction coupling in mammalian skeletal muscle. Clin Exp Pharmacol Physiol. 2008;35:1482–1487. doi: 10.1111/j.1440-1681.2008.05050.x. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov A, Olivares J, Kay L, Saks V. Mitochondrial regular arrangement in muscle cells: a "crystal-like" pattern. Am J Physiol Cell Physiol. 2005;288:C757–C767. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- Verburg E, Murphy R, Stephenson G, Lamb G. Disruption of excitation-contraction coupling and titin by endogenous Ca2+-activated proteases in toad muscle fibres. J Physiol. 2005;564:775–789. doi: 10.1113/jphysiol.2004.082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg E, Dutka T, Lamb G. Long-lasting muscle fatigue: partial disruption of excitation-contraction coupling by elevated cytosolic Ca2+ concentration during contractions. Am J Physiol. 2006;290:C1199–C1208. doi: 10.1152/ajpcell.00469.2005. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet J. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, DeHaven W, Bird G, Billingsley J, Wang H, Rao P, Hutchings A, Jouvin M, Putney J, Kinet J. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T, Grassucci R, Frank J, Saito A, Inui M, Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989;338:167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T, Hsieh C-E, Rath B, Fleischer S, Marko M. Electron tomography of frozen-hydrated isolated triad junctions. Biophys J. 2002;83:2491–2501. doi: 10.1016/S0006-3495(02)75260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zheng Z, Messi M, Delbono O. Muscle fibers from senescent mice retain excitation-contraction coupling properties in culture. In vitro Cell Dev Biol. 2007;43:222–234. doi: 10.1007/s11626-007-9047-z. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tang S, Messi M, Yang J, Delbono Residual sarcoplasmic reticulum Ca2+ concentration after Ca2+ release in skeletal myofibers from young adult and old mice. Pflugers Arch. 2012;463:615–624. doi: 10.1007/s00424-012-1073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C, Prosser B, Greiser M, Westerblad H, Khairallah R, Lederer W. A novel assay of mechano-transduction in single muscle cells. Biophys J. 2011;100:589a. [Google Scholar]
- Weber A. On the role of calcium in the activity of adenosine 5′-triphosphate hydrolysis by actomyosin. J Biol Chem. 1959;234:2764–2769. [PubMed] [Google Scholar]
- Weber A, Herz R. The relationship between caffeine contracture in intact muscle and the effect of caffeine on Reticulum. J Gen Physiol. 1968;52:750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Varsányi M, Dulhunty A, Beard N. The conformation of calsequestrin determines its ability to regulate skeletal ryanodine receptors. Biophys J. 2006;91:1288–1301. doi: 10.1529/biophysj.106.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder N, Zhou J, Ma J. Detection of calcium sparks in intact and permeabilized skeletal muscle fibers. Methods Mol Biol. 2012;798:395–410. doi: 10.1007/978-1-61779-343-1_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H. The role of pH and inorganic phosphate ions in skeletal muscle fatigue. Chapter 12. In: Hargreaves M, Thompson M, editors. Biochemistry of exercise X. Human Kinetics: Champaign, USA; 1999. pp. p147–p154. [Google Scholar]
- Westerblad H, Allen D. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. Slowing of relaxation during fatigue in single mouse muscle fibres. J Physiol. 1991;434:323–336. doi: 10.1113/jphysiol.1991.sp018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci. 2002;17:17–21. doi: 10.1152/physiologyonline.2002.17.1.17. [DOI] [PubMed] [Google Scholar]
- Williams D, Head S, Bakker A, Stephenson G. Resting calcium concentrations in isolated skeletal muscle fibres of dystrophic mice. J Physiol. 1990;428:243–256. doi: 10.1113/jphysiol.1990.sp018210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J Gen Physiol. 1968;51:65–83. doi: 10.1085/jgp.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium E, Dulhunty A, Beard N. A skeletal muscle ryanodine receptor interaction domain in triadin. PLoS One. 2012;7:e43817. doi: 10.1371/journal.pone.0043817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Baddeley D, Bushong E, Yu Z, Ellisman M, Hoshijima M, Soeller C. Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: dilation of T-tubules near the junctions. Biophys J. 2013;104:L22–L24. doi: 10.1016/j.bpj.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D, Zollman J, Reuben J. Human skeletal muscle properties of the “chemically skinned” fiber. Science. 1975;187:1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]
- Woods C, Novo D, DiFranco M, Vergara J. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol. 2004;557:59–75. doi: 10.1113/jphysiol.2004.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yi J, Ma C, Li Y, Weisleder N, Rios E, Ma J, Zhou J. Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (E-C) coupling. J Biol Chem. 2011;286:32436–32443. doi: 10.1074/jbc.M110.217711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L, Nikaido H, Kagawa Y. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J Biol Chem. 1980;255:1771–1774. [PubMed] [Google Scholar]
- Zhang S, Yu Y, Roos J, Kozak J, Deerinck T, Ellisman M, Stauderman K, Cahalan M. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Launikonis B, Ríos E, Brum G. Regulation of Ca2+ sparks by Ca2+ and Mg2+ in mammalian and amphibian muscle. An RyR isoform-specific role in excitation-contraction coupling? J Gen Physiol. 2004;124:409–428. doi: 10.1085/jgp.200409105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Yi J, Royer L, Launikonis B, González A, García J, Ríos E. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am J Physiol Cell Physiol. 2006;290:C539–C553. doi: 10.1152/ajpcell.00592.2004. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim Biophys Acta. 2005;1706:40–52. doi: 10.1016/j.bbabio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Zorzato F, Fujii J, Otsu M, Phillips M, Green N, Lai F, Meissner G, MacLennan D. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]