Abstract
Chromosomes undergoing meiosis are defined by a macromolecular protein assembly called the synaptonemal complex which holds homologs together and carries out important meiotic functions. By retaining the molecular specificity, multiplexing ability, and in situ imaging capabilities of fluorescence microscopy, but with vastly increased resolution, 3D-SIM and other superresolution techniques are poised to make significant discoveries about the structure and function of the synaptonemal complex. This review discusses recent developments in this field and poses questions approachable with current and future technology.
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-013-0116-0) contains supplementary material, which is available to authorized users.
Keywords: Meiosis, Synaptonemal complex, Superresolution microscopy, 3D-SIM
Visualizing subcellular structures beyond the diffraction limit
Significant technical advances in microscope resolution have occurred in recent years. Precision control of excitation light and fluorophore characteristics, combined with computational techniques for reconstructing image information from raw data, have enabled the development of several superresolution imaging modalities (Schermelleh et al. 2010) capable of resolving objects more finely than the Abbe diffraction limit. One superresolution technique, three-dimensional structured illumination microscopy (3D-SIM) (Gustafsson et al. 2008; Schermelleh et al. 2008), enables enhancement of resolution by a factor of two in both the lateral and axial directions, to 100 nm in the x–y direction and 250 nm in the z-direction. Because of its resolution range, 3D-SIM is most effectively applied in visualizing subcellular structures that fall between 100 and 200 nm. Meiotic chromosomes in particular are attractive targets for 3D-SIM (Carlton 2008) due to several of their structural features.
Meiosis and the SC
Sexually reproducing organisms create their gametes through meiosis, a series of two cell divisions which partition a diploid genome between two haploid daughter cells, each obtaining a complementary half of the genetic material. This precise partitioning is accomplished by the de novo pairing of homologous chromosomes before their segregation, followed by the establishment of exchanges between homologs that orient them in opposite directions at metaphase I. Meiotic chromosomes appear as long, thin threads, separately distinguishable yet not as condensed as during the mitotic cell cycle. It has long been hypothesized that this striking appearance and its underlying structural basis reflect functional requirements.
A near-universal structural component of meiotic chromosomes is the synaptonemal complex (SC), a zipper-like protein macroassembly that localizes between paired homologous chromosomes along their lengths (reviewed extensively in Zickler and Kleckner 1999). The SC is composed of two major parts. The first is a protein core that extends the length of each individual chromosome; the cores are referred to as axial elements (AEs) before the intimate association (synapsis) of chromosomes, and as lateral elements (LEs) afterwards. The second part is the central element (CE), a zipper-like structure that polymerizes between opposed AEs when homologous chromosomes synapse (Fig. 1). The SC plays key roles in enabling chromosome pairing and recombination, is necessary for proper chromosome morphology, and likely has additional roles in the control of recombination (Hayashi et al. 2010). Any defects in SC formation are likely to disrupt proper gamete formation, causing sterility. Thus, the formation and disassembly of the SC are under strict regulation.
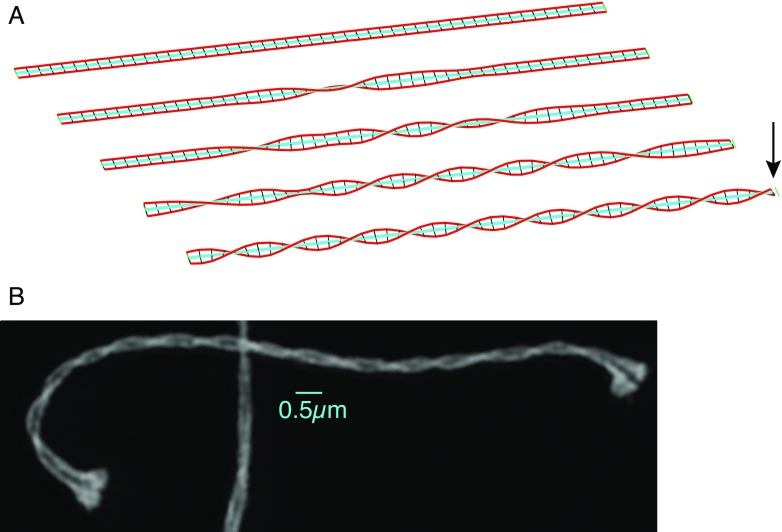
Fig. 1.
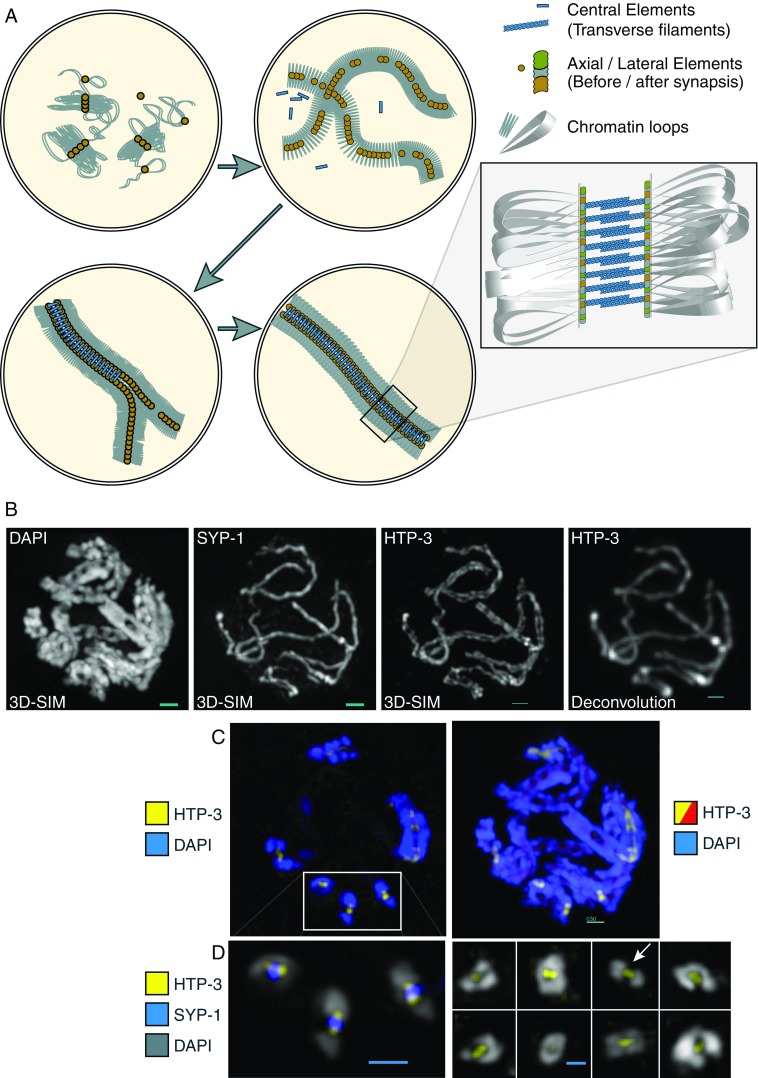
a Formation of the synaptonemal complex (SC). Arrows indicate chronological sequence, beginning at upper left. (1) Chromatin loops begin assembling together with axial element proteins, forming loop domains; (2) continued assembly forms the characteristic leptotene chromosome morphology; (3) synapsis begins as axes continue to form; chromatin is excluded from the interface between the two axial elements; (4) completed synapsis; lateral elements (LEs) and the central element (CE) forms regular, railroad track-like axis throughout the length of the paired homologs. Inset: One model of the arrangement of various components. b Visualization of Caenorhabditis elegans meiotic chromosomes with three-dimensional structured illumination microscopy (3D-SIM) microscopy. Chromatin [stained with 4′,6-diamidino-2-phenylindole (DAPI)], the transverse filament protein SYP-1, and the axial element protein HTP-3, are displayed. For comparison, the image at far right shows the axial elements under conventional deconvolution microscopy. Scale bar: 0.5 μm. c Left Highlight of a single optical section (thickness 125 nm) from a meiotic nucleus, showing the two LEs in cross-section in three locations (boxed inset); right entire nucleus in projection, displaying the DNA stained blue, the LEs (HTP-3 protein) stained red, with LEs from the highlighted section shown in yellow. Scale bars: 0.5 μm. The same nucleus is shown as a rotating movie in the Electronic Supplementary Material (movie 1). d 3D-SIM images of cross-sections of C. elegans chromosomes, showing axial element protein HTP-3 (yellow), transverse filament protein SYP-1 (blue), and DAPI (gray). Left Cross-sections highlighted in the nucleus above; right a montage of cross-sections from several nuclei, showing various arrangements of chromatin: either surrounding the entire axis to various extents, or completely bilobed (arrow). Scale bars: 0.5 μm
The SC was first discovered over 50 years ago by electron microscopy (EM) studies of meiotic chromosomes (Fawcett 1956; Moses 1956). EM images revealed the basic dimensions of the complete SC: LEs are spaced from 100 to 200 nm apart, and CE filaments (the “teeth” of the zipper) are spaced roughly 20–30 nm apart (Fig. 2). The ability to reconstruct entire SC complements of meiotic cells from serial-sectioned EM images was a significant advance in cytogenetics, as the paths of synapsed and unsynapsed chromosomes could be seen in their entirety in situ. However, the nature of the SC itself remained mysterious until its molecular components could be identified. We now know that the SC is composed of many different proteins, interlocking in a complicated mesoscale polymer framework. Protein components of both LEs and CEs have been identified in animals, fungi, and plants (Table 1). CE components include the transverse filaments (Solari and Moses 1973), coiled-coil proteins that extend from the LEs to meet at the center, as well as other proteins that localize to the central region (Bolcun-Filas et al. 2007; Fraune et al. 2012; Page et al. 2008). LE components include SC-specific proteins, including many proteins with HORMA domains (Aravind and Koonin 1998; Couteau et al. 2004), as well as cohesin proteins that make up the axial core (Eijpe et al. 2003). One of the most comprehensive recent studies of SC structure has been performed in the nematode Caenorhabditis elegans, using a combination of genetic, biochemical, and immuno-electron microscopy image data of the CE (Schild-Prüfert et al. 2011). The resulting model is likely the most detailed yet provided in any organism, and defines which of the known CE proteins of C. elegans (SYP-1, -2, -3, and -4) make direct contact with each other at which end (N– or C-terminus). The actual structure or structures that satisfy the constraints provided by this consensus model, as well as their higher order of organization within the SC, remain to be elucidated. While the SC is grossly similar between different species, considerable variation exists in protein makeup, connectivity, and overall architecture (reviewed in Hawley 2011).
Fig. 2.
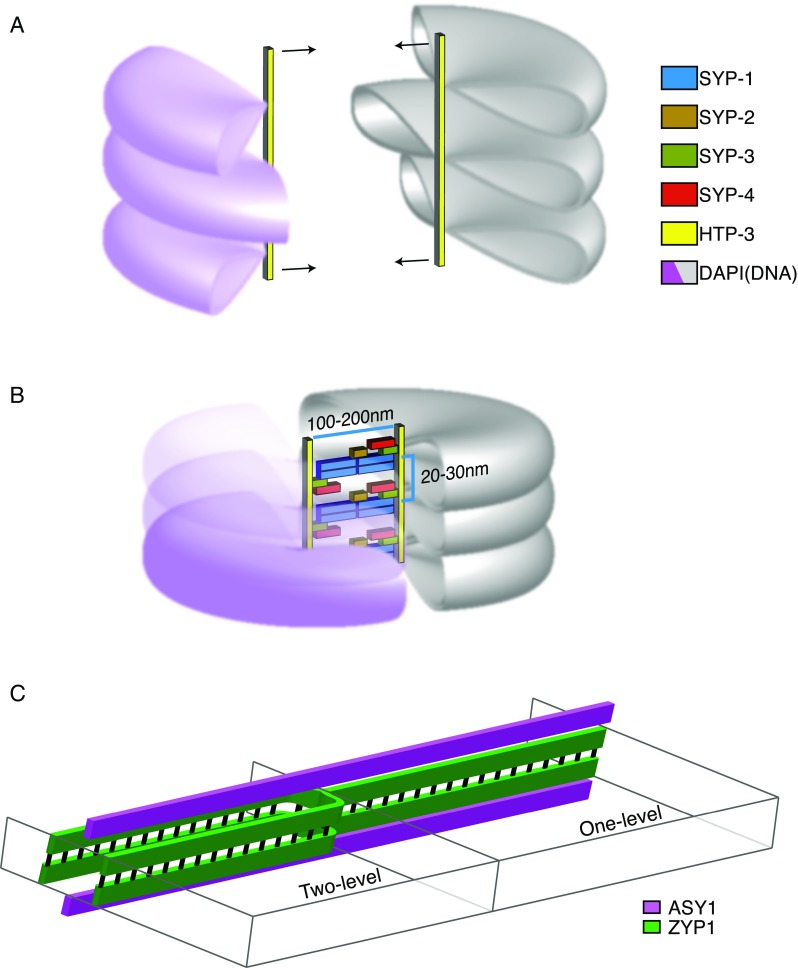
a. Before synapsis, homologous chromosomes consist of chromatin loops (violet, gray) attached to an axial core. The size and extent of individual loops are not well-characterized. To allow synapsis, the loops of chromatin must somehow become oriented to allow intimate synapsis at a distance of <200 nm. b. When chromosomes have synapsed, chromatin is often visible as more densely packed and pushed to either side. The CE proteins polymerized between the axes are shown in one possible conformation consistent with the model in (Schild-Prüfert et al. 2011). c. Schematic of the two kinds of SC structure in barley visualized with 3D-SIM in Phillips et al. (2012). Models of alternate SC structure are taken from Phillips et al. 2012. In barley meiocytes, the SC was observed by 3D-SIM to exist both in a canonical one-level configuration (right) as well as a two-level configuration (left)
Table 1.
A partial list of synaptonemal complex proteins
| Source | Transverse filament (TF) | Central element (CE) | Lateral element (LE) | Cohesin core |
|---|---|---|---|---|
| Yeast | ZIP1 | Zip2 | Hop1 | Rec8 |
| Zip3 | Mek1 | |||
| Zip4 | Red1 | |||
| Drosophila | C(3)G | Cona | C(2)M | ORD |
| Caenorhabditis elegans | SYP-1 | SYP-2 | HIM-3 | Rec8 |
| SYP-3 | HTP-1/2 | |||
| SYP-4 | HTP-3 | |||
| Mouse | SYCP1 | Tex12 | Sycp2 | Rec8 |
| Syce1 | Sycp3 | |||
| Syce2 | ||||
| Syce3 | ||||
| Arabidopsis | ZYP1 | Asy1 | SYN1 | |
| Asy2 | ||||
| Asy3 |
Like most biological structures, the SC is capable of dynamic reorganization and modification, including assembly, disassembly, and covalent modification of specific subunits, such as, for example, through phosphorylation (Bailis and Roeder 1998; Fukuda et al. 2012) or SUMOylation (Watts and Hoffmann 2011). However, while biochemical and genetic studies have greatly increased our knowledge of the components that make up the SC, a complete structural and functional explanation, including how it interfaces with meiotic chromatin, remains elusive. Part of the difficulty has arisen from the fact that the dimensions of the SC lie below the diffraction limit of visible light (250–350 nm), rendering optical microscopy relatively ineffective as a tool to reveal its detailed composition. Recently, new forms of superresolution microscopy have begun to be successfully applied to the study of the SC, bridging the gap between the molecular specificity of fluorescence microscopy and the high resolution required for structural information to be uncovered.
3D-SIM applied to meiotic chromosomes
3D--SIM has been successfully used to observe meiotic chromosomes in plant and animal meiosis. Wang et al. (2009) used immunostaining of AFD1, a homolog of kleisin Rec8, to demonstrate 3D-SIM’s ability to optically resolve both LEs in maize. The spacing of AFD1 was measured at 190 nm, consistent with previous EM studies of total SC width (Gillies 1973). Additional structural details of the SC, such as its coiling (see below) and the resolution of entanglements, were also quantitatively measured. In a recent study, Phillips et al. (2012) examined the SCs of barley with 3D-SIM, examining not only the LE HORMA-domain protein ASY1 (Armstrong et al. 2002) but also ZYP1, a transverse filament protein (Higgins et al. 2005). By simultaneously viewing both proteins with 3D-SIM, these authors were able to discern two different types of SC structure coexisting in the same nucleus. In addition to a canonical tripartite structure, where LEs and CEs lie in the same plane, they also discovered a two-level organization of ZYP-1 both above and below the LE plane (Fig. 2c). The authors performed immunostaining with antibodies raised against ZYP1’s C-terminus, which localizes near the LEs. 3D-SIM was sufficient to show that ZYP1 localized internally to ASY1, demonstrating its ability to quantitatively measure substructures within the SC. It will be interesting to determine whether the observed variation between the two SC structures has any functional consequence. In animal systems, genetic studies have added further information to what can be inferred by cytology. Qiao et al. (2012) used 3D-SIM to examine the SCs of mouse chromosomes. Their cytological data provided evidence for new roles of the CE transverse filament protein Sycp1 in regulating exchanges between LEs at crossover sites and preventing unregulated axis associations. With the higher resolution of 3D-SIM it was also apparent that centromeric ends of LEs displayed malformations in the absence of Sycp1. In C. elegans, Zhang et al. (2012) employed 3D-SIM to investigate the localization of CE transverse filament protein SYP-1 (MacQueen et al. 2002) in wild-type animals and in a strain carrying a mutation (hal-2) which prevents homologous pairing. In nuclei missing the HAL-2 protein, CE proteins abnormally loaded onto single, unsynapsed LEs (detected with immunostaining against HTP-3, one of four HORMA domain-containing proteins in the C. elegans LE). The LEs in hal-2 mutants appeared as single tracks by 3D-SIM, in contrast to those of wild-type animals in which 3D-SIM can resolve both LE tracks. In the above examples, 3D-SIM microscopy enabled quantitative measurements of 3D higher order structural details of specific protein molecules, a significant improvement over previous forms of microscopy.
4pi microscopy applied to meiotic chromosomes
Another superresolution modality recently applied to the study of meiotic chromosomes is 4pi microscopy (Schrader et al. 1998). 4pi microscopy uses two objective lenses to capture emitted fluorescence from both sides of the sample, thereby increasing the axial resolution by a factor ranging from 2 to 7, down to approximately 80 nm. Since the axial resolution of conventional microscopy is so poor (500–700 nm), it is often a limiting factor in the interpretation of cell image data. In the case of the SC, two paths that may be distinguishable in the XY plane can merge into ambiguity when viewed from the side. With 4pi microscopy, however, Fritsche et al. (2012) were able to trace the paths of mouse SCs with extremely high accuracy and to incorporate these data into a physical model of large-scale chromosome organization. This example highlights the utility of superresolution not only for obtaining sub-diffraction structural data, but also for overcoming long-standing barriers to quantitative imaging associated with limited-resolution systems.
Other superresolution techniques applicable to meiotic chromosomes
Higher resolution with localization microscopy and stimulated emission depletion microscopy
Although 3D-SIM and 4pi microscopy offer great improvements over conventional widefield imaging, the dimensions of the SC as seen by EM (around 100 nm) can lie near or past the limit of their resolution capabilities. Especially when longer wavelengths of light are used, the resolution of 3D-SIM may be insufficient to resolve LEs or transverse filament proteins as two-track structures. Additionally, since the axial resolution of 3D-SIM is still limited to approximately 250 nm, only a subset of views of the SC within a sample are amenable to 3D-SIM analysis. To achieve even more detailed views of the SC, it will be necessary to make use of higher resolution imaging techniques. Two promising techniques for continued studies of the SC are (1) single-molecule localization microscopy, also known as fPALM (fluorescence photoactivated localization microscopy) or STORM (stochastic optical reconstruction microscopy), and (2) stimulated emission depletion (STED) microscopy (both reviewed in Toomre and Bewersdorf 2010).
Localization microscopy can achieve tremendous resolution, on the order of approximately 20–50 nm, in both the lateral and axial directions. By imaging the individual positions of a large number of stochastically activated single molecules within a structure and then fitting a mathematical distribution to the image data, the positions of the molecules in space can be localized to a very high precision. Axial (Z) information may be obtained via several enhancements to the basic technique, such as the use of astigmatism (Huang et al. 2008), simultaneous recording of several focal planes (Juette et al. 2008), or, in the iPALM technique, exploiting the interference in emitted photons passing through opposing objective lenses (Shtengel et al. 2009). The precision of localization is limited in principle only by the number of photons that can be detected above the background level. Recent advances in chemical fluorophore modification have provided further improvements in probe brightness, potentially allowing localization to just several nanometers (Vaughan et al. 2012). Localization microscopy can be performed with several probes simultaneously, making it a strong technique for exploring the structural composition of the SC in three dimensions.
STED microscopy is a superresolution extension of confocal scanning microscopy. It achieves superresolution by adding a shaped fluorescence-depleting beam to the normal excitation beam. The depletion beam shrinks the effective size of the excitation spot to below the diffraction limit, theoretically as small as desired but practically limited to the same range as localization microscopy, 50 ± 30 nm. STED and 4pi microscopy can be combined to enable isotropic multi-wavelength resolution of approximately 30 nm in both the lateral and axial directions (Schmidt et al. 2009). Multicolor 4pi-STED could potentially acquire images of the SC on the same size scale as localization microscopy. As the resolution of these techniques approaches and may surpass the approximately 20–30 nm spacing of transverse filaments seen in many EM preparations (Schmekel et al. 1993), they have incredible potential to unlock the remaining structural ambiguities of the SC.
Unanswered questions about SC structure
Chromatin accommodation within the SC
Before synapsis can occur, AEs must be properly formed in close association with chromatin. Surprisingly little is known about the mechanism of forming early meiotic prophase chromosomes. Meiotic chromosomes before synapsis consist of loops of chromatin bound to a cohesin core (Moens and Pearlman 1988). The spacing of loops is relatively constant between species, covering a range from 15 to 45 loops per micron (Kleckner 2006). Chromatin immunoprecipitation (ChIP) experiments have revealed chromosome locations that are reproducibly enriched for axial associations (Blat and Kleckner 1999; Glynn et al. 2004) in yeast. These sites represent a population consensus; actual loop formation sites in any given cell are likely to be somewhat variable. The proteins that make up the chromosome axis include the cohesin family (Eijpe et al. 2003). With the discovery of Rad21L (Ishiguro et al. 2011; Lee and Hirano 2011), it was also shown that the order of different cohesins along the meiotic chromosome was largely mirrored between homologs, even before synapsis. It should be possible to further elucidate this heterogeneity in SC structure with higher resolution imaging studies.
The chromatin loops themselves may present an underappreciated barrier to synapsis. It has been suggested that if chromatin loop sizes are large compared to their spacing, then the loops would surround the axial element with a halo of chromatin as a result of the loops’ tendency to assume an equilibrium conformation (Marko and Siggia 1997). For two AEs to synapse, they must approach within <200 nm of each other; consequently, this halo of chromatin must be displaced, i.e., the chromatin located between the AEs must somehow move out of the way. If the properties of the chromatin do not otherwise change at this time, this displacement would cause an increase in the density of chromatin loops, which would be resisted by “chromatin pressure”, a manifestation of the chromatin fiber’s tendency to resume its equilibrium conformation. A feature common to meiotic chromosomes at this stage of initial pairing is rapid dynamic movement of chromosome ends mediated by the cytoskeleton (Baudrimont et al. 2010; Chikashige et al. 2009; Koszul et al. 2008; Morimoto et al. 2012; Wynne et al. 2012); it is conceivable that this movement could provide some of the necessary force to make axes coincide. Alternatively, several observations of chromosomes before and after synapsis suggest that instead of a radial arrangement, the loops of chromatin point away from the axis, as co-oriented arrays (reviewed in Kleckner et al. 2004). This arrangement, which could conceivably be brought about by specific interactions between chromatin and the axis, would be more favorable to pairing.
Twisting of the synaptonemal complex
Twisting as a general feature of SC structure has been known from EM reconstructions from serial sections (see e.g. (Lin 1979) ). Using 3D-SIM, Wang et al. observed SC twisting in maize, with a strong bias toward left-handed helicity. Phillips et al. also observed twisting in their preparations of barley SCs, though without a pronounced handedness bias. The mechanism behind twisting is not known, but the progressive nature of twisting in maize as well as the handedness bias seen in some organisms indicates it may be the result of an active process, and not simply a random or equilibrium configuration. Among the mechanisms that could result in twisting is differential length change: if one part of the meiotic chromosome (the central element, for example) were to contract relative to another part, then twisting could be induced if LE components had a strong tendency to maintain a constant relative spacing (Fig. 3). The amount of length contraction necessary would be quite small: given the relation of helix extension z to path length s, 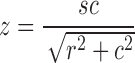 , and the SC’s helix radius r (100 nm), a length contraction of only 1 % would suffice to give a visible half-gyre spacing c of approximately 2.5 μm. A hypothesized relationship of SC twisting to differential lengths of SC components is predicated upon the prevalence of twists in situations of heterologous synapsis—for example, differently sized sex chromosomes (Solari 1992) or a resizing inversion loop (Moses et al. 1982). Alternatively, twisting may be induced by the dynamic movement of chromosome ends at the nuclear envelope (Hiraoka and Dernburg 2009), if there were any net rotation of chromosome ends in addition to the translational movement. Contrasting synapsis in species with and without biased twisting using superresolution microscopy may shed light on the mechanisms involved.
, and the SC’s helix radius r (100 nm), a length contraction of only 1 % would suffice to give a visible half-gyre spacing c of approximately 2.5 μm. A hypothesized relationship of SC twisting to differential lengths of SC components is predicated upon the prevalence of twists in situations of heterologous synapsis—for example, differently sized sex chromosomes (Solari 1992) or a resizing inversion loop (Moses et al. 1982). Alternatively, twisting may be induced by the dynamic movement of chromosome ends at the nuclear envelope (Hiraoka and Dernburg 2009), if there were any net rotation of chromosome ends in addition to the translational movement. Contrasting synapsis in species with and without biased twisting using superresolution microscopy may shed light on the mechanisms involved.
Fig. 3.
a Relationship between contraction of the center filament (cyan) of an SC-like tripartite ribbon and twisting in the outer axes (red) if they are unable to contract (i.e., if their arc length is constant). In this example simulation, with a radius set to 100 nm and original length of 25 μm, a 1 % contraction in CE length (arrow) results in approximately 5.6 full LE twists, approx. 4.4 μm apart. The original position of the right end is indicated by a green line. Full-length movie of this simulation is shown in the Electronic Supplementary Material (movie 2). b 3D-SIM image of a mouse pachytene SC stained with antibodies against Sycp3. Twisting is apparent at a pitch of approx. 2 μm per full turn. Scale bar: 0.5 μm
Dynamic behavior of the synaptonemal complex
Even less well understood than the SC’s structure is the question of its dynamics: how it is assembled, what its behavior is when fully formed, and how it is disassembled. SC initiation is not random: in budding yeast, it initiates at sites bound by the synapsis initiation complex (SIC), which includes proteins Zip2, Zip3, and Zip4 (Fung et al. 2004; Rockmill et al. 1995). The progression of synapsis after initiation has been presumed to be mostly processive, lacking significant desynapsis, in the normal meiotic program. However, this model awaits direct confirmation by in vivo observation of synapsis. Little is known about the dynamics of the complete SC. That the SC must be able to dynamically change is inferred by several observations, such as (1) adjustment of inversion loops or deletions (Moses and Poorman 1981; Poorman et al. 1981; Tease and Fisher 1986); (2) correction of synapsis in polyploids from groups of three or four to pairs of two (Rasmussen 1977; Rasmussen and Holm 1979); (3) extensive desynapsis and resynapsis in response to ionizing radiation (Couteau and Zetka 2011). Additionally, a recent study in budding yeast, in which a fluorescent Zip1 protein was expressed in prophase-arrested cells with complete synapsis (Voelkel-Meiman et al. 2012), found that new Zip1p molecules continually accumulate in the mature SC, indicating a dynamic flexibility in SC protein stoichiometry. Whether accumulated Zip1p molecules pack into an existing structure or whether they assume an alternate configuration, remains to be determined. Superresolution imaging of transverse filament molecules that have been differentially labeled could shed light on this question.
Further questions
While we understand SC structure and function in broad outline, several outstanding questions still have only rudimentary answers.
What is the stoichiometry and precise position of the various CE proteins in a given sub-assembly, and how do these vary over the length of the SC?
Do variant CE substructures have functional differences?
What is the higher order 3D orientation of each sub-assembly? Various possibilities are: (1) subcomplexes always arrange in a polar fashion (i.e., all oriented the same way); (2) always lining up head-to-head and thus without any overall large-scale polarity; (3) randomly oriented, exhibiting short domains of different polarity over varying lengths.
How are the various LE proteins arranged with respect to each other? Several different LE proteins are known to exist, including four in C. elegans. Whether these form a regularly patterned structure, or separate continuous axes, is not known.
How is meiotic chromatin incorporated within the axis? If loops are constrained to point away from the AE, rather than surrounding it, how is this achieved?
In all cases, superresolution optical techniques will be essential tools for obtaining information necessary to answer these questions. Combined with molecular and genetic tools for understanding the functional roles of its protein subunits, the near future will likely see a continued unraveling of the puzzle of how the structure of the SC allows it to carry out such diverse and essential functions in meiosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(Nucleus). The nucleus from Fig. 1c in maximum-intensity projection. DAPI staining of DNA is shown in blue; the lateral elements stained with anti-HTP-3 in red; and a single section of LE staining is highlighted in yellow. Scale bar, 0.5 μm. (MPG 1406 kb)
(Movie of twisting). A frame-by-frame animation of the twisting as shown in Fig. 3. In this movie, contraction of the central element (cyan) begins at the midpoint and proceeds simultaneously to both ends, to a final value of 1 % total contraction. Source code in the Processing language is available at: https://github.com/pmcarlton/Chromosomics. (MPG 1140 kb)
Acknowledgments
The author thanks Aya Sato for discussion and critical comments on the manuscript, and Shinichiro Chuma for the mouse spermatocyte sample and anti-Sycp3 antibodies. This work was supported in part by a JSPS Grant-in-Aid for Young Scientists A (#24687024). The iCeMS is supported by World Premier International Research Center Initiative (WPI), MEXT, Japan.
Conflict of interest
None
Abbreviations
- SC
Synaptonemal complex
- CE
Central elements
- LE
Lateral elements
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-013-0116-0) contains supplementary material, which is available to authorized users.
References
- Aravind L, Koonin EV. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem Sci. 1998;23(8):284–286. doi: 10.1016/S0968-0004(98)01257-2. [DOI] [PubMed] [Google Scholar]
- Armstrong SJ, Caryl AP, Jones GH, Franklin FCH. Asy1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brassica. J Cell Sci. 2002;115(18):3645–3655. doi: 10.1242/jcs.00048. [DOI] [PubMed] [Google Scholar]
- Bailis JM, Roeder GS. Synaptonemal complex morphogenesis and sister-chromatid cohesion require Mek1-dependent phosphorylation of a meiotic chromosomal protein. Genes Dev. 1998;12(22):3551–3563. doi: 10.1101/gad.12.22.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudrimont A, Penkner A, Woglar A, Machacek T, Wegrostek C, Gloggnitzer J, Fridkin A, Klein F, Gruenbaum Y, Pasierbek P, Jantsch V. Leptotene/zygotene chromosome movement via the SUN/KASH protein bridge in Caenorhabditis elegans. PLoS Genet. 2010;6(11):e1001219. doi: 10.1371/journal.pgen.1001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blat Y, Kleckner N. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell. 1999;98(2):249–259. doi: 10.1016/S0092-8674(00)81019-3. [DOI] [PubMed] [Google Scholar]
- Bolcun-Filas E, Costa Y, Speed R, Taggart M, Benavente R, De Rooij DG, Cooke HJ. SYCE2 is required for synaptonemal complex assembly, double strand break repair, and homologous recombination. J Cell Biol. 2007;176(6):741–747. doi: 10.1083/jcb.200610027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton PM. Three-dimensional structured illumination microscopy and its application to chromosome structure. Chromosom Res. 2008;16(3):351–365. doi: 10.1007/s10577-008-1231-9. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Yamane M, Okamasa K, Tsutsumi C, Kojidani T, Sato M, Haraguchi T, Hiraoka Y. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J Cell Biol. 2009;187(3):413–427. doi: 10.1083/jcb.200902122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteau F, Zetka M. DNA damage during meiosis induces chromatin remodeling and synaptonemal complex disassembly. Dev Cell. 2011;20(3):353–363. doi: 10.1016/j.devcel.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Couteau F, Goodyer W, Zetka M. Finding and keeping your partner during meiosis. Cell Cycle. 2004;3(8):1012–1014. doi: 10.4161/cc.3.8.1077. [DOI] [PubMed] [Google Scholar]
- Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1β and SMC3. J Cell Biol. 2003;160(5):657–670. doi: 10.1083/jcb.200212080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW. The fine structure of chromosomes in the meiotic prophase of vertebrate spermatocytes. J Biophys Biochem Cytol. 1956;2(4):403–406. doi: 10.1083/jcb.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraune J, Schramm S, Alsheimer M, Benavente R. The mammalian synaptonemal complex: protein components, assembly and role in meiotic recombination. Exp Cell Res. 2012;318(12):1340–1346. doi: 10.1016/j.yexcr.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Fritsche M, Reinholdt LG, Lessard M, Handel MA, Bewersdorf J, Heermann DW. The impact of entropy on the spatial organization of synaptonemal complexes within the cell nucleus. PLOS One. 2012;7(5):e36282. doi: 10.1371/journal.pone.0036282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Pratto F, Schimenti JC, Turner JMA, Camerini-Otero RD, Höög C. Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis. PLoS Genet. 2012;8(2):e1002485. doi: 10.1371/journal.pgen.1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung JC, Rockmill B, Odell M, Roeder GS. Imposition of crossover interference through the nonrandom distribution of synapsis initiation complexes. Cell. 2004;116(6):795–802. doi: 10.1016/S0092-8674(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Gillies C. Ultrastructural analysis of maize pachytene karyotypes by three dimensional reconstruction of the synaptonemal complexes. Chromosoma. 1973;43(2):145–176. doi: 10.1007/BF00483376. [DOI] [Google Scholar]
- Glynn EF, Megee PC, Yu HG, Mistrot C, Unal E, Koshland DE, DeRisi JL, Gerton JL. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol. 2004;2(9):e259. doi: 10.1371/journal.pbio.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MGL, Shao L, Carlton PM, Wang RCJ, Golubovskaya IN, Cande WZ, Agard DA, Sedat JW. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys J. 2008;94(12):4957–4970. doi: 10.1529/biophysj.107.120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley RS. Solving a meiotic LEGO\textsuperscript® puzzle: transverse filaments and the assembly of the synaptonemal complex in \textitCaenorhabditis elegans. Genetics. 2011;189(2):405–409. doi: 10.1534/genetics.111.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Mlynarczyk-Evans S, Villeneuve AM. The synaptonemal complex shapes the crossover landscape through cooperative assembly, crossover promotion and crossover inhibition during Caenorhabditis elegans meiosis. Genetics. 2010;186(1):45–58. doi: 10.1534/genetics.110.115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JD, Sanchez-Moran E, Armstrong SJ, Jones GH, Franklin FCH. The Arabidopsis synaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over. Genes Dev. 2005;19(20):2488–2500. doi: 10.1101/gad.354705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Dernburg AF. The SUN rises on meiotic chromosome dynamics. Dev Cell. 2009;17(5):598–605. doi: 10.1016/j.devcel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Huang B, Wang W, Bates M, Zhuang X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science. 2008;319(5864):810–813. doi: 10.1126/science.1153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro KI, Kim J, Fujiyama-Nakamura S, Kato S, Watanabe Y. A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep. 2011;12(3):267–275. doi: 10.1038/embor.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juette MF, Gould TJ, Lessard MD, Mlodzianoski MJ, Nagpure BS, Bennett BT, Hess ST, Bewersdorf J. Three-dimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat Methods. 2008;5(6):527–529. doi: 10.1038/nmeth.1211. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Chiasma formation: chromatin/axis interplay and the role(s) of the synaptonemal complex. Chromosoma. 2006;115(3):175–194. doi: 10.1007/s00412-006-0055-7. [DOI] [PubMed] [Google Scholar]
- Kleckner N, Zickler D, Jones GH, Dekker J, Padmore R, Henle J, Hutchinson J. A mechanical basis for chromosome function. Proc Natl Acad Sci USA. 2004;101(34):12592–12597. doi: 10.1073/pnas.0402724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszul R, Kim KP, Prentiss M, Kleckner N, Kameoka S. Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell. 2008;133(7):1188–1201. doi: 10.1016/j.cell.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hirano T. RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol. 2011;192(2):263–276. doi: 10.1083/jcb.201008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Fine structure of meiotic prophase chromosomes and modified synaptonemal complexes in diploid and triploid Rhoeo spathacea. J Cell Sci. 1979;37(1):69. doi: 10.1242/jcs.37.1.69. [DOI] [PubMed] [Google Scholar]
- MacQueen AJ, Colaiacovo MP, McDonald K, Villeneuve AM. Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev. 2002;16(18):2428–2442. doi: 10.1101/gad.1011602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko JF, Siggia ED. Polymer models of meiotic and mitotic chromosomes. Mol Biol Cell. 1997;8(11):2217–2231. doi: 10.1091/mbc.8.11.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Pearlman RE. Chromatin organization at meiosis. BioEssays. 1988;9(5):151–153. doi: 10.1002/bies.950090503. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Shibuya H, Zhu X, Kim J, Ishiguro K-I, Han M, Watanabe Y. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol. 2012;198(2):165–172. doi: 10.1083/jcb.201204085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses MJ. Chromosomal structures in crayfish spermatocytes. J Biophys Biochem Cytol. 1956;2(2):215–218. doi: 10.1083/jcb.2.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses M, Poorman P. Synaptonemal complex analysis of mouse chromosomal rearrangements. Chromosoma. 1981;81(4):519–535. doi: 10.1007/BF00285847. [DOI] [PubMed] [Google Scholar]
- Moses M, Poorman P, Roderick T, Davisson M. Synaptonemal complex analysis of mouse chromosomal rearrangements. Chromosoma. 1982;84(4):457–474. doi: 10.1007/BF00292848. [DOI] [PubMed] [Google Scholar]
- Page SL, Khetani RS, Lake CM, Nielsen RJ, Jeffress JK, Warren WD, Bickel SE, Hawley RS. Corona is required for higher-order assembly of transverse filaments into full-length synaptonemal complex in Drosophila oocytes. PLoS Genet. 2008;4(9):e1000194. doi: 10.1371/journal.pgen.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D, Nibau C, Wnetrzak J, Jenkins G. High resolution analysis of meiotic chromosome structure and behaviour in Barley (Hordeum vulgare L.) PLoS One. 2012;7(6):e39539. doi: 10.1371/journal.pone.0039539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorman P, Moses M, Russell L, Cacheiro N. Synaptonemal complex analysis of mouse chromosomal rearrangements. Chromosoma. 1981;81(4):507–518. doi: 10.1007/BF00285846. [DOI] [PubMed] [Google Scholar]
- Qiao H, Chen JK, Reynolds A, Höög C, Paddy M, Hunter N. Interplay between synaptonemal complex, homologous recombination, and centromeres during mammalian meiosis. PLoS Genet. 2012;8(6):e1002790. doi: 10.1371/journal.pgen.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SW. Chromosome pairing in triploid females of Bombyx mori analyzed by three dimensional reconstructions of synaptonemal complexes. Carlsb Res Commun. 1977;42(3):163–197. doi: 10.1007/BF02910461. [DOI] [Google Scholar]
- Rasmussen SW, Holm PB. Chromosome pairing in autotetraploid Bombyx females. Mechanism for exclusive bivalent formation. Carlsb Res Commun. 1979;44(2):101–125. doi: 10.1007/BF02906525. [DOI] [Google Scholar]
- Rockmill B, Sym M, Scherthan H, Roeder GS. Roles for two RecA homologs in promoting meiotic chromosome synapsis. Genes Dev. 1995;9(21):2684–2695. doi: 10.1101/gad.9.21.2684. [DOI] [PubMed] [Google Scholar]
- Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso CM, Agard DA, Gustafsson MG, Leonhardt H, Sedat JW. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320(5881):1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh L, Heintzmann R, Leonhardt H. A guide to super-resolution fluorescence microscopy. J Cell Biol. 2010;190(2):165–175. doi: 10.1083/jcb.201002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild-Prüfert K, Saito TT, Smolikov S, Gu Y, Hincapie M, Hill DE, Vidal M, McDonald K, Colaiacovo MP. Organization of the synaptonemal complex during meiosis in Caenorhabditis elegans. Genetics. 2011;189(2):411–421. doi: 10.1534/genetics.111.132431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmekel K, Skoglund U, Daneholt B. The three-dimensional structure of the central region in a synaptonemal complex: a comparison between rat and two insect species, Drosophila melanogaster and Blaps cribrosa. Chromosoma. 1993;102(10):682–692. doi: 10.1007/BF00650894. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Wurm CA, Punge A, Egner A, Jakobs S, Hell SW. Mitochondrial cristae revealed with focused light. Nano Lett. 2009;9(6):2508–2510. doi: 10.1021/nl901398t. [DOI] [PubMed] [Google Scholar]
- Schrader M, Bahlmann K, Giese G, Hell SW. 4Pi-confocal imaging in fixed biological specimens. Biophys J. 1998;75(4):1659–1668. doi: 10.1016/S0006-3495(98)77608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtengel G, Galbraith JA, Galbraith CG, Lippincott-Schwartz J, Gillette JM, Manley S, Sougrat R, Waterman CM, Kanchanawong P, Davidson MW, et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc Natl Acad Sci USA. 2009;106:3125–3130. doi: 10.1073/pnas.0813131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari A. Equalization of Z and W axes in chicken and quail oocytes. Cytogenet Genome Res. 1992;59(1):52–56. doi: 10.1159/000133199. [DOI] [PubMed] [Google Scholar]
- Solari AJ, Moses MJ. The structure of the central region in the synaptonemal complexes of hamster and cricket spermatocytes. J Cell Biol. 1973;56(1):145–152. doi: 10.1083/jcb.56.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tease C, Fisher G. Further examination of the production-line hypothesis in mouse foetal oocytes. Chromosoma. 1986;93(5):447–452. doi: 10.1007/BF00285827. [DOI] [PubMed] [Google Scholar]
- Toomre D, Bewersdorf J. A new wave of cellular imaging. Annu Rev Cell Dev Biol. 2010;26:285–314. doi: 10.1146/annurev-cellbio-100109-104048. [DOI] [PubMed] [Google Scholar]
- Vaughan JC, Jia S, Zhuang X. Ultrabright photoactivatable fluorophores created by reductive caging. Nat Methods. 2012;9:1181–1184. doi: 10.1038/nmeth.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman K, Moustafa SS, Lefrançois P, Villeneuve AM, Macqueen AJ. Full-length synaptonemal complex grows continuously during meiotic prophase in budding yeast. PLoS Genet. 2012;8(10):e1002993. doi: 10.1371/journal.pgen.1002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-JR, Carlton PM, Golubovskaya IN, Cande WZ. Interlock formation and coiling of meiotic chromosome axes during synapsis. Genetics. 2009;183(3):905–915. doi: 10.1534/genetics.109.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts F, Hoffmann E. SUMO meets meiosis: an encounter at the synaptonemal complex. Bioessays. 2011;33(7):529–537. doi: 10.1002/bies.201100002. [DOI] [PubMed] [Google Scholar]
- Wynne DJ, Rog O, Carlton PM, Dernburg AF. Dynein-dependent processive chromosome motions promote homologous pairing in C. elegans meiosis. J Cell Biol. 2012;196(1):47–64. doi: 10.1083/jcb.201106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Miley N, Zastrow MS, Macqueen AJ, Sato A, Nabeshima K, Martinez-Perez E, Mlynarczyk-Evans S, Carlton PM, Villeneuve AM. HAL-2 promotes homologous pairing during Caenorhabditis elegans meiosis by antagonizing inhibitory effects of synaptonemal complex precursors. PLoS Genet. 2012;8(8):e1002880. doi: 10.1371/journal.pgen.1002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Nucleus). The nucleus from Fig. 1c in maximum-intensity projection. DAPI staining of DNA is shown in blue; the lateral elements stained with anti-HTP-3 in red; and a single section of LE staining is highlighted in yellow. Scale bar, 0.5 μm. (MPG 1406 kb)
(Movie of twisting). A frame-by-frame animation of the twisting as shown in Fig. 3. In this movie, contraction of the central element (cyan) begins at the midpoint and proceeds simultaneously to both ends, to a final value of 1 % total contraction. Source code in the Processing language is available at: https://github.com/pmcarlton/Chromosomics. (MPG 1140 kb)