Abstract
Background
Ischemic stroke occurs in a significant subset of patients with blunt traumatic cerebrovascular injury (TCVI). The patients are victims of motor vehicle crashes, assaults or other high-energy collisions, and suffer ischemic stroke due to injury to the extracranial carotid or vertebral arteries.
Summary
An increasing number of patients with TCVI are being identified, largely because of the expanding use of computed tomography angiography for screening patients with blunt trauma. Patients with TCVI are particularly challenging to manage because they often suffer polytrauma, that is, numerous additional injuries including orthopedic, chest, abdominal, and head injuries. Presently, there is no consensus about optimal management.
Key Messages
Most literature about TCVI and stroke has been published in trauma, general surgery, and neurosurgery journals; because of this, and because these patients are managed primarily by trauma surgeons, patients with stroke due to TCVI have been essentially hidden from view of neurologists. This review is intended to bring this clinical entity to the attention of clinicians and investigators with specific expertise in neurology and stroke.
KeyWords: Trauma, Dissection, Stroke
Traumatic Cerebrovascular Injury Is a Distinct Clinical Entity
Traumatic cerebrovascular injury (TCVI) is markedly different from spontaneous dissections or dissections occurring after low-energy trauma. Truly spontaneous dissections and dissections that occur after coughing fits, rapid head turning or rapid neck extension are an important cause of stroke and have been studied extensively. The risk of stroke with spontaneous and low-energy dissections was recently assessed in the Cervical Artery Dissection in Stroke Study (CADISS) [1]. However, TCVI differs from spontaneous dissection in several ways. Specifically, (1) most TCVIs are initially asymptomatic and discovered by screening imaging; (2) high-energy TCVI is likely biologically distinct from spontaneous dissections, which are frequently related to underlying connective tissue abnormalities [2]; (3) most blunt trauma patients experience a hypercoagulable state soon after injury [3] that coincides with the time course of ischemic stroke with TCVI, mostly occurring within 72 h of the injury [4]; (4) spontaneous cervical arterial dissections tend to primarily affect the outer arterial wall [5], in contrast to TCVI, in which intimal disruptions are relatively common [6, 7, 8]; and (5) patients with TCVI often have other traumatic injuries, making their management more complex than patients with spontaneous dissection.
Epidemiology and Risk Factors
Extracranial TCVI is present in approximately 1–2% of patients admitted after blunt trauma [9, 10]. Based on an average annual number of blunt trauma admissions in the US of 2,405,000 [11], up to 48,000 patients with TCVI are admitted each year. Based on studies reporting that 10–20% of patients with TCVI have a stroke [4, 10, 12], TCVI may be responsible for up to 9,600 ischemic strokes in the US per year.
Patients admitted with blunt trauma and a risk factor for TCVI routinely undergo a screening neck computed tomography angiography (CTA) to look for TCVI. Due to the increasing availability and use of CTA, the incidence of the diagnosis of TCVI has been rising over the last decade [13, 14]. Approximately 20% of all blunt trauma patients have a risk factor for TCVI and thus are candidates for a screening CTA; based on the annual number of blunt trauma admissions in the US, this accounts for 480,000 screening CTAs each year. With an average cost of a screening neck CTA estimated at USD 708 [15], this translates into USD 339,840,000 spent on screening neck CTAs annually.
Motor vehicle crashes are the most common cause of TCVI, followed by assaults, falls, and hanging. Risk factors for TCVI are established and include cervical spine fracture, skull fracture, closed head injury, cervical seat belt bruising, mandible fracture, and a high injury severity score [9, 16, 17]. Additional risk factors include intracranial hemorrhage, hanging, facial injury, hemotympanum, thoracic or lumbar spine fracture, Horner syndrome, and clavicular fracture [9]. It is believed that the use of screening CTA for all blunt trauma patients with a risk factor for TCVI captures approximately 80% of all TCVIs.
Pathophysiology
High-energy, nonpenetrating trauma can result in a disruption in one of more layers of the cervical carotid or vertebral artery wall. These defects can be solitary or multiple, with one or more patterns of injury (Fig. 1). Excessive and rapid neck motion causing stretching of the artery, or a direct blow to the artery, may produce intimal tears and expose subendothelial collagen, resulting in platelet activation and thrombosis with a potential for thromboembolism or occlusion of the artery (Fig. 2). This thrombotic process is likely exacerbated by a trauma-induced hypercoagulable state occurring early after injury [3]. An intimal defect can provide a pathway for blood to enter the layers of the artery wall, causing stenosis or occlusion of the artery. Disruption of the elastic laminae may lead to expansion of the adventitia with traumatic aneurysm formation [8]. The cervical internal carotid artery (ICA) is most vulnerable to stretch injury in the region between the soft tissue of the neck and the petrous canal. The distal cervical ICA is also vulnerable to stretch injury over the second and third cervical vertebrae during extremes of hyperextension, lateral flexion, or rotation. Furthermore, hyperflexion and significant rotation place the ICA at risk for compression by the angle of the mandible and styloid process. The majority of vertebral artery injuries are associated with subluxation, fracture of cervical vertebrae 1–3, and fractures involving the foramen transversarium. More than one cervical artery is affected in about one-third of cases.
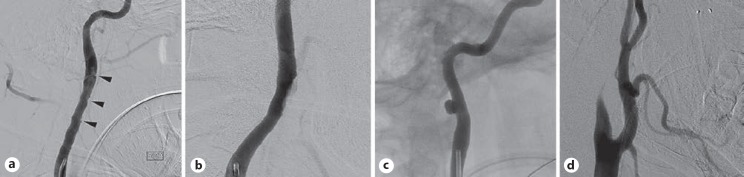
Fig. 1.
Traumatic cerebrovascular injuries type 1–4. a Type 1; multiple intimal disruptions (arrow heads). b Type 2. c Type 3. d Type 4.
Fig. 2.
Traumatic cerebrovascular injuries. a Intimal disruption. b Intimal disruption with thrombus formation. c Elastic laminae disruption allowing traumatic aneurysm formation (arrows). d Hematoma within the artery wall with luminal stenosis.
Imaging
Digital subtraction angiography (DSA) has been conventionally considered the gold standard for imaging TCVI. Angiography can produce high-resolution images of the injury, detect subtle intimal defects, and recognize intraluminal thrombus and intracranial arterial occlusions more accurately than any other modality. It also affords the opportunity to undertake endovascular treatment if necessary. However, DSA has limitations in terms of practicality (particularly in patients with polytrauma), expense, and availability. Presently, DSA is usually reserved for patients with uncertain noninvasive imaging findings or with a clear indication for a therapeutic endovascular procedure.
Advancements in CT technology over the last 2 decades have made rapid high-resolution neck and head CTA feasible at most trauma centers. A screening CTA can be done in several minutes and can be included with other CT examinations during the initial imaging of patients with blunt trauma. Improvements in CT technology, coupled with growing experience, have led to greater accuracy of CTA in the diagnosis of TCVI. According to the Blunt Cerebrovascular Injury Practice Management Guidelines of the Eastern Association for the Surgery of Trauma, CTA is the preferred method for screening for TCVI [18]. A recent systematic review of 8 studies on the accuracy of CTA for the diagnosis of TCVI as compared to DSA found a combined pooled sensitivity and specificity of 66 and 97%, respectively [19]. Despite the limited sensitivity of CTA, it remains the preferred method of TCVI screening among North American trauma surgeons, neurosurgeons, stroke neurologists, and interventional radiologists [20], and is routinely employed at most trauma centers for patients with TCVI risk factors.
Magnetic resonance imaging (MRI) and angiography (MRA) can be a useful adjunct for the detection of ischemic brain injury, especially in the acute period. The accuracy of MRA in this setting may be limited; an early study comparing MRA to DSA reported sensitivities for the detection of carotid and vertebral artery injuries of 50 and 47%, respectively [21]. More contemporary reports of the accuracy of MRA in detecting TCVI are lacking. MRI also has practical limitations as a screening tool regarding time of acquisition, MRI compatibility, and patient transport; currently, it is most useful in trauma patients for evaluating stroke.
Ultrasound is not useful for screening for TCVI due to an inability to visualize the upper cervical ICA and vertebral arteries. However, transcranial Doppler (TCD) ultrasonography for the detection of microemboli can be useful as an adjunctive imaging technique to identify TCVI patients at high risk of embolic stroke [22, 23].
TCVI Classification
A widely used classification scheme for blunt TCVI divides carotid and vertebral artery injuries into 5 subtypes (Table 1) [24, 25]. It is important to note that this system was developed based on DSA and that it is difficult to distinguish type 1, 2, and 3 TCVI with CTA alone. Many contemporary studies investigating the frequency and prognosis of type 1–3 lesions have primarily employed CTA alone [4, 23, 26, 27, 28, 29]; therefore, the reliability of these reports, in precisely distinguishing between TCVI types, is limited. However, CTA can reliably identify type 4 lesions (complete artery occlusion). Type 5 injuries, transection of the artery, are rarely seen and not usually survivable.
Table 1.
Traumatic cerebrovascular classification scheme and relative frequencies1
| Injury type | Description | Proportion of all blunt traumatic carotid artery injuries [24], % | Proportion of all blunt traumatic vertebral artery injuries [25], % |
|---|---|---|---|
| 1 | Luminal irregularity or dissection with <25% luminal narrowing | 61 | 53 |
| 2 | Dissection or intramural hematoma with ≥25% luminal narrowing | 17 | 19 |
| 3 | Traumatic aneurysm | 15 | 6 |
| 4 | Occlusion | 5 | 21 |
| 5 | Transection | 4 | 0 |
By digital subtraction angiography.
Mechanism and Risk of Ischemic Stroke
The most common mechanism of ischemic stroke due to TCVI is thromboembolism; this is corroborated by the pattern of ischemic injury and the presence of microembolic signals on TCD ultrasonography [12]. The finding of at least 8 emboli per hour is strongly associated with embolic stroke in patients with both type 3 [23] and type 4 TCVI [22]. Hemodynamic failure is likely a factor in ICA occlusion and bilateral vertebral artery occlusion [22].
The precise risk of ischemic stroke with TCVI is difficult to discern. Many studies of stroke in this setting were limited by factors including a lack of a precise definition of ischemic stroke, a lack of patient evaluation by investigators with neurological expertise, a failure to distinguish patients presenting with a stroke due to TCVI from patients with initially asymptomatic TCVI who go on to develop a stroke, and a failure to distinguish stroke from other causes of neurological injury in patients with polytrauma. It is clear that the majority of ischemic strokes due to TCVI occur relatively early, either prior to admission or within 72 h of the injury [4, 10, 12, 30]. It is useful to divide patients with TCVI into 2 categories: (1) patients with an ischemic stroke at the time of initial diagnosis of TCVI and (2) patients who are found to have an initially asymptomatic TCVI, discovered with screening, and who are at risk of stroke.
Stroke at the Time of Diagnosis of TCVI
Several studies have found that a significant subset of patients with TCVI-related stroke suffered the ischemic event prior to admission to the hospital or prior to initial vascular imaging [10, 12, 30]. In these patients, the stroke likely occurred at the time of the original arterial injury, during transport to the hospital, or during the early phase following admission. In a report of blunt trauma patients in 2 level I trauma centers, 9 out of 11 patients (82%) with TCVI-related stroke had the stroke within 2 h of the injury [30]. Two other similar studies identified an ischemic stroke on initial imaging in 44% [10] and 67% [12] of patients.
Stroke in Patients with Initially Asymptomatic TCVI
Patients who are found to have an asymptomatic TCVI on screening imaging and then are treated with antithrombotic therapy appear to have an overall risk of ischemic stroke of up to 20% [10, 12, 21, 25, 26, 31, 32, 33, 34, 35]. Studies reporting on initially asymptomatic patients treated with antiplatelet agents indicate a risk of stroke of up to 11% [10, 12, 21, 26, 31, 32, 33, 34, 36]. Studies reporting on initially asymptomatic patients treated with anticoagulation indicate a risk of stroke of up to 20% [10, 32]. It cannot be determined, based on the available data, whether treatment with antiplatelet agents or anticoagulation is more effective in preventing ischemic stroke in this setting. No prospective studies comparing anticoagulation to antiplatelet agents have been published.
Risk of Stroke according to Type of Injury
The risk of ischemic stroke appears to correlate, to some extent, with the type of arterial injury. Biffl et al. [24, 25], the originators of the TCVI grading system, reported that the risk of stroke was greater with each ascending type. They reported stroke rates with type 1, type 2, type 3, and type 4 ICA injuries of 3, 11, 33, and 44%, respectively [24]. It seems intuitive that type 2 injuries (i.e., dissection with ≥25% narrowing of the lumen) would carry a higher risk of stroke compared to type 1 injuries (irregularity of the vessel wall or a dissection with <25% narrowing of the lumen). However, early reports of an even higher risk of stroke with type 3 injuries (traumatic aneurysms) [24, 33] have not been supported by more recent studies, which have found that the risk of stroke with traumatic aneurysms is not different from the risk of stroke with type 1 and type 2 injuries [37]. Moreover, the grading system popularized by Biffl et al. [24, 25] was developed based on the appearance of TCVI lesions on DSA. It has become clear in recent years that CTA cannot reliably distinguish type 1, type 2, and type 3 lesions. CTA can, however, reliably identify type 4 lesions (complete arterial occlusion), which are also the least common and carry the highest risk of stroke [22, 33, 38, 39]. All pieces of evidence indicate that the risk of stroke with traumatic vessel occlusion (type 4) exceeds the risk of stroke with type 1–3 injuries: unilateral occlusion of the vertebral artery (9–20%) [10, 22, 33, 39], unilateral occlusion of the ICA (>50%) [10, 22, 33, 38], and bilateral occlusion of the vertebral arteries (50%) [22, 39]. Therefore, in the present era in which CTA is the most commonly used imaging technique for TCVI [20], the risk of stroke with initially asymptomatic TCVI can be divided into 3 relative categories: low (arterial injury without occlusion), medium (single vertebral artery occlusion), and high (ICA occlusion and bilateral vertebral artery occlusion).
Antithrombotic Therapy
Given that thromboembolism is the primary mechanism of ischemic stroke in TCVI, treatment with antithrombotic medication seems prudent. Data from nonrandomized, uncontrolled studies suggest that antithrombotic therapy is protective against ischemic stroke in this setting [10, 12, 33, 40]. A survey of US clinicians found clinicians divided between favoring anticoagulation (42.8%), antiplatelet agents (32.5%), or both (17.1%) [20].
The most widely reported antithrombotic regimen is anticoagulation with intravenous heparin. However, hemorrhagic complication rates of anticoagulation in this setting range from 8 to 16% [21, 41], and about one-third of patients with TCVI are not candidates for systemic anticoagulation due to polytrauma [10, 42]. Although low-molecular-weight heparin may be safer than unfractionated heparin, it has not been studied in a systematic fashion.
Treatment with antiplatelet agents offers advantages over systemic anticoagulation in that antiplatelet agents are easier to maintain and appear to be relatively well tolerated in patients with polytrauma. In a study of 68 TCVI patients treated with aspirin, 325 mg per day, no adverse events attributable to the aspirin were identified [12]. Two studies found lower rates of bleeding complications with antiplatelet agents when compared to anticoagulation [42, 43]. Furthermore, antiplatelet agents carry a theoretical advantage in that the mechanism of stroke in TCVI is usually artery-to-artery embolism, and antiplatelet agents may be more appropriate in the platelet-rich arterial environment. Several retrospective studies have suggested that antiplatelet therapy is equivalent to anticoagulation in terms of neurological outcomes [10, 36, 42].
Traumatic Aneurysms: A Unique Subset
Extracranial traumatic aneurysms (i.e., type 3 lesions) result from disruption of the elastic laminae with preservation and stretching of the adventitia. The colloquial term pseudoaneurysm implies a complete disruption of all layers of the artery wall; although some aneurysms caused by trauma may contain a complete disruption of the artery wall, the term traumatic aneurysm is more precise in this setting and is preferable. Traumatic aneurysms of the extracranial carotid system are present in 10% of cases [10, 24, 37] and tend to occur in the upper cervical segment, at or just below the skull base. Traumatic aneurysms of the vertebral artery are less frequent and appear in 6.5% of cases [10, 37].
The risk of ischemic stroke in the acute phase after injury for traumatic aneurysms is probably similar to the risk of stroke with type 1 and 2 lesions. The risk of stroke with traumatic aneurysms ranges up to 15.4% [10, 22, 37].
A distinctive aspect of traumatic aneurysms is that they can be dynamic in shape and size. A study of the DSA appearance of TCVI lesions found that 8% of lesions consisting only of a luminal irregularity later progressed to a traumatic aneurysm [33]. Some 19–38% of traumatic aneurysms resolve completely on follow-up imaging and 18–28% enlarge [23, 37]. This variability in morphology over time likely reflects differences in severity of the artery wall injury among traumatic aneurysms. Traumatic aneurysms fall into 2 categories according to shape: saccular and fusiform (Fig. 3). Saccular traumatic aneurysms have a distinct neck and a roundish dome and resemble intracranial saccular aneurysms. Saccular traumatic aneurysms likely arise from a significant disruption of the elastic laminae, are more prone to enlarge over time, and are more highly associated with stroke [37]. Fusiform traumatic aneurysms, in contrast, have a smooth, tapering shape and likely arise from stretching, or a less extensive disruption, of the elastic laminae. More than half of fusiform traumatic aneurysms resolve over time [37].
Fig. 3.
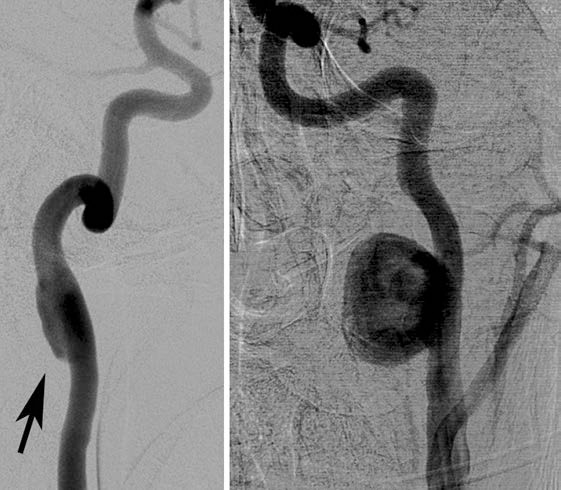
Traumatic aneurysms. Left: fusiform ICA aneurysm (arrow). Right: saccular ICA aneurysm.
Endovascular Management
The emergence of endovascular techniques for the treatment of cerebrovascular disorders has provided an array of options for the management of TCVI, but these techniques also carry unique challenges. Endovascular procedures in this setting fall into 4 categories.
Mechanical Thrombectomy
Endovascular clot removal is an attractive option for TCVI patients with acute ischemic stroke, particularly as most trauma patients are not candidates for intravenous alteplase due to other injuries (Fig. 4). However, effective use of mechanical thrombectomy in this setting depends on prompt recognition of symptoms or imaging evidence of large vessel occlusion. Formidable barriers exist. First, trauma patients are often intubated or have orthopedic injuries that obscure neurological changes. Second, trauma personnel often lack specific training in neurological examination and stroke management. Third, polytrauma patients are frequently managed with pharmacological paralysis and continuous narcotic infusions, further complicating the detection of neurological change. These obstacles to recognition of stroke in trauma patients make it essential that an ongoing coordinated effort exist between trauma, stroke neurology, and neurointerventional services.
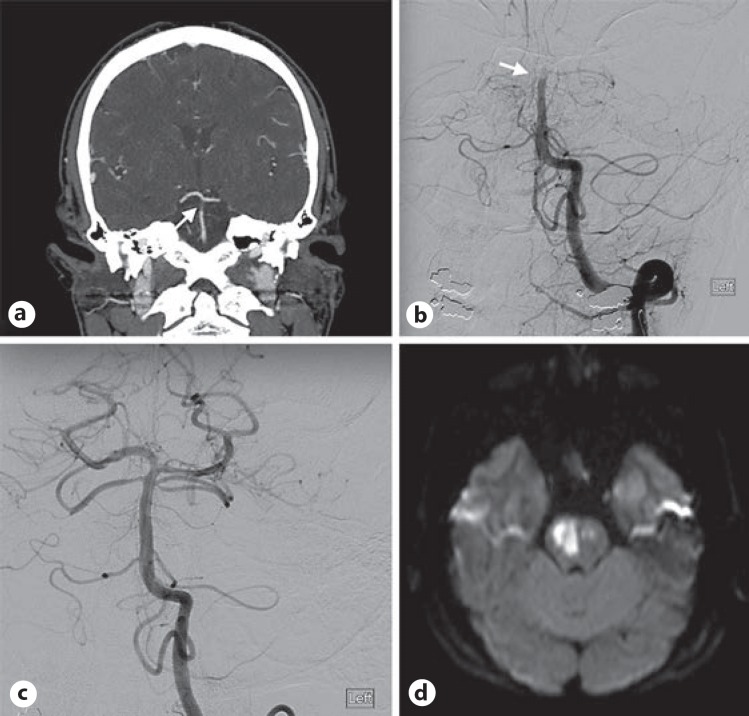
Fig. 4.
Traumatic basilar artery (BA) occlusion treated with mechanical thrombectomy. a CTA demonstrating thrombosis of the BA (arrow). b Anteroposterior DSA demonstrating BA occlusion (arrow). c Recanalization of the BA following mechanical thrombectomy. d DWI sequence demonstrating acute ischemic stroke.
Stenting
Stent placement for TCVI, usually for the treatment of carotid artery dissections, has become increasingly common in recent years. Although the technical success rate is high (>99%), there is no consensus about the indications for the use of stenting in this setting, and a significant percentage of stent procedures are done for asymptomatic traumatic carotid artery dissections. Prospective studies of TCVI stenting to assess efficacy are lacking. Importantly, intra-arterial stent placement requires antiplatelet medication, usually with dual antiplatelet coverage. In a series of TCVI carotid stent cases in which antiplatelet agents were not used routinely, acute thrombosis occurred in 45% [44]. Because the use of dual antiplatelet agents can be problematic in trauma patients, it seems prudent to limit stenting to symptomatic lesions and to coordinate the treatment decision with the other services involved in the patient's care.
Endovascular Treatment of Traumatic Aneurysms
Aside from a risk of thromboembolic stroke due to disruption of the intima, extracranial carotid and vertebral artery aneurysms are only rarely symptomatic. Moreover, since the majority of traumatic aneurysms resolve spontaneously or remain stable on follow-up imaging, it seems reasonable to limit endovascular treatment to larger (>15 mm) aneurysms or aneurysms that display significant enlargement on follow-up [37]. Covered stenting, coiling, stent-assisted coiling, and flow diverter placement techniques carry a high rate of technical success. The need for antiplatelet therapy depends on the endovascular treatment strategy. Long-term studies of the endovascular management of traumatic aneurysms are lacking.
Therapeutic Arterial Occlusion
Complete thrombotic arterial occlusion (i.e., type 4 lesions) carries not only a risk of hemodynamic failure but also of migration of the intraluminal thrombus into the intracranial circulation. The high clot burden in type 4 lesions likely contributes to the higher risk of ischemic stroke. Anecdotal evidence suggests that manipulation of the cervical spine during surgery to repair a fracture may cause migration of an intraluminal vertebral artery clot and ischemic stroke (Fig. 5). However, an analysis of 52 cases of traumatic vertebral artery occlusions actually found that spine surgery was associated with a lower risk of stroke [39]. Coil embolization of the ICA [22] and of the vertebral artery proximal to the TCVI lesion prior to cervical spine surgery [32] to reduce the theoretical risk of embolization has been reported. Comparative studies are needed to determine if this strategy is worthwhile.
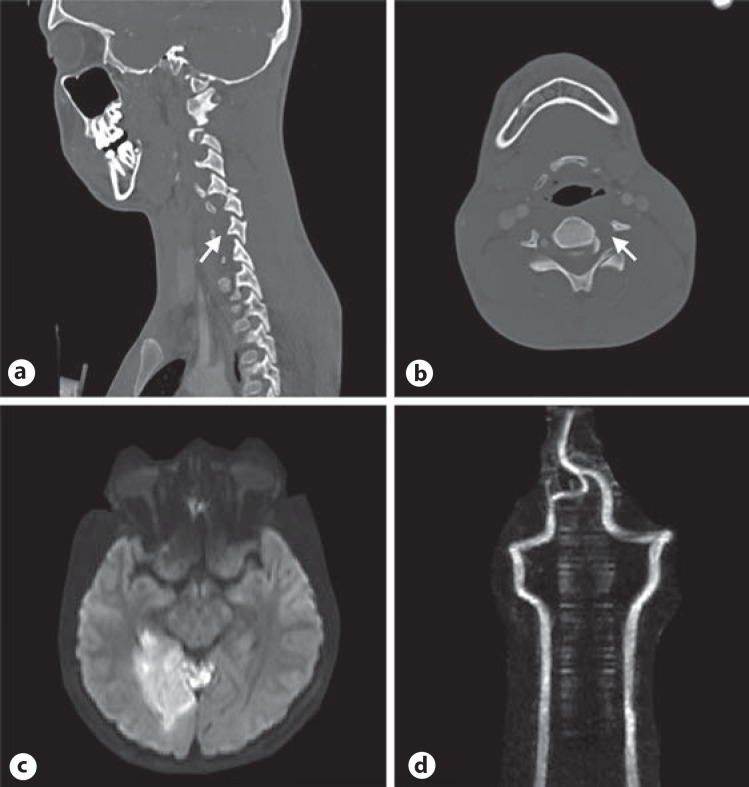
Fig. 5.
Type 4 left vertebral artery injury with acute ischemic stroke following corrective spinal surgery. a CTA demonstrating a cervical spine fracture/subluxation with occlusion of the left vertebral artery (arrow). b CTA demonstrating occlusion of the left vertebral artery (arrow). c DWI sequence obtained after corrective cervical spine surgery with evidence of an acute ischemic stroke. d MRA demonstrating restored patency of the previously occluded vertebral artery.
Conclusions and Future Directions
Although TCVI is present in only 1–2% of all blunt trauma admissions, it is becoming clear that TCVI is an important cause of ischemic stroke. The use of screening CTA for patients with blunt trauma has become ubiquitous in the US despite an absence of a consensus, guidelines, or level I data to guide the management of patients with TCVI. The widespread use of screening neck CTAs therefore represents a significant public health expense without a firm clinical or scientific foundation. Furthermore, although antithrombotic therapy is widely accepted, there is no consensus regarding the agent, dose, or duration of therapy. This field is ripe for collaborative research by investigators with expertise in stroke neurology, trauma, and neurointervention. The stage is set for a prospective multicenter adjudicated study to produce sufficient data to determine standards of care in the management of TCVI.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
P.M.F. participated in the literature search, manuscript production, manuscript revision, table production, figure production, and reviewed the final version of the submitted manuscript. M.R.H. developed the idea for the manuscript, participated in the literature search, manuscript production, manuscript revision, and reviewed the final version of the submitted manuscript.
References
- 1.Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection: a randomised trial. Lancet Neurol. 2015;14:361–367. doi: 10.1016/S1474-4422(15)70018-9. [DOI] [PubMed] [Google Scholar]
- 2.Brandt T, Orberk E, Weber R, Werner I, Busse O, Muller BT, Wigger F, Grau A, Grond-Ginsbach C, Hausser I. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. 2001;57:24–30. doi: 10.1212/wnl.57.1.24. [DOI] [PubMed] [Google Scholar]
- 3.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE, Mann KG, Holcomb JB. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–275. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford JD, Allan KM, Patel KU, Hart KD, Schreiber MA, Azarbal AF, Liem TK, Mitchell EL, Moneta GL, Landry GJ. The natural history of indeterminate blunt cerebrovascular injury. JAMA Surg. 2015;150:841–847. doi: 10.1001/jamasurg.2015.1692. [DOI] [PubMed] [Google Scholar]
- 5.Volker W, Dittrich R, Grewe S, Nassenstein I, Csiba L, Herczeg L, Borsay BA, Robenek H, Kuhlenbaumer G, Ringelstein EB. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. 2011;76:1463–1471. doi: 10.1212/WNL.0b013e318217e71c. [DOI] [PubMed] [Google Scholar]
- 6.Hoit DA, Schirmer CM, Weller SJ, Lisbon A, Edlow JA, Malek AM. Angiographic detection of carotid and vertebral arterial injury in the high-energy blunt trauma patient. J Spinal Disord Tech. 2008;21:259–266. doi: 10.1097/BSD.0b013e318141fce8. [DOI] [PubMed] [Google Scholar]
- 7.Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28:1711–1728. doi: 10.1148/rg.286085512. [DOI] [PubMed] [Google Scholar]
- 8.Griessenauer CJ, Foreman PM, Deveikis JP, Harrigan MR. Optical coherence tomography of traumatic aneurysms of the internal carotid artery: report of 2 cases. J Neurosurg. 2016;124:305–309. doi: 10.3171/2015.1.JNS142840. [DOI] [PubMed] [Google Scholar]
- 9.Franz RW, Willette PA, Wood MJ, Wright ML, Hartman JF. A systematic review and meta-analysis of diagnostic screening criteria for blunt cerebrovascular injuries. J Am Coll Surg. 2012;214:313–327. doi: 10.1016/j.jamcollsurg.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Stein DM, Boswell S, Sliker CW, Lui FY, Scalea TM. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009;66:132–143. doi: 10.1097/TA.0b013e318142d146. [DOI] [PubMed] [Google Scholar]
- 11.Velopulos CG, Enwerem NY, Obirieze A, Hui X, Hashmi ZG, Scott VK, Cornwell EE, 3rd, Schneider EB, Haider AH. National cost of trauma care by payer status. J Surg Res. 2013;184:444–449. doi: 10.1016/j.jss.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griessenauer CJ, Fleming JB, Richards BF, Cava LP, Cure JK, Younan DS, Zhao L, Alexandrov AV, Barlinn K, Taylor T, Harrigan MR. Timing and mechanism of ischemic stroke due to extracranial blunt traumatic cerebrovascular injury. J Neurosurg. 2013;118:397–404. doi: 10.3171/2012.11.JNS121038. [DOI] [PubMed] [Google Scholar]
- 13.Harrigan MR, Falola MI, Shannon CN, Westrick AC, Walters BC. Incidence and trends in the diagnosis of traumatic extracranial cerebrovascular injury in the nationwide inpatient sample database, 2003–2010. J Neurotrauma. 2014;31:1056–1062. doi: 10.1089/neu.2013.3309. [DOI] [PubMed] [Google Scholar]
- 14.Newhall K, Gottlieb DJ, Stone DH, Goodney PP. Trends in the diagnosis and outcomes of traumatic carotid and vertebral artery dissections among medicare beneficiaries. Ann Vasc Surg. 2016;36:145–152. doi: 10.1016/j.avsg.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye D, Brasel KJ, Neideen T, Weigelt JA. Screening for blunt cerebrovascular injuries is cost-effective. J Trauma. 2011;70:1051–1056. doi: 10.1097/TA.0b013e318211857d. [DOI] [PubMed] [Google Scholar]
- 16.Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Elliott JP, Burch JM. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178:517–522. doi: 10.1016/s0002-9610(99)00245-7. [DOI] [PubMed] [Google Scholar]
- 17.Berne JD, Cook A, Rowe SA, Norwood SH. A multivariate logistic regression analysis of risk factors for blunt cerebrovascular injury. J Vasc Surg. 2010;51:57–64. doi: 10.1016/j.jvs.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 18.Bromberg WJ, Collier BC, Diebel LN, Dwyer KM, Holevar MR, Jacobs DG, Kurek SJ, Schreiber MA, Shapiro ML, Vogel TR. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68:471–477. doi: 10.1097/TA.0b013e3181cb43da. [DOI] [PubMed] [Google Scholar]
- 19.Roberts DJ, Chaubey VP, Zygun DA, Lorenzetti D, Faris PD, Ball CG, Kirkpatrick AW, James MT. Diagnostic accuracy of computed tomographic angiography for blunt cerebrovascular injury detection in trauma patients: a systematic review and meta-analysis. Ann Surg. 2013;257:621–632. doi: 10.1097/SLA.0b013e318288c514. [DOI] [PubMed] [Google Scholar]
- 20.Harrigan MR, Weinberg JA, Peaks YS, Taylor SM, Cava LP, Richman J, Walters BC. Management of blunt extracranial traumatic cerebrovascular injury: a multidisciplinary survey of current practice. World J Emerg Surg. 2011;6:11. doi: 10.1186/1749-7922-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, Qaisi WG, Felker RE, Timmons SD. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386–393. doi: 10.1097/01.SLA.0000027174.01008.A0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton RP, Hanak BW, Levitt MR, Fink KR, Peterson EC, Vilela MD, Kim LJ, Chesnut RM. Blunt traumatic occlusion of the internal carotid and vertebral arteries. J Neurosurg. 2014;120:1446–1450. doi: 10.3171/2014.2.JNS131658. [DOI] [PubMed] [Google Scholar]
- 23.Morton RP, Levitt MR, Emerson S, Ghodke BV, Hallam DK, Sekhar LN, Kim LJ, Chesnut RM. Natural history and management of blunt traumatic pseudoaneurysms of the internal carotid artery: the Harborview algorithm based off a 10-year experience. Ann Surg. 2016;263:821–826. doi: 10.1097/SLA.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 24.Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845–853. doi: 10.1097/00005373-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Biffl WL, Moore EE, Elliott JP, Ray C, Offner PJ, Franciose RJ, Brega KE, Burch JM. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231:672–681. doi: 10.1097/00000658-200005000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott WW, Sharp S, Figueroa SA, Eastman AL, Hatchette CV, Madden CJ, Rickert KL. Clinical and radiographic outcomes following traumatic grade 1 and 2 carotid artery injuries: a 10-year retrospective analysis from a level I trauma center. The Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg. 2015;122:1196–1201. doi: 10.3171/2015.1.JNS14642. [DOI] [PubMed] [Google Scholar]
- 27.Scott WW, Sharp S, Figueroa SA, Eastman AL, Hatchette CV, Madden CJ, Rickert KL. Clinical and radiographic outcomes following traumatic grade 3 and 4 carotid artery injuries: a 10-year retrospective analysis from a level 1 trauma center. The Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg. 2015;122:610–615. doi: 10.3171/2014.10.JNS14875. [DOI] [PubMed] [Google Scholar]
- 28.Scott WW, Sharp S, Figueroa SA, Eastman AL, Hatchette CV, Madden CJ, Rickert KL. Clinical and radiological outcomes following traumatic grade 3 and 4 vertebral artery injuries: a 10-year retrospective analysis from a level I trauma center. The Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg. 2015;122:1202–1207. doi: 10.3171/2014.9.JNS1461. [DOI] [PubMed] [Google Scholar]
- 29.Laser A, Kufera JA, Bruns BR, Sliker CW, Tesoriero RB, Scalea TM, Stein DM. Initial screening test for blunt cerebrovascular injury: validity assessment of whole-body computed tomography. Surgery. 2015;158:627–635. doi: 10.1016/j.surg.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 30.Mayberry JC, Brown CV, Mullins RJ, Velmahos GC. Blunt carotid artery injury: the futility of aggressive screening and diagnosis. Arch Surg. 2004;139:609–612. doi: 10.1001/archsurg.139.6.609. [DOI] [PubMed] [Google Scholar]
- 31.Cothren CC, Moore EE, Ray CE, Jr, Ciesla DJ, Johnson JL, Moore JB, Burch JM. Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg. 2005;190:845–849. doi: 10.1016/j.amjsurg.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Berne JD, Norwood SH. Blunt vertebral artery injuries in the era of computed tomographic angiographic screening: incidence and outcomes from 8,292 patients. J Trauma. 2009;67:1333–1338. doi: 10.1097/TA.0b013e31818888c7. [DOI] [PubMed] [Google Scholar]
- 33.Biffl WL, Ray CE, Jr, Moore EE, Franciose RJ, Aly S, Heyrosa MG, Johnson JL, Burch JM. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235:699–706. doi: 10.1097/00000658-200205000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra AK, Camacho M, Ivatury RR, Davis IC, Komorowski DJ, Leung DA, Grizzard JD, Aboutanos MB, Duane TM, Cockrell C, Wolfe LG, Borchers CT, Martin NR. Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg. 2007;246:632–642. doi: 10.1097/SLA.0b013e3181568cab. [DOI] [PubMed] [Google Scholar]
- 35.Biffl WL, Moore EE, Ryu RK, Offner PJ, Novak Z, Coldwell DM, Franciose RJ, Burch JM. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg. 1998;228:462–470. doi: 10.1097/00000658-199810000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cothren CC, Biffl WL, Moore EE, Kashuk JL, Johnson JL. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144:685–690. doi: 10.1001/archsurg.2009.111. [DOI] [PubMed] [Google Scholar]
- 37.Foreman PM, Griessenauer CJ, Falola M, Harrigan MR. Extracranial traumatic aneurysms due to blunt cerebrovascular injury. J Neurosurg. 2014;120:1437–1445. doi: 10.3171/2014.3.JNS131959. [DOI] [PubMed] [Google Scholar]
- 38.Lauerman MH, Feeney T, Sliker CW, Saksobhavivat N, Bruns BR, Laser A, Tesoriero R, Brenner M, Scalea TM, Stein DM. Lethal now or lethal later: the natural history of grade 4 blunt cerebrovascular injury. J Trauma Acute Care Surg. 2015;78:1071–1074. doi: 10.1097/TA.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 39.Foreman PM, Griessenauer CJ, Chua M, Hadley MN, Harrigan MR. Corrective spinal surgery may be protective against stroke in patients with blunt traumatic vertebral artery occlusion. J Neurosurg Spine. 2015:1–6. doi: 10.3171/2015.1.SPINE141174. [DOI] [PubMed] [Google Scholar]
- 40.Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE, Jr, Johnson JL, Moore JB, Burch JM. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg. 2004;139:540–545. doi: 10.1001/archsurg.139.5.540. [DOI] [PubMed] [Google Scholar]
- 41.Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, Croce MA. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51:279–285. doi: 10.1097/00005373-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Wahl WL, Brandt MM, Thompson BG, Taheri PA, Greenfield LJ. Antiplatelet therapy: an alternative to heparin for blunt carotid injury. J Trauma. 2002;52:896–901. doi: 10.1097/00005373-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Edwards NM, Fabian TC, Claridge JA, Timmons SD, Fischer PE, Croce MA. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from long-term follow-up. J Am Coll Surg. 2007;204:1007–1013. doi: 10.1016/j.jamcollsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 44.Cothren CC, Moore EE, Ray CE, Jr, Ciesla DJ, Johnson JL, Moore JB, Burch JM. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg. 2005;140:480–486. doi: 10.1001/archsurg.140.5.480. [DOI] [PubMed] [Google Scholar]