Summary
Although it is important to clarify the pathogenic functions of T cells in human samples, their examination is often limited due to difficulty in obtaining sufficient numbers of dendritic cells (DCs), used as antigen-presenting cells, especially in autoimmune diseases. We describe the generation of DCs from induced pluripotent stem cells derived from T cells (T-iPSCs). We reprogrammed CD4+ T cell clones from a patient with Sjögren's syndrome (SS) into iPSCs, which were differentiated into DCs (T-iPS-DCs). T-iPS-DCs had dendritic cell-like morphology, and expressed CD11c, HLA-DR, CD80, CD86, and also BDCA-3. Compared with monocyte-derived DCs, the capacity for antigen processing was similar, and T-iPS-DCs induced the proliferative response of autoreactive CD4+ T cells. Moreover, we could evaluate T cell functions of the patient with SS. In conclusion, we obtained adequate numbers of DCs from T-iPSCs, which could be used to characterize pathogenic T cells in autoimmune diseases such as SS.
Keywords: iPSCs, Sjögren's syndrome, T cell, dendritic cells
Highlights
-
•
Dendritic cells were generated from iPSCs derived from CD4+ T cells (T-iPS-DCs)
-
•
Adequate numbers of functional DCs were generated from a small blood sample
-
•
The comparison between T-iPS-DCs and monocyte-derived DCs was evaluated
-
•
The functional assays of T cells in Sjögren's syndrome were analyzed by T-iPS-DCs
In this article, Sumida and colleagues demonstrate the generation of iPSCs from CD4+ T cell clones of Sjögren's syndrome (SS), which differentiated into dendritic cells (DCs) (T-iPS-DCs). Adequate numbers of functional DCs were obtained and used for the analysis of T cell functions in SS. T-iPS-DCs could be used to characterize pathogenic T cells in autoimmune diseases such as SS.
Introduction
Sjögren's syndrome (SS) is an autoimmune disease characterized by infiltration of lymphocytes into lacrimal and salivary glands (Fox and Stern, 2002). Immunohistochemical studies have shown that most infiltrating lymphocytes are CD4+ αβT cells. With respect to the cytokine profile, overexpression of interferon gamma (IFN-γ) has been described in salivary glands of SS, and CD4+ T cells, which produce IFN-γ, activate salivary gland epithelial cells (Kawakami et al., 2012, Iizuka et al., 2012). Moreover, a high proportion of IFN-γ-producing CD4+ T cells is present among peripheral blood mononuclear cells (PBMCs), suggesting that they play an important role in the pathogenesis of SS (Koarada et al., 2006). Although many reports support the involvement of IFN-γ-producing CD4+ T cells in SS, there are only a few reports that provide precise analysis of CD4+ T cells, establishing monoclonal T cell lines from patients with SS.
Dendritic cells (DCs), used as antigen-presenting cells (APCs) in the analysis of CD4+ T cells, usually differentiate from monocytes in vitro. However, monocytes cannot be propagated, and obtaining sufficient number of these cells is not feasible since it requires an abundant amount of blood. Thus, versatile methods for obtaining sufficient numbers of APCs are needed.
Induced pluripotent stem cells (iPSCs) are generated from various cell types upon enforced expression of transcription factors, such as Oct4, Klf4, Sox2, and c-Myc (Takahashi et al., 2007). T cells could also convert into iPSCs (T-iPSCs), retaining rearranged TCR genes from the original T cell, and then re-differentiate into functional T cells (Nishimura et al., 2013, Vizcardo et al., 2013, Ando et al., 2015). Recently, differentiation of DCs from human iPSCs (iPS-DCs) derived from fibroblasts has been reported (Choi et al., 2009, Senju et al., 2011). There is no information, however, whether T-iPSCs, especially from patients with autoimmune diseases such as SS, could differentiate into functional DCs. For this reason, we tried to establish DCs from T-iPSCs via Sacs. This approach would be critically useful not only as a less invasive approach for patients but also due to the lower cost and less effort than other methods. Thus, it could replace conventional methods in which DCs are prepared from only a few monocytes or from iPS-DCs obtained from non-PBMCs such as fibroblasts.
In this study, we established DCs from T-iPSCs of patients with SS. This could be used in the functional examination of pathogenic T cells without any concern regarding the preparation of monocytes or the background of donors.
Results
Generation of T-iPSCs from T Cell Clones
First, to establish CD4+ T cell clones, we isolated single CD4+ T cells from PBMCs of a patient with primary SS. The patient had not been treated, and satisfied the Japanese Ministry of Health criteria for the diagnosis of SS (Fujibayashi et al., 2004).
From 384 wells of a single CD4+ cell, we obtained 32 clones. To rule out the possibility that clones were derived from feeder cells (irradiated allogenic PBMCs), we checked the haplotype of HLA-DRB1 and confirmed they were identical to that of the patient with SS. Since IFN-γ producing CD4+ T cells are thought to be involved in the pathogenesis of SS (Singh and Cohen, 2012), we checked IFN-γ production from the clones and selected four clones, SS1-9, SS3-6, SS4-6, and SS4-7, which produced more IFN-γ than the others (Figure S1A). These clones were all CD4+ T cells and did not express CD8 molecules (Figure S1B). Moreover, we confirmed their monoclonality from the TCR Vβ repertoire (Figures S1C and S1D).
Next, we transduced these clones with reprogramming factors via Sendai virus vectors, and developed iPSCs (T-iPSCs) (Nishimura et al., 2013). We obtained 12 colonies from the above four T cell clones, and of these, four colonies could be stably cultured without feeder cells (TkSST1-2, TkSST1-3, TkSST1-4 from SS3-6, and TkSST2-2 from SS4-6). These T-iPSCs had embryonic stem cell (ESC)-like morphology (Figure S2A, and data not shown) and overexpressed pluripotency markers of SSEA4, Oct4, and Nanog (Figure S2B, and data not shown). They were cultured on C3H10T1/2 cells and checked on CD34+CD43+ early hematopoietic progenitor cells, which had comparable capacity to differentiate into myeloid cells (Vodyanik et al., 2006) (Figure S2C). Since the highest proportion of CD34+CD43+ cells were TkSST2-2 compared with the other three T-iPSCs, they were selected for the following experiments. The usage of TCR Vβ and the sequence of the CDR3 region in TkSST2-2 were consistent with those of the original T cell clone (SS4-6) (Figures S1D, S2D, and S2E), demonstrating that T-iPSCs were developed from the above-mentioned single CD4+ T cell clone.
Differentiation of T-iPSCs into DCs
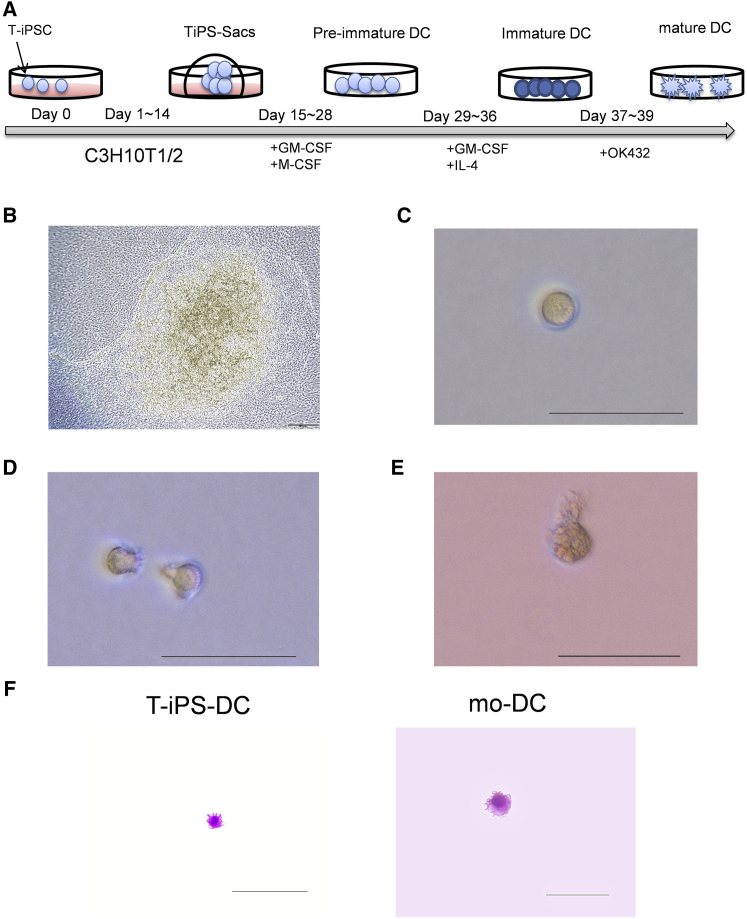
We induced the differentiation of T-iPSCs into DCs (T-iPS-DCs) (Figure 1A). T-iPSCs were cultured on C3H10T1/2 cells to differentiate into hematopoietic cells through TiPS-Sacs (Figure 1B). TiPS-Sacs consisted of a morphologically distinct external layer that enveloped thousands of spherical cells. TiPS-Sacs were dissociated into single cells and cultured in the presence of GM-CSF and M-CSF without feeder cells (pre-immature cells of T-iPS-DCs [preDCs]) (Figure 1C). To acquire more CD11c+ mature DCs, we evaluated the culture period (Figure S3A). As a culture period of 14 days with GM-SCF and M-SCF induced the greatest numbers of CD11c+ DCs constantly, we used that for the following protocol (Figure S3B). The expression of CD117, also known as c-kit, in the cells cultured with these cytokines for 14 days had already disappeared, and the cells did not fit the phenotype of precursor DCs (Breton et al., 2015) (Figures S3C and S3D). We added GM-CSF and interleukin-4 (IL-4) to generate immature T-iPS-DCs (Figure 1D), and finally, OK432 was added (Figure 1E). Mature T-iPS-DCs, and mo-DCs by way of comparison, were analyzed with May-Grunwald-Giemsa staining and found to have DC-like morphology (Figure 1F). Thus, mature DCs were assumed to be established from T-iPSCs.
Figure 1.
Differentiation of T-iPS-DCs from T-iPSCs
(A) Schematic illustration of differentiation of T-iPSCs into T-iPS-DCs.
(B–E) Phase-contrast images of TiPS-Sacs on day 13 on C3H10T1/2 feeder cells (B), pre-immature cells of T-iPS-DCs (C), immature T-iPS-DCs (D), and mature T-iPS-DCs (E). Original magnification (B) 200×, bars represent 20 μm; (C–E) 400×, bars represent 50 μm. Data are representative of ten cultured samples.
(F) May-Grunwald-Giemsa staining of mature T-iPS-DCs and mo-DCs. Original magnification 400×, bars represent 100 μm. Data are representative of three stained samples.
Characterization of T-iPS-DCs
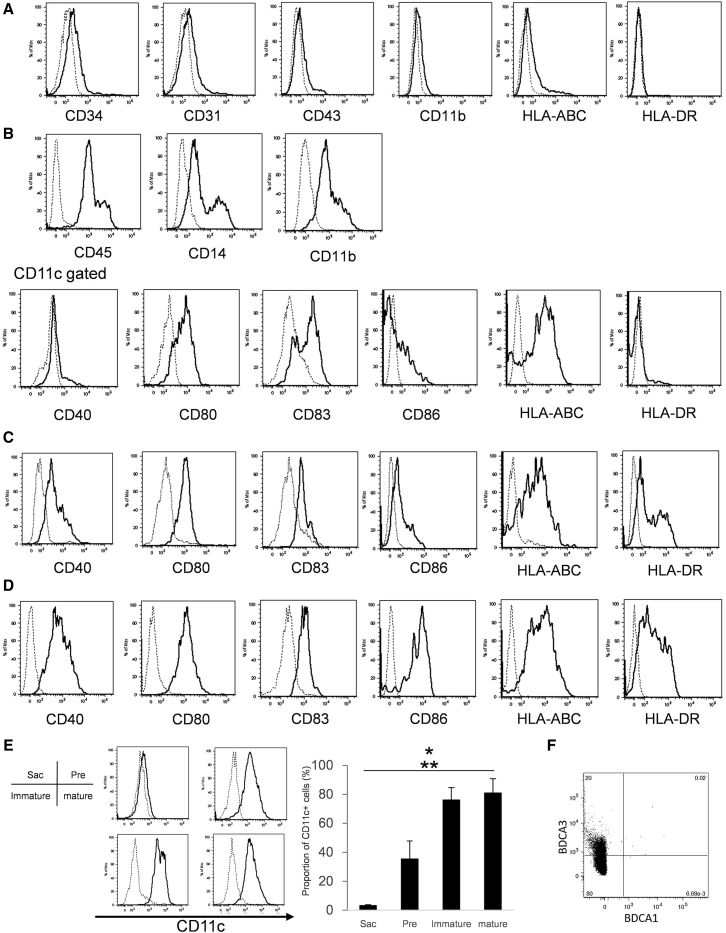
We examined cell surface markers specific for DCs. The cells within TiPS-Sacs expressed CD34, CD43 (hematopoietic cells), and CD31 (endothelial cells) (Figure 2A). This result suggests that the cells were multipotent hematopoietic progenitors. In TiPS-Sacs, HLA-DR was not detected, but CD11b, CD11c, and HLA-ABC were slightly expressed. In preDCs, CD11b and CD45 expressed higher and CD14 was detected, which is related to myeloid precursor cells (Figure 2B). In addition, CD80, CD83, and CD86 were slightly expressed but not HLA-DR. When advancing the step of immature T-iPS-DCs, not only co-stimulatory molecules (CD40, CD80, and CD86) but also HLA-DR expression were clearly observed (Figures 2C and S4A). Mature T-iPS-DCs expressed a higher level of CD40 than immature T-iPS-DCs (Figure 2D). As for the proportion of CD11c+ cells, mature and immature T-iPS-DCs were significantly higher than preDCs and TiPS-Sacs (Figure 2E). To further evaluate the phenotype of T-iPS-DCs, we checked the expression of both BDCA-1 and BDCA-3 (Figure 2F). Intriguingly, T-iPS-DCs expressed BDCA-3 but had little BDCA-1.
Figure 2.
Characterization of Differentiated T-iPS-DCs
(A–D) Flow cytometric analysis of surface molecules on (A) Sacs, (B) pre-immature cells of T-iPS-DCs, (C) immature T-iPS-DCs, and (D) mature T-iPS-DCs. (C) and (D) were gated on CD11c+ cells.
(E) The expression levels and the proportion of CD11c+ cells. Error bars denote SD.
(F) BDCA-1 and BDCA-3 on mature iPS-DCs. ∗p < 0.05 versus mature T-iPS-DCs, ∗∗p < 0.05 versus immature T-iPS-DCs by Kruskal-Wallis test. N = 3 independent experiments. The dotted line represents the isotype control.
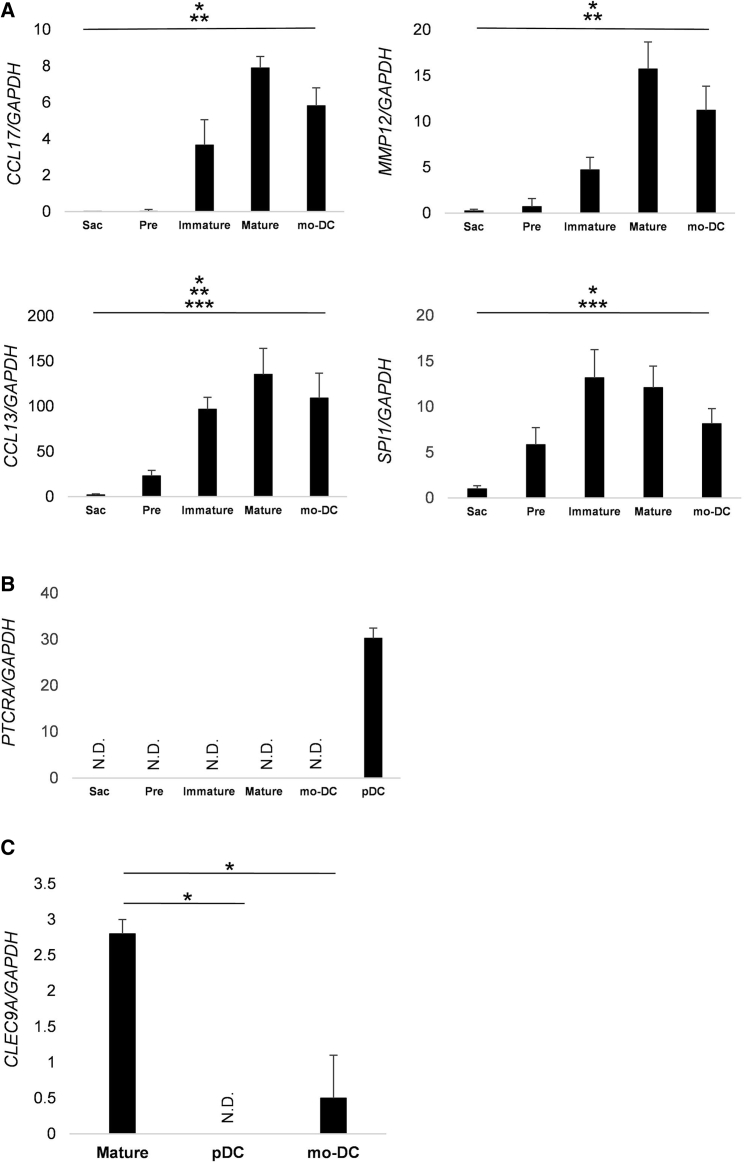
We also analyzed the chemokines and transcription factors specific for DCs. Although CCL17, CCL13, and MMP12 were rarely expressed in TiPS-Sacs and preDCs, they were significantly upregulated in the mature T-iPS-DCs and mo-DC (Figure 3A). The expression of MMP12 was significantly higher on mature T-iPS-DCs than immature DCs. PU.1, which is higher in myeloid DCs (mDC) compared with plasmacytoid DCs (pDC), was also detected in mature T-iPS-DCs (Nutt et al., 2005). On the other hand, pre-T cell receptor α expression, expressed in pDCs, but not in mDCs, was not detected (Figure 3B). It has been reported that BDCA-3+ DCs expressed the signature marker of CLEC9A (Haniffa et al., 2012); T-iPS-DCs expressed it significantly higher than pDCs and mo-DCs (Figure 3C). These findings indicated that T-iPS-DCs, typical of the mDC phenotype, were generated.
Figure 3.
Gene Expression Analysis of T-iPS-DCs
(A and B) qPCR analysis in Sacs, pre-immature cells of T-iPS-DCs (Pre), immature T-iPS-DCs (Immature), mature T-iPS-DCs (Mature), mature monocytes-derived DCs (mo-DC), and plasmacytoid DCs (pDCs). Data were normalized to the reference gene, GAPDH. Values are means ± SD. ∗p < 0.05 versus mature T-iPS-DCs, ∗∗p < 0.05 versus mo-DCs, ∗∗∗p < 0.05 versus immature T-iPS-DCs by Kruskal-Wallis test.
(C) qPCR analysis of CLEC9A. ∗p < 0.05 by Mann-Whitney's test. N = 3 independent experiments.
Error bars denote SD.
Functional Analysis of T-iPS-DCs
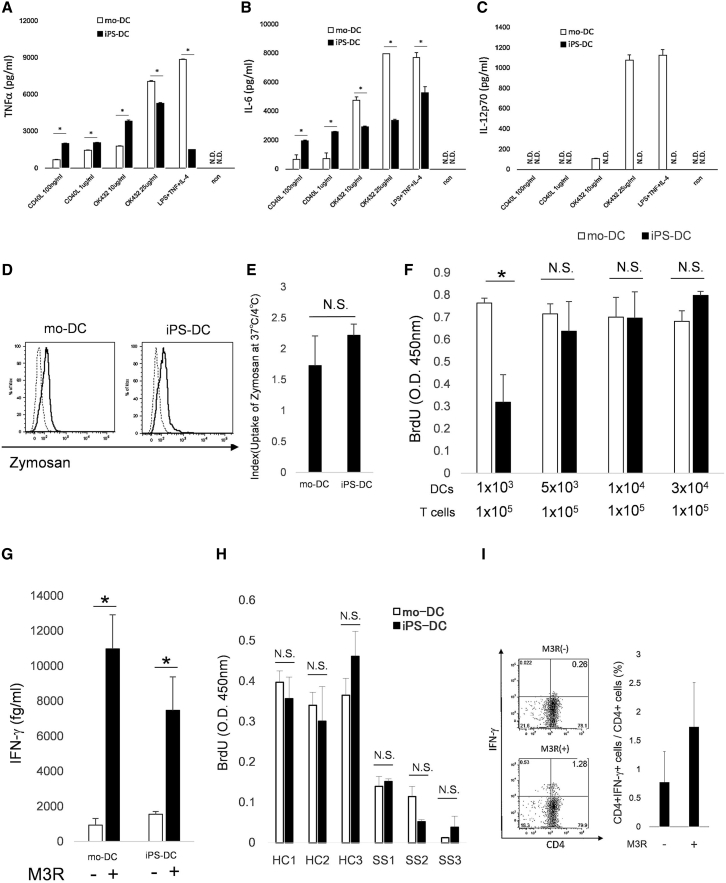
Since mo-DCs produced pro-inflammatory cytokines such as TNF-α and IL-6, we checked these cytokines. Although the levels of TNF-α and IL-6 from T-iPS-DCs were comparatively different from those in mo-DCs with the kind of stimulations, we detected them in mature T-iPS-DCs (Figures 4A and 4B). On another front, they, unexpectedly, did not produce IL-12p70 with any stimulations (Figure 4C).
Figure 4.
Functional Analysis of T-iPS-DCs
(A–C) Production of (A) TNF-α, (B) IL-6, and (C) IL-12p70 in mature T-iPS-DCs and mo-DCs stimulated for 72 hr with TNF-α, lipopolysaccharide, and IL-4 or OK432 (10 μg/mL, 25 μg/mL) or CD40L (100 ng/mL, 1 μg/mL). Values are means ± SD. ∗p < 0.05 by Mann-Whitney test.
(D) Flow cytometric analysis of CD11c gated T-iPS-DCs incubated with green-labeled zymosan for 60 min at 37°C (solid line) or 4°C (dotted line).
(E) Index mean value of the ratio of zymosan incorporation at 37°C and 4°C. Values are means ± SD. N.S., not significant.
(F) T-iPS-DCs or mo-DCs were co-cultured with allogeneic T cells for 5 days. Proliferation of T cells was measured based on bromodeoxyuridine uptake in the last 20 hr of the culture. ∗p < 0.05 by Mann-Whitney's test. Data were run in triplicate.
(G) T-iPS-DCs or mo-DC pre-incubated with or without M3R peptide (10 μg/mL) were co-cultured with the M3R-reactive T cell line (5 × 104 cells/well). Autoreactive CD4+ T cell responses were evaluated. ∗p < 0.05 by Mann-Whitney test. N = 3 independent experiments.
(H) T-iPS-DCs or mo-DCs (5 × 103 cells/well) were co-cultured with allogeneic T cells (3 × 104 cells/well) of healthy donors (HC1-3) or patients with SS (SS1-3) for 5 days. Proliferation of T cells was measured.
(I) T-iPS-DCs (1 × 104 cells/well) were pre-incubated with or without M3R peptide (10 μg/mL) and co-cultured with auto-CD4+ T cells (1 × 105 cells/well). M3R-reactive CD4+ T cells were evaluated.
Error bars denote SD.
To check the ability of phagocytosis, mature T-iPS-DCs were treated with green-labeled zymosan. It was phagocytosed by mature T-iPS-DCs (Figure 4D), and there was no significant difference in phagocytosis properties between T-iPS-DCs and mo-DCs obtained from the same patient (Figure 4E). These findings indicated that the ability of phagocytosis in T-iPS-DCs was equal to that in mo-DCs.
We also examined allogeneic T cell responses, the capacity to stimulate naive T cells. Mo-DCs could stimulate allogenic T cells even with a lower numbers of cells. Meanwhile, mature T-iPS-DCs induced a robust proliferative response of allogeneic T cells in proportion to the cell number, and at greater numbers (1/15 ratio of T cells), there was no significant difference between T-iPS-DCs and mo-DCs (Figure 4F). This implied that when evaluating the functional studies of T cells using T-iPS-DCs, especially the reactions of allogenic T cells, we need to take care of the number of T-iPS-DCs, and over a certain level, they might have the capacity to stimulate T cells the same as mo-DCs.
Finally, we evaluated the antigen-presenting capacity of T-iPS-DCs to autologous CD4+ T cells (Figure 4G). M3R is known as one of the etiologic auto-antigens of SS, and the important role of the autoimmune response against M3R has been clarified (Naito et al., 2006, Iizuka et al., 2010). M3R reactive CD4+ T cells were detected in PBMCs of patients with SS, and the proportion of these cells was significantly higher than in HLA-DRB1-matched healthy donors (Figure S4B). Thus, we checked IFN-γ production from M3R reactive CD4+ T cell lines under M3R peptide stimulation using T-iPS-DCs. A significantly higher level of IFN-γ was produced when cultured with M3R antigens, the same as mo-DCs. These results indicated that T-iPS-DCs were able to process antigens.
The Analyses of T Cell Function in SS Using T-iPS-DCs
Although T cells from patients with systemic lupus erythematosus (SLE) were reported to be poorer responders to allogenic non-T cells (Russell et al., 1983), the response in patients with SS remains unknown. Thus, we evaluated the response of allogenic T cells, using T-iPS-DCs. As for patients with SS, not only proliferation but also activation, such as IFN-γ production, of allogenic T cells had a propensity to be lower than in healthy donors (Figures 4H and S4C).
Moreover, we tried to detect M3R-reactive CD4+ T cells. As expected, we were able to detect M3R-reactive T cells using T-iPS-DCs, and they were comparable with mo-DCs (Figures 4I and S4B).
These results indicate that, using T-iPS-DCs, we can analyze some of the T cell functions of SS, which could elucidate the steps in its pathology.
Discussion
The results indicate that T-iPSCs were developed from a single CD4+ T cell from a patient with SS, through the establishment of CD4+ T cell clones, and were differentiated into an adequate amount of functional DCs via TiPS-Sacs.
First, we established CD4+ T cell clones from PBMCs of a patient with SS. Although various T cell clones have been established from other autoimmune diseases (Greidinger et al., 2004), as far as we know, there are only a few reports on the development of CD4+ T cell clones from SS. SS is characterized by the appearance of lymphopenic conditions and a decrease in T cell response to IL-2 (Goules and Tzioufas, 2016, Alcocer-Varela et al., 1984). Under these unfavorable conditions, we were able to develop CD4+ T cell clones smoothly. Since IFN-γ-producing CD4+ T cells are thought to be involved in the pathogenesis of SS (Singh and Cohen, 2012), we checked IFN-γ production and selected clones.
When evaluating the immune responses, especially in patients with uncommon diseases such as SS, it is critical to acquire sufficient numbers of mature DCs periodically. Haruta et al. (2013) developed a method to amplify monocytes by lentivirus-mediated transduction of cMYC along with BMI1. This method is easy and solid, but is dependent on the efficiency of virus transduction. In fact, it was difficult to transduce these genes into CD14 cells from our patient with SS efficiently (data not shown). Their development from ESCs or iPSCs derived from human fibroblasts of healthy donors has been also reported (Choi et al., 2009, Senju et al., 2007, Senju et al., 2011). In the latter case, mouse embryonic fibroblasts were used to maintain human iPSCs, and feeder layers of mouse OP9 were used to induce hematopoietic cells. In our study, T-iPSCs were maintained under feeder-free conditions, and they were transferred on C3H10T1/2 feeder cells to generate TiPS-Sacs. Takayama et al. (2008) found sac-like structures after human ESCs were cultured on C3H10T1/2 cells, which contained multipotent hematopoietic progenitors. Our data showed that TiPS-Sacs also included CD34-, CD31-, and CD43-positive cells. Unexpectedly, the slight expression of CD11c, which is evident on both mature and precursor DCs (Diao et al., 2006), was confirmed on TiPS-Sacs.
Under the maturation of T-iPS-DCs, we detected high expression levels of CCL17, CCL13, and MMP12, which were comparable with mo-DCs. They are selectively produced by mDCs, but not by pDCs, and are upregulated after the immature state of mDCs (Penna et al., 2002, Tang and Saltzman, 2004). PU.1, which is required for the generation of myeloid-derived DCs, was also detected (Guerriero et al., 2000). On the other hand, T-iPS-DCs did not express pre-T cell receptor α, expressed with pDCs (Spits et al., 2000). Furthermore T-iPS-DCs expressed BDCA-3 but not BDCA-1, and in fact higher expression of CLEC9A was detected (Haniffa et al., 2012). Considered together, our results indicate that T-iPS-DCs were myeloid lineage cells, consistent with those of previous studies on the generation of DCs from ESCs or iPSCs (Slukvin et al., 2006, Senju et al., 2011), and differentiated into BDCA-3+ DCs.
The ability of antigen uptake in T-iPS-DCs was equal to that of mo-DCs, but there was a slight difference in cytokine production. T-iPS-DCs produced much more IL-6 and TNF-α than mo-DCs, especially when cultured with CD40L stimulation, but much less with other stimulations. T-iPS-DCs had a tendency to express CD40 higher than mo-DCs, and also express other co-molecules such as CD80 and CD86 less (data not shown), which might explain the differences. Moreover, T-iPS-DCs did not produce IL-12p70 at all. As discussed above, T-iPS-DCs expressed BDCA-3, and it was reported that BDCA-3+DCs produced very little or no IL-12 (Jongbloed et al., 2010, Haniffa et al., 2012), which might be one of the reasons.
There was also a slight difference in allogenic T cell stimulation. Although T-iPS-DCs in a ratio of more than 1/15 to T cells could stimulate them as well as mo-DCs, the lower number of T-iPS-DCs (1/100 of T cells) was insufficient compared with that of mo-DCs. One of the reasons for this difference might be the expression levels of co-stimulatory molecules, which are known to play critical roles in T cell activation (Orabona et al., 2004). In addition, BDCA3+ DCs were reported to stimulate allogenic T cells less than mo-DCs, depending on the number of DCs (MacDonald et al., 2002, Chu et al., 2012). Although further studies are needed, these data might support the slight difference in T cell stimulation between T-iPS-DCs and mo-DCs.
It is not difficult to prepare a large amount of T-iPS-DCs because there are no limitations to expanding T-iPSCs. From only one well of 80% confluent T-iPSCs (6-well plate; 9.6 cm2), we obtained about 3–5 × 105 cells of DCs, repeatedly (Figure S3B). On the other hand, about 10 mL of blood must be collected to obtain the same number of DCs from CD14+ monocytes, each time. Thus, we were able to obtain stable and functional DCs from only a small blood sample from the donor.
Taking into account the functional analyses of T-iPS-DCs, we evaluated T cell functions of SS. As expected from the genetic similarities between SLE and SS (Teruel and Alarcon-Riquelme, 2016), we confirmed that T cells from patients with SS, like SLE, were poorer responders to allogenic non-T cells than T cells from healthy donors (Russell et al., 1983). Furthermore, we could evaluate M3R-reactive CD4+ T cells, which are thought to be one of the pathogenic T cells for SS.
The present study describes the generation of functional DCs from T-iPSCs prepared from a single patient with SS. T-iPS-DCs could be useful for characterization of pathogenic T cells in autoimmune diseases such as SS and elucidate the pathogenicity of autoreactive T cells.
Experimental Procedures
Differentiation of T-iPS-DCs from T-iPSCs
T-iPSCs were differentiated into hematopoietic cells as previously reported (Nishimura et al., 2013). They were first cultured in αMEM with 20% fetal bovine serum (FBS), GM-CSF (100 ng/mL), and M-CSF (50 ng/mL), and cultured again in RPMI-1640 medium with 10% FBS, GM-CSF (100 ng/mL), and IL-4 (10 ng/mL) to generate immature T-iPS-DCs. OK432 (10 μg/mL) was added to induce mature T-iPS-DCs. This study was approved by the local ethics committees of each participating institution and a signed informed consent was obtained from each subject.
Statistical Analysis
Data are expressed as the mean ± SD. Differences between groups were examined using the Mann-Whitney test and Kruskal-Wallis test. A p value <0.05 denotes the presence of a statistically significant difference.
Author Contributions
M.I.-K. and H.A. conceived the study, designed and performed experiments, analyzed data, and wrote the draft of the manuscript. M.A., C.-Y.L., S.M., and M.N. designed and performed experiments, and analyzed the data. T.N., H. Tsuboi, T.H., H. Takahashi, and I.M. provided expert advice. M.O. supervised and supported the study. T.S. conceived, supervised, supported the study, and finalized the manuscript.
Acknowledgments
We thank Dr. F.G. Issa for critical reading of the manuscript. M.N. is a founder and CTO of TOKIWA Bio Inc. This work was supported by the Research Program for Intractable Diseases, Health and Labor Sciences Research Grants (26870078 and 16K20928) from the Ministry of Health, Labour and Welfare, Japan.
Published: May 9, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.04.010.
Supplemental Information
References
- Alcocer-Varela J., Laffón A., Alarcón-Segovia D. Differences in the production of and/or the response to interleukin-2 by T lymphocytes from patients with the various connective tissue disease. Rheumatol. Int. 1984;4:39–44. doi: 10.1007/BF00683884. [DOI] [PubMed] [Google Scholar]
- Ando M., Nishimura T., Yamazaki S., Yamaguchi T., Kawana-Tachikawa A., Hayama T., Nakauchi Y., Ando J., Ota Y., Takahashi S. A safeguard system for induced pluripotent stem cell-derived rejuvenated T cell therapy. Stem Cell Rep. 2015;13:597–608. doi: 10.1016/j.stemcr.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton G., Lee J., Liu K., Nussenzweig M.C. Defining human dendritic cell progenitors by multiparametric flow cytometry. Nat. Protoc. 2015;10:1407–1422. doi: 10.1038/nprot.2015.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.D., Vodyanik M.A., Slukvin I.I. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J. Clin. Invest. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.C., Ali N., Karagiannis P., Di Meglio P., Skowera A., Napolitano L., Barinaga G., Grys K., Sharif-Paghaleh E., Karagiannis S.N. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J. Exp. Med. 2012;209:935–945. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J., Winter E., Cantin C., Chen W., Xu L., Kelvin D., Phillips J., Cattral M.S. In situ replication of immediate dendritic cell (DC) precursors contributes to conventional DC homeostasis in lymphoid tissue. J. Immunol. 2006;176:7196–7206. doi: 10.4049/jimmunol.176.12.7196. [DOI] [PubMed] [Google Scholar]
- Fox R.I., Stern M. Sjogren's syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand. J. Rheumatol. Suppl. 2002;116:3–13. [PubMed] [Google Scholar]
- Fujibayashi T., Sugai S., Miyasaka N., Hayashi Y., Tsubota K. Revised Japanese criteria for Sjogren’s syndrome (1999): availability and validity. Mod. Rheumatol. 2004;14:425–434. doi: 10.3109/s10165-004-0338-x. [DOI] [PubMed] [Google Scholar]
- Goules A.V., Tzioufas A.G. Primary Sjogren's syndrome: clinical phenotypes, outcome and the development of biomarkers. Immunol. Res. 2016 doi: 10.1007/s12026-016-8844-4. [DOI] [PubMed] [Google Scholar]
- Greidinger E.L., Gazitt T., Jaimes K.F., Hoffman R.W. Human T cell clones specific for heterogeneous nuclear ribonucleoprotein A2 autoantigen from connective tissue disease patients assist in autoantibody production. Arthritis Rheum. 2004;50:2216–2222. doi: 10.1002/art.20287. [DOI] [PubMed] [Google Scholar]
- Guerriero A., Langmuir P.B., Spain L.M., Scott E.W. PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood. 2000;95:879–885. [PubMed] [Google Scholar]
- Haniffa M., Shin A., Bigley V., McGovern N., Teo P., See P., Wasan P.S., Wang X.N., Malinarich F., Malleret B. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M., Tomita Y., Imamura Y., Matsumura K., Ikeda T., Takamatsu K., Nishimura Y., Senju S. Generation of a large number of functional dendritic cells from human monocytes expanded by forced expression of cMYC plus BMI1. Hum. Immunol. 2013;74:1400–1408. doi: 10.1016/j.humimm.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Wakamatsu E., Tsuboi H., Nakamura Y., Hayashi T., Matsui M., Goto D., Ito S., Matsumoto I., Sumida T. Pathogenic role of immune response to M3 muscarinic acetylcholine receptor in Sjögren's syndrome-like sialoadenitis. J. Autoimmun. 2010;35:383–389. doi: 10.1016/j.jaut.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Tsuboi H., Matsuo N., Kondo Y., Asashima H., Matsui M., Matsumoto I., Sumida T. The crucial roles of IFN-γ in the development of M3 muscarinic acetylcholine receptor induced Sjögren's syndrome-like sialadenitis. Mod. Rheumatol. 2012;23:614–616. doi: 10.1007/s10165-012-0785-8. [DOI] [PubMed] [Google Scholar]
- Jongbloed S.L., Kassianos A.J., McDonald K.J., Clark G.J., Ju X., Angel C.E., Chen C.J., Dunbar P.R., Wadley R.B., Jeet V. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Sawaki T., Sakai T., Miki M., Iwao H., Nakajima A., Nakamura T., Sato T., Fujita Y., Tanaka M. Skewed production of IL-6 and TGF-b by cultured salivary gland epithelial cells from patients with Sjogren’s syndrome. PLoS One. 2012;7:e45689. doi: 10.1371/journal.pone.0045689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koarada S., Haruta Y., Mitamura M., Morito F., Tada Y., Ohta A., Nagasawa K. Ex vivo CD4(+) T cell cytokine expression from patients with Sjogren’s syndrome following in vitro stimulation to induce proliferation. Rheumatology (Oxford) 2006;45:392–399. doi: 10.1093/rheumatology/kei182. [DOI] [PubMed] [Google Scholar]
- MacDonald K.P., Munster D.J., Clark G.J., Dzionek A., Schmitz J., Hart D.N. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Naito Y., Matsumoto I., Wakamatsu E., Goto D., Ito S., Tsutsumi A., Sumida T. Altered peptide ligands regulate muscarinic acetylcholine receptor reactive T cells of patients with Sjögren’s syndrome. Ann. Rheum. Dis. 2006;65:269–271. doi: 10.1136/ard.2005.039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Kaneko S., Kawana-Tachikawa A., Tajima Y., Goto H., Zhu D., Nakayama-Hosoya K., Iriguchi S., Uemura Y., Shimizu T. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Nutt S.L., Metcalf D., D’Amico A., Polli M., Wu L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J. Exp. Med. 2005;201:221–231. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orabona C., Grohmann U., Belladonna M.L., Fallarino F., Vacca C., Bianchi R., Bozza S., Volpi C., Salomon B.L., Fioretti M.C. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat. Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- Penna G., Vulcano M., Roncari A., Facchetti F., Sozzani S., Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J. Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- Russell P.J., Doolan T.J., Webb J., Carr G.A. Studies of autologous mixed lymphocyte reactions in patients with systemic lupus erythematosus. Pathology. 1983;15:37–43. doi: 10.3109/00313028309061400. [DOI] [PubMed] [Google Scholar]
- Senju S., Suemori H., Zembutsu H., Uemura Y., Hirata S., Fukuma D., Matsuyoshi H., Shimomura M., Haruta M., Fukushima S. Genetically manipulated human embryonic stem cell-derived dendritic cells with immune regulatory function. Stem Cells. 2007;25:2720–2729. doi: 10.1634/stemcells.2007-0321. [DOI] [PubMed] [Google Scholar]
- Senju S., Haruta M., Matsumura K., Matsunaga Y., Fukushima S., Ikeda T., Takamatsu K., Irie A., Nishimura Y. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–883. doi: 10.1038/gt.2011.22. [DOI] [PubMed] [Google Scholar]
- Singh N., Cohen P.L. The T cells in Sjogren’s syndrome: force majeure, not spectateur. J. Autoimmun. 2012;39:229–233. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin I.I., Vodyanik M.A., Thomson J.A., Gumenyuk M.E., Choi K.D. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J. Immunol. 2006;176:2924–2932. doi: 10.4049/jimmunol.176.5.2924. [DOI] [PubMed] [Google Scholar]
- Spits H., Couwenberg F., Bakker A.Q., Weijer K., Uittenbogaart C.H. Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (pre-DC) 2 but not into pre-DC1 Evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takayama N., Nishikii H., Usui J., Tsukui H., Sawaguchi A., Hiroyama T., Eto K., Nakauchi H. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- Tang Z., Saltzman A. Understanding human dendritic cell biology through gene profiling. Inflamm. Res. 2004;53:424–441. doi: 10.1007/s00011-004-1283-z. [DOI] [PubMed] [Google Scholar]
- Teruel M., Alarcon-Riquelme M.E. Genetics of systemic lupus erythematosus and Sjögren’s syndrome: an update. Curr. Opin. Rheumatol. 2016;28:506–514. doi: 10.1097/BOR.0000000000000310. [DOI] [PubMed] [Google Scholar]
- Vizcardo R., Masuda K., Yamada D., Ikawa T., Shimizu K., Fujii S., Koseki H., Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Vodyanik M.A., Thomson J.A., Slukvin I.I. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.