Abstract
We argue that dynamic changes in DNA supercoiling in vivo determine both how DNA is packaged and how it is accessed for transcription and for other manipulations such as recombination. In both bacteria and eukaryotes, the principal generators of DNA superhelicity are DNA translocases, supplemented in bacteria by DNA gyrase. By generating gradients of superhelicity upstream and downstream of their site of activity, translocases enable the differential binding of proteins which preferentially interact with respectively more untwisted or more writhed DNA. Such preferences enable, in principle, the sequential binding of different classes of protein and so constitute an essential driver of chromatin organization.
Keywords: DNA supercoiling, DNA translocases, Chromatin organization, Superhelicity gradients, DNA untwisting, DNA writhing
Dynamic changes of DNA supercoiling act as a driving force behind the alterations of genetic activity and DNA compaction both in eukaryotes and prokaryotes. Both these processes involve formation of spatially organized nucleoprotein structures by DNA architectural proteins. Whereas in eukaryotes the paradigmal example is the histone octamer, in prokaryotes a similar function is attributed to a small class of highly abundant nucleoid-associated proteins. We argue that, depending on the primary sequence organization, the changes of superhelicity elicit various alterations of local DNA geometry, while these, in turn, facilitate the assembly of distinct nucleoprotein structures pertinent to genetic function. Genome-wide conversion of the superhelical energy into various three-dimensional DNA structures thus acts as an analogue code coordinating the energy supply with the genetic expression of the chromosome.
Recent studies make it increasingly clear that the DNA double helix carries at least two types of encoded information and that these include the well-known genetic code and the structural information determining the form and physical properties of the double helix itself. Since both these information types are inscribed in the same DNA sequence, and yet one is discrete whereas the other is continuous, they have been respectively dubbed the digital and analog DNA codes (Marr et al. 2008; Travers et al. 2012; Muskhelishvili and Travers 2013). The potential of the DNA to adopt distinct configurations is strongly modulated by DNA supercoiling, which is increasingly recognized as one of the major forces coordinating the cellular DNA transactions in both prokaryotes (including Archaea) and eukaryotes.
DNA supercoiling can be generated by the simple application of torque to the double helix (Strick et al. 1998) but, importantly, because the DNA molecule itself possesses chirality—it is, after all, a right-handed double helix—the local structures stabilized by changes in superhelical density depend on both the sign and strength of the applied torque. For example, both positive and negative torque (respectively over- and underwinding of the double helix) can change the intrinsic coiling of the double helix itself but in opposite senses. At high superhelical densities, negative torque results ultimately in strand separation, often in short flexible regions flanked by rigid base steps, but can also induce the further coiling of the DNA duplex to form a superhelix writhing. Likewise, positive torque can also increase the writhing of the opposite sense in extended regions with flexible DNA but potentially also induces P DNA configuration with intertwined backbones inside and bases outside (Allemand et al. 1996). Another example of the effects of chirality is the stability of DNA crossovers, which varies depending on their handedness (Harris et al. 2008), an effect likely due to difference in the interacting surfaces of the juxtaposed helices (Varnai and Timsit 2010). The sign of the crossover can have biological consequences, since it may in turn determine the efficiency of protein binding. For example, DNA gyrase wraps the DNA, preferentially stabilizing a positive crossover (Papillon et al. 2013), whereas increased stiffness of the DNA due to changing the base composition and sequence substantially reduces the efficiency of gyrase binding and function (Oram et al. 2006; Fernández-Sierra et al. 2015).
The configuration of supercoiled DNA is extremely sensitive to physical factors, such as, for example, temperature or salt concentration. Whereas increased temperature unwinds the DNA, negatively supercoiled small DNA circles adopt either a planar or plectonemic configuration in low and high salt, respectively (Bednar et al. 1994) because the screening counterions reduce both the effective diameter of the DNA and the repulsion between the negatively charged phosphate backbones of the juxtaposed duplexes in the superhelix (Fig. 1). Again, the DNA in small circles has higher configurational entropy than in larger circles, which accommodate writhing by adopting a plectonemic form (Mitchell and Harris 2013). DNA supercoiling may thus affect the number of configurations adopted by the DNA (Irobalieva et al. 2015), or the volume of the configurational space (Bednar et al. 1994; Travers et al. 2012). In other words, the repertoire of possible local structures will depend on the superhelical state of the DNA and the sign (either negative or positive) of coiling. The changing degree of superhelicity can be seen as a continuous mode of increased acquisition of particular geometries facilitated by the sign of coiling. For negative supercoiling, these geometries could be subsumed under the changes of writhe and twist*,1 the distribution of which will depend on the sequence composition. Thus, while the DNA polymer is a superb molecule for sensing environmental conditions, the adopted gross configurations will have different consequences for the repertoire of available local geometries depending on the base sequence and composition. It is this repertoire, which is used by the DNA binding proteins for DNA transactions governing the genetic activity.
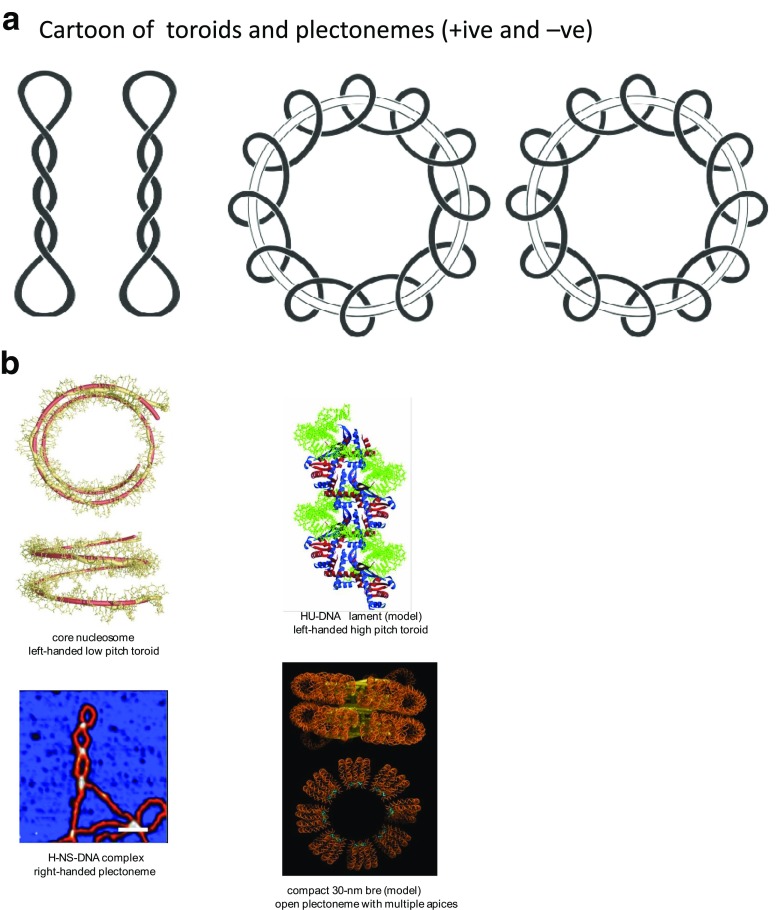
Fig. 1.
a Plectonemic and toroidal forms of supercoiled DNA showing lefthanded and right-handed forms of each. Adapted from Calladine et al. (2004) with permission. b Modes of constraint of negative superhelicity in protein complexes. Top left nucleosome core particle (Richmond and Davey 2003); top right model of Hu-DNA nucleofilament (Guo and Adhya 2007); bottom left AFM visualization of H-NS-plectoneme complex (Maurer et al. 2009); bottom right model of compact 30-nm fibre with 217-bp NRL (Wu et al. 2016; reproduced with permission
Effects of DNA sequence and base composition on DNA superhelical structures
DNA is long, moderately flexible and heterogeneous polymer, with different DNA sequences conferring different physical properties. These properties—elasticity, melting and stacking energies, and bending anisotropy—respond differently to imposed torsion and accordingly influence protein binding to supercoiled DNA. One consequence of this heterogeneity is that, when torque is applied to a DNA molecule of mixed sequence, the superhelical energy is not distributed uniformly but instead is concentrated in structurally deformable sequences. One example is the transient strand separation observed in short regions of low melting energy, as exemplified by the −10 hexamers of certain prokaryotic promoters (Drew et al. 1985). In contrast, increased negative superhelicity can also increase the writhing (forming a true supercoil) of more elastic extended regions containing flexible base steps. The untwisting resulting in strand separation normally extends over a much shorter sequence than writhing and, consequently, the local superhelical density in such regions is much greater than where the superhelicity is manifest only as writhing. To take an extreme example, the superhelical density of a 10-bp transcription bubble is ∼−1 while the same superhelicity constrained by writhing in a nucleosome requires ∼125 bp with a resultant superhelical density of ∼−0.08. Since the average superhelical density in both the nucleoid and nucleus is lower (∼−0.06 to −0.07) inevitably some other DNA sequences will be less affected by the torsion.
These considerations have led to the concept of superhelicity-sensitive sequences (Hatfield and Benham 2002), but analyses based simply on helix stability or a related parameter do not necessarily distinguish between sequences that writhe and those that preferentially untwist in response to negative supercoiling. This is likely because the responses of the DNA polymer are multiscalar (Fig. 2). Untwisting resulting in strand separation is normally restricted to about a single double helical turn and local writhing to very approximately 10 double helical turns. The formation of higher order superhelical structures is also scale-dependent, with simple ‘open’ plectonemes with loops formed in supercoiled circular DNA containing several kb, while larger circles (above ∼15–20 kb) of the same superhelical density fold to form ‘hyperplectonemes’ with no prominent loops (Japaridze 2015). For protein binding, it is the distinction between preferential untwisting and preferential writhing that is the most crucial. In part, this is simply a consequence of the multiscale response, as the elasticity of DNA limits the extent of bending that can accommodate writhing and so, for a given change in linkage number, a longer sequence is required. Additionally, the unstable sequences most susceptible to untwisting—and therefore able to accommodate a high superhelical density—are those which are bounded by short much more stable sequences which are less susceptible to torsional deformation. Good examples of such sequences are the −10 hexamers of certain stable RNA promoters in E. coli and also TBP binding sites (TATA boxes) in eukaryotic promoters. Both are typically flanked by highly G/C-rich sequences. The topoisomerase II binding sites at the 3′ ends of some Saccharomyces cerevisiae and Drosophila melanogaster genes are an excellent counterexample. These regions contain multiple overlapping topoisomerase II binding sites (Burden and Osheroff 1999), comprising long runs (up to 20) of TA, the most unstable dinucleotide (SantaLucia 1998), and are among the least stable 50-bp regions of DNA in the yeast genome (Adryan et al., unpublished observations). Yet the DNA substrate for topoisomerase II is writhed, not melted, DNA (Dong and Berger 2007).
Fig. 2.
Multiscale effects of analog information in DNA. The structural consequences of negative superhelicity are length-dependent. Image by A. Japaridze reproduced with permission.
Another property relevant to the superhelical wrapping of DNA on a protein surface is bending anisotropy. This specifies the preferred directional bending of specific sequences, typically encompassing at least 5 or more successive base-steps. In contrast, the local deviations of the superhelical axis correlate with the roll deformations of individual base-steps. The extensive nature of bending anisotropy is reflected in the superhelical geometry. Thus, DNA wrapped on the surface of the histone octamer or around the three FIS (factor for inversion stimulation) dimers in tyrT UAS complex has a surface helical repeat of 10.2 bp. This number, being less than the intrinsic twist of 10.4–10.5 bp, reflects the writhing of DNA as a left-handed superhelix (Drew and Travers 1985). This sequence periodicity (in the range 10.1–10.3 bp) is observed both in natural and SELEX-selected octamer binding sites. The periodicity is manifest as an averaged alternating pattern of A/T-rich and G/C-rich sequences spaced every helical turn, so that the A/T-rich sequences are located where the minor groove of DNA points toward the octamer and the G/C-rich sequences point away from the octamer. Such sequences can adopt a preferred direction (anisotropic) of bending and so occupy less configurational space than a fully flexible isotropically bendable sequence (Fig. 3). Because this preferred bending direction conforms to the binding surface of the histone octamer, the resulting nucleosome is thermodynamically more stable (Anselmi et al. 2000), reflecting the additional information encoded in the DNA. This represents a classic example of how increased spatial recurrence of readable information (sequence periodicity)—and therefore decreased Shannon entropy (Shannon 1948; Nigatu et al. 2016)—is manifested as decreased thermodynamic entropy.
Fig. 3.
Anisotropic bending reduces the configurational space available to a DNA chain. A preferred direction of bending—anisotropy—is conferred by particular sequence organisations and periodicity (and hence, more information) such as, for example, the alternating blocks of A/T and G/C rich sequences associated with DNA wrapping on the histone octamer. One consequence of reducing the degrees of configurational freedom is an increase in the summed vectorial stiffness of the duplex (the bicycle chain effect). In principle, the directionality of bending anisotropy can act either to promote or antagonize wrapping on a given protein surface
Negative DNA superhelicity can also induce the formation of other DNA structures that depart from the canonical DNA helix. These include cruciforms, slipped loops, Z-DNA, H-DNA and two-stranded structures containing the G-quadruplex motif (Lilley 1980; Singleton et al. 1982; Voloshin et al. 1992; Mace et al. 1983; Minyat et al. 1995; Sun and Hurley 2009; Kouzine et al. 2013; Selvam et al. 2014). While these serve as binding sites for particular proteins, they may also function as topological switches to absorb or release superhelicity in topologically sensitive regions such as promoters (Travers and Muskhelishvili 2015).
Gradients of superhelicity and protein binding
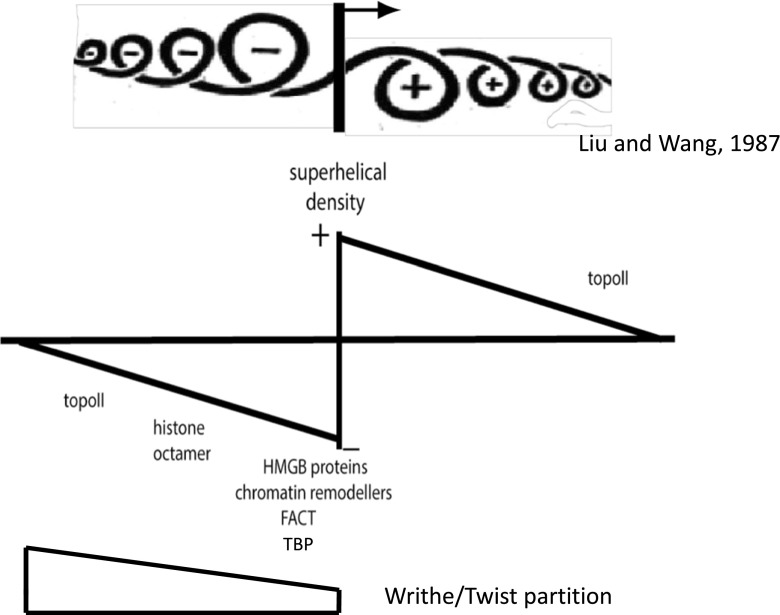
The application of torque to supercoiled DNA requires energy and, consequently, as has long been reognized, supercoiled DNA has a higher intrinsic energy than relaxed DNA. In both eukaryotes and bacteria, the average superhelical density of DNA—including both constrained and unconstrained regions—is ∼0.06–0.07. That is, each double-helical turn of turn is underwound by, on average, ∼6.5 %. This raises the questions of how the DNA acquires this superhelicity and the nature of the necessary energy source. In bacteria, the topoisomerase DNA gyrase uses ATP to insert negative superhelical turns into DNA (Gellert et al. 1976), but in eukaryotes, although present in chloroplasts and at least some mitochondria (Salvador et al. 1998; Stirdivant et al. 1985; Wall et al. 2004), this enzyme is apparently absent from the nucleus. Another mechanism, arguably more important, even in bacteria, for generating superhelicity is the processive motion of certain DNA translocases. Of these, RNA polymerase is probably the most abundant and the most powerful (Yin et al. 1995). In a ground-breaking paper, Liu and Wang (1987) pointed out that, while topoisomerases can establish and maintain an equilibrium state of supercoiling, transcription has the potential to generate transient changes in DNA supercoiling following the translocation of the protein complexes along the DNA. These transients arise as a direct consequence of the double helical structure of DNA. When proteins such as RNA polymerase move along DNA, they do not track linearly along the molecule, but instead, by following one or other of the grooves, rotate along a helical path (Harada et al. 2001). Many protein complexes are much more bulky than DNA and their freedom to rotate around the DNA may be constrained by molecular crowding creating viscous drag (Wang and Liu 1990). In some cases, the translocating enzymes may even be restrained in a fixed spatial position by secondary physical attachment to extensive structures, such as membranes (Lodge et al. 1989; Lynch and Wang 1993; Bowater et al. 1994; Ma et al. 1994). Under these circumstances, provided the rotation of the DNA molecule is also constrained, torsional strain is generated such that the DNA is overwound downstream of the advancing enzyme and underwound upstream (Fig. 4). This twin-supercoil domain model plays a key role in the genetic and structural stabilization of chromosomes.
Fig. 4.
Generation of gradients of superhelicity ahead and behind a processive DNA translocase. The twist–writhe partition across a gradient will dpepend in part on the superhelical density. Upper cartoon adapted from Liu and Wang (1987)
In principle, the net amount of superhelicity generated by translocase motion is zero, the upstream underwinding being equal and opposite to the downstream overwinding. Thus, the motion does not of itself explain why DNA in the nucleus is on average negatively supercoiled. A conversion of symmetric generation to an asymmetric steady state requires that the trapping and/or relaxation rates of positive and negative supercoils differ. Notably, nearly all the most abundant DNA binding proteins in both nuclei (e.g., histones) and bacterial nucleoids (e.g., HU, H-NS, FIS) constrain negatively supercoiled DNA. One exception is the DNA-binding protein Dps, which accumulates to high levels in bacterial nucleoids in the late stationary phase (Azam et al. 1999; Frenkiel-Krispin et al. 2001), correlating with low energy availability and low average negative superhelical density (Bordes et al. 2003). In contrast, no abundant DNA binding proteins that constrain positive supercoils have been described. The differential binding of negative supercoils by constraining proteins provides at least a partial explanation for the asymmetric retention of negative, rather than positive, superhelicity. Topological sinks at the 3′-end of transcribed genes can also facilitate the relaxation of positive superhelicity (Adryan et al., unpublished observations). Preferential storage of the DNA as a negative supercoil can also, on removal of the constraint, potentially facilitate the DNA unwinding required for the initiation of transcription and DNA replication. Another consequence of the Liu–Wang model is that, because the progression of a translocase such as RNA polymerase is unidirectional, so also is the eventual accumulation, if any, of the generated superhelicity. Such directionality is again consistent with the presence of a topological sink at the 3′-end of a transcribed gene.
An important feature of the Liu-Wang model—shown but not emphasised in the original publication (Liu and Wang 1987)—is that the superhelicity consequent on the motion forms a density gradient outward from the site of generation (Fig. 4) to a point where it is dissipated by diffusion and/or relaxation. This implies that the physical properties of the supercoiled DNA—and hence the interactions with proteins—will depend on the physical distance from the active translocase. The propagation of superhelicity from its site of origin has profound implications for the regulation of both gene expression and chromatin structure. Any effects would depend on how far such gradients extend. Current estimates suggest that, depending on the strength of the signal, gradients may extend for up to a few kilobases (Krasilnikov et al. 1999; Moulin et al. 2005; Kouzine et al. 2008), and therefore their influence will be local rather than global (Sobetzko 2016; Meyer and Beslon 2014). Since transcription is a dominant source of the local generation of superhelicity, the relative orientation of neighboring genes determines how transcription of one may affect the activity of its neighbor by supercoiling-mediated coupling. For divergently transcribed genes, negative superhelicity may be transmitted from an active gene to the neighboring promoter and thereby facilitate its activation (Hatfield and Benham 2002). Conversely, for convergently transcribed genes, positive superhelicity would be propagated between the neighbors. In the E. coli chromosome, these effects are pervasive (Sobetzko 2016). The expression of about half of the E. coil genes is sensitive to variations in DNA superhelicity (Blot et al. 2006), such that some (hyp) genes require high superhelical densities for activation, whereas other (rel) genes respond to low superhelical densities. Analysis of these genes showed that hyp genes were enriched in divergently oriented direct neighbors with small intergenic regions, whereas rel genes were enriched in arrangements with a close convergent direct neighbor. The close proximity of divergent neighbors for hyp genes and close convergent neighbors for rel genes indicated an efficient transmission of positive and negative supercoiling for transcription-mediated coupling of sensitive genes (Sobetzko 2016). Nevertheless, in bacteria, in addition to transcription-dependent torsional coupling, the maintenance of negative superhelical density by DNA gyrase is also crucial for the regulation of gene expression. Notably, the colocalization of gyrase binding sites with highly active and highly supercoil-dependent rrn genes (Sobetzko et al. 2012, 2013) implies a more nuanced complexity of regulation.
In eukaryotes, genome-wide expression analysis studies have demonstrated the coexpression of adjacent genes in yeast (Kruglyak and Tang 2000) as well as in plants (Williams and Bowles 2004) and mammals (Semon and Duret 2006). Among these coexpressed pairs, divergent genes were found to be the most frequent as well as more strongly expressed and highly correlated (Cohen et al. 2000a; Wang et al. 2011). However, in higher eukaryotes, the gene length may exceed the distance for superhelicity propagation and so minimize the effects of convergent transcription. By focusing on Drosophila melanogaster gene pairs, whose relative promoter spacing would be expected to preclude any transcriptionally induced torsional effects from other neighboring genes, Meyer and Beslon (2014) demonstrated that coupled expression correlated strongly with divergently transcribed pairs consistent with torsional coupling.
In this context, relative gene orientation has consequences not only for expression but also for chromatin structure. This is particularly relevant to convergently transcribed genes, where, if the intervening distance is short, positive superhelicity transmitted into the neighboring gene could disrupt chromatin structure by affecting the stability of protein–DNA complexes. For example, positive superhelicity could potentially unwrap nucleosomal DNA. In both Saccharomyces cerevisiae and Drosophila melanogaster, the occurrence of topological barriers in the form of topoisomerase II binding sites in the intergenic region would potentially minimize such disruption and, at the same time, reduce torsional coupling of expression (Adryan et al., unpublished observations).
In addition to their effects on local chromatin structure and coupled gene expression, gradients of superhelicity generated by DNA translocases may affect both the nature of bound proteins and the ordering of their assembly. This is because how DNA binding proteins constrain negative superhelicity can differ substantially, in particular in the context of the twist–writhe partition. For example, both the HMG domain and the TATA binding protein (TBP) bind to untwisted DNA while proteins such as the bacterial FIS protein, as well as histones stabilize negative writhe with little, if any, change in the intrinsic DNA twist (Zivanovic et al. 1988). Yet others, for example the bacterial HU protein, potentially stabilize negatively supercoiled DNA, which is both untwisted and negatively writhed (Guo and Adhya 2007). The bacterial RNA polymerase is different again. At certain promoters, initial contacts constrain negative writhe but subsequently the DNA in the −10 promoter region is untwisted, resulting in a greater constraint of negative superhelicity (Amouyal and Buc 1987). In contrast, during transcription elongation, the principal constraint is imparted by the unwound transcription bubble.
Differences in the twist–writhe ratio recognized by proteins constraining negative superhelicity can provide important clues to function. The existence of local gradients of superhelical density in the vicinity of DNA translocases implies that, as the DNA superhelical density varies over a wide range, the nature of the unconstrained superhelicity—the twist–writhe partition—must also vary, with writhe favored at the lower densities. However, in principle, proteins that constrain negative superhelicity might be expected to bind preferentially to DNA within a narrow range of superhelical density, as has been shown for the FIS protein, which favors a low negative superhelical density (Schneider et al. 1997). There is unfortunately little information available for other proteins, but another possible example would be the eukaryotic HMG domain, which, in addition to being the principal component of the small HMGB proteins (Thomas and Travers 2001), is found in certain subunits of chromatin remodeling complexes (Quinn et al. 1996) and of transcription elongation complexes (Orphanides et al. 1999; Brewster et al. 2001; Formosa et al. 2001; Mason and Struhl 2003). The small HMGB proteins themselves may also play an essential role in these processes (Moreira and Holmberg 2000). Common to these processes is their mediation by powerful DNA translocases, e.g., RNA polymerase or the motor subunit of remodeling complexes. The negative superhelical density produced by such assemblies is likely to be highest closest to the generation site and so will favor the binding of proteins which preferentially interact with supercoiled DNA with a twist–writhe partition more favorable to untwisting. In eukaryotes, upstream binding factor, a multisubunit assembly of simple HMG domain proteins, is preferentially associated with highly active transcriptons, including both rRNA genes and certain genes transcribed by RNA polymerase II (Diesch et al. 2015). In bacteria, HU may play, in part, a similar role to the HMG domains. However, unlike HMGB proteins, there is evidence that HU can also compact DNA into a fiber (Rouvière-Yaniv et al. 1979) in which the DNA is wrapped in a left-handed sense (Guo and Adhya 2007). Although negative superhelicity can promote transient strand separation in particular sequences (Drew et al. 1985), it is only one of several mechanisms that may potentially create short stretches of DNA lacking continuous base pairing. Both HU and the HMGB proteins also bind with high affinity to, for example, Holliday junctions, DNA bulges and cis-platin-modified DNA (Balandina et al. 2002; Thomas and Travers 2001; Malarkey and Churchill 2012). Many studies of HMG domain-containing proteins have emphasized their ability to bend DNA by ∼90° (Jamieson et al. 1999; Lorenz et al. 1999). Such bending results from intercalation into a single DNA strand and, as in the case of DNA bulges, may simply be a concomitant of untwisting.
Any differentiation of the binding preferences of proteins that stabilize different superhelical densities could be reflected in the timing of their binding after the passage of a DNA translocase. Such proteins would stabilize DNA structures that are naturally present in DNA as a consequence of translocase activity rather than simply inducing such structures. For example, like the HMG domain, TBP intercalates aminoacids between base-pairs and stabilizes untwisted DNA (Kim et al. 1993a, b; Juo et al. 1996). This structural preference for untwisted DNA is emphasized by its potentiation by flanking untwisted cis-platin lesions (Cohen et al. 2000b). In contrast, the DNA wrapped on the histone octamer is writhed but its intrinsic twist differs little, if at all, from that of relaxed DNA (Zivanovic et al. 1988). The different structural binding preferences of TBP and the histone octamer imply that, where both are present, TBP will likely bind closer to, for example, a transcribing polymerase than the histone octamer, and in so doing maintain chromatin organization.
Because both negative and potentially superhelicities are generated by moving translocases, the maintenance of DNA compaction upstream and downstream could be facilitated by protein–DNA complexes that have the potential to constrain both positive and negative supercoils. The chromatin fiber containing histone octamers, with or without the linker histone, has this property (Recouvreux et al. 2011). However, positive superhelicity may also promote the dissociation of H2A/H2B dimers, thus promoting transcription elongation by loosening DNA wrapping (Sheinin et al. 2013). In bacteria, the octameric form of a mutant HUα protein has also been shown to constrain positive supercoils (Kar et al. 2006), although there is no direct evidence that the wild-type protein has this capability. In this context, an important parameter would be the relative stability of the complexes constraining positive or negative supercoils.
Nucleoprotein complexes
The crucial role of the superhelical density of DNA as a determinant of DNA binding means that it is also a critical factor in the structure and function of higher-order nucleoprotein complexes. Not only can the negative superhelicity stored in packaging complexes be released as unconstrained superhelicity facilitating DNA untwisting but also constraint in higher-order structures maintains DNA compaction. These twin functions are potentially coordinated by DNA translocases, which, in bacteria, may act in concert with DNA gyrase. Superhelicity per se is not essential to maintain DNA compaction. In E. coli, the nucleoid body in late stationary phase is very compact, even though the average superhelical density is low (Frenkiel-Krispin et al. 2001). Similarly, when HeLa cells are deprived of energy, the chromatin compacts, yet, in this case, there is no evidence for a structurally distinct chromatin fibre (Visvanathan et al. 2013).
FIS–RNA polymerase complex
One of the first examples of the effect of superhelical density on the structure of higher-order nucleoprotein assemblies was reported about two decades ago in a study of a nucleoprotein complex formed by the abundant bacterial nucleoid- associated protein (NAP) FIS. It was shown that FIS preferentially binds the supercoiled DNA from the intermediate fraction and much less so from the extremes of the topological spectrum (Schneider et al. 1997). This preference is associated with geometrical constraints imposed by local DNA geometry on the binding of closely spaced helix-turn-helix DNA-binding motifs of FIS (Stella et al. 2010), such that the FIS molecule can be conceived as a wedge fixing a particular DNA geometry in which the two adjacent major groves are in close proximity. This local geometry can be achieved by DNA bending, which in turn requires strategically placed flexible base-steps characteristic of the consensus FIS binding motif (Lazarus and Travers 1993).
The functional relevance of this local feature has been demonstrated on the example of upstream activating sequences (UAS) of stable RNA promoters containing three or more FIS binding sites arranged in helical register. The activity of such promoters, including those directing both ribosomal and transfer RNA synthesis, is highly dependent on negative superhelicity (Oostra et al. 1981; Lamond 1985; Rochman et al. 2002). FIS activates these promoters by binding highly cooperatively (Pemberton et al. 2002) and constrains a particular three-dimensional configuration of the UAS as a higher-order nucleoprotein complex. In this complex, about 80 bp of DNA are wrapped as a tightly bent DNA microloop with low helical pitch. The DNA is wrapped as a single turn of a left-handed supercoil of similar dimensions to the superhelical turns in the eukaryotic nucleosome (Maurer et al. 2006). Disruption of the phasing of FIS binding sites abolishes both the higher-order complex formation and activation by FIS (Muskhelishvili et al. 1995, 1997). Consistent with this structure, the absence of FIS can be compensated by increased negative superhelicity leading to the hypothesis that FIS acts as a topological homeostat, stabilizing the local writhe conducive for untwisting of the GC-rich stable RNA promoters (Rochman et al. 2002; Muskhelishvili and Travers 2003). At the same time, FIS was found to repress the expression of the genes encoding DNA gyrase (Schneider et al. 1999). Thus, in this case, a major DNA architectural protein coordinates the global and local structure of the DNA. Repression of gyrase under high ATP/ADP ratios in rich medium prevents accumulation of excessive negative superhelicity, which is not only energy consuming but also potentially detrimental for the cell, whereas stabilization of the writhed configuration of the DNA in the UAS regions of stable RNA promoters ensures high activity of the rrn operons required for fast growth. Since, as mentioned above, the binding of FIS itself depends on negative superhelicity, it can be readily seen in this example how the variation of supercoiling level can homeostatically regulate itself by determining the activity of distinct components (FIS, in this example) of the genetic system.
The nucleoprotein complex formed at the tyrT promoter comprises not only FIS but also RNA polymerase. The formation of this complex is initiated by a cooperative interaction between the polymerase and a FIS dimer positioned at the proximal binding site leading to the recruitment of further FIS dimers (Pemberton et al. 2002). Yet, even in the absence of FIS, polymerase can interact with the upstream region constraining a negative superhelical loop comprising about 150 bp of DNA (Pemberton et al. 2002; Maurer et al. 2006). This interaction is itself potentiated by negative superhelicity (Pemberton et al. 2002).
The complex of three FIS dimers on the tyrT-UAS is dynamic and is strongly influenced by the process of transcription initiation. The cooperative binding of FIS to three sites coincides precisely with its stimulation of transcription initiation. However, polymerase binding is accompanied by an initial loss in the characteristic footprinting signature of FIS bound to site II, suggesting a change in the interaction, perhaps a dissociation, of FIS bound at that site (Pemberton et al. 2002). One possibility is that this change accompanies untwisting of the DNA in the −10 promoter region by RNA polymerase, a process that would require transmission of negative superhelicity within the complex (Travers and Muskhelishvili 1998) (Fig. 5).
Fig. 5.
Transcription initiation at the tyrT promoter and utilization of DNA wrapping by FIS and RNA polymerase. Conversion of the DNA analog information into the digital code by transmission of the torque stored in the microloop stabilised at the tyrT gene promoter. Left-handed (lh) toroidal coils are metastable to right-handed (rh) plectonemic coils, activation involves conversion of the left-handed into a right-handed coil thus transmitting the supercoil energy to the promoter region and facilitating untwisting of the DNA at the transcription startpoint. a RNA polymerase binding. b Cooperative binding of transcriptional activator FIS to site I proximal to polymerase. c Cooperative binding of FIS to the upstream sites II and III and stabilization of the closed polymerase complex. d Untwisting of the transcription start site (promoter opening) and ejection of FIS at site II. e Escape of polymerase from the promoter and initiation of transcription generating the digital linear message. Right panel shows the AFM images of nucleoprotein complexes assembled at the E. coli tyrT promoter containing three FIS binding sites arranged in helical register and corresponding to depicted stages A and C (courtesy S. Maurer). Reproduced from Muskhelishvili and Travers (2013) with permission
The loop of upstream DNA in an E. coli RNA polymerase-promoter complex is not restricted to stable RNA promoters. It has also been inferred to be present in polymerase complexes formed at the lac promoter (Amouyal and Buc 1987; Buckle et al. 1992; Rivetti et al. 1999), although in these cases the loop is likely considerably shorter (∼90 bp compared to 140–150 bp for stable RNA promoters) and no dependence on DNA superhelicity has been tested.
The dependence of promoter activity in vitro on the superhelical density is strongly influenced by structural modifications induced by DNA supercoiling (Bertrand-Burggraf et al. 1984). In vitro, optimal activity is observed over a narrow range of superhelical density (Stirdivant et al. 1985; Auner et al. 2003). As the density increases, activity reaches a maximum and then declines such that the activity maximum is different for different promoters. For example, the E. coli fis promoter has a higher optimum superhelical density than the wild-type tyrT promoter (Schneider et al. 2000; Auner et al. 2003). Similarly, mutations in the tyrT promoter that improve the fit of the promoter sequence to the consensus lower the optimal value of the superhelical density (Auner et al. 2003). A possible explanation of this effect is that the effect of negative superhelicity on promoter activity is a balance between the facilitation of open complex formation and reduction in the rate of promoter clearance.
30-nm fiber
In mammalian nuclei, the decompaction of chromatin is accompanied by the release of unconstrained negative superhelicity (Naughton et al. 2013). As in bacteria, this phenomenon is indicative of torsional storage in compact chromatin. How is this torsion stored and how might it be generated?
The first hierarchical folding of an array of nucleosomes is the 30-nm fiber (Olins and Olins 1974; Oudet et al. 1975; Finch and Klug 1976). In this structure, nucleosomes are coiled into one or more helical stacks with the linker histone located in the interior of the fiber (Dimitrov et al. 1987; Graziano et al. 1994). The unfolding of the fiber is believed to be a necessary step for transcriptional competence, while further folding to higher-order structures is required to achieve the degree of compaction necessary for most nuclear packaging. Nevertheless, although the fiber is well characterized in vitro, the question of its in vivo significance is still much debated (Maeshima et al. 2010; Fussner et al. 2011; Hansen 2012; Ghirlando and Felsenfeld 2013). In particular, no higher order structures, including the 30-nm fiber, are observed by cryo-electron microscopy in native mitotic chromosomes (Eltsov et al. 2009; Nishino et al. 2012), nor are they apparent in nuclei of tissue-culture cells analyzed by correlative electron spectroscopic imaging (Ahmed et al. 2010). As originally described, the 30-nm fibrr contains one or more helical stacks of nucleosomes with an eponymous diameter of approximately 30 nm (Finch and Klug 1976) (Fig. 6). Such a structure could, in principle, constrain superhelicity in addition to that constrained directly by nucleosomes. A direct demonstration of such constraint was provided by the mechanical application of negative torsion to a regular array of nucleosomes with a 200-bp nucleosome repeat length (NRL) in the presence of a linker histone (Recouvreux et al. 2011). Under these conditions, the maximal negative constraint was ∼1.7 superhelical turns/nucleosome. This value is comparable to that obtained for the superhelicity constrained by an isolated chromatosome containing a single histone octamer and one molecule of linker histone (Zivanovic et al. 1990), and greater than the value of ∼1.1 for an octamer alone (Zivanovic et al. 1988).
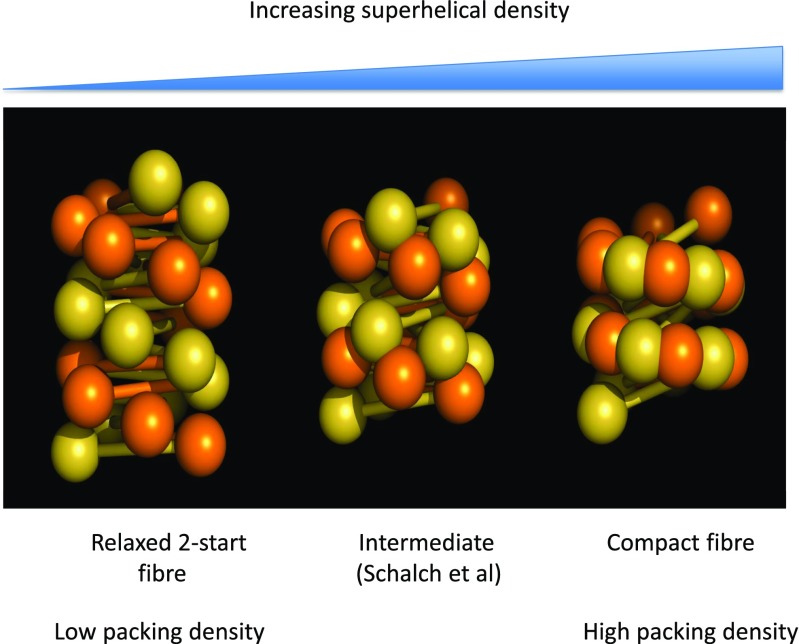
Fig. 6.
Formation of compact 30-nm fibers driven by increasing negative superhelicity. With increasing coiling, the two nucleosome stacks of a ‘relaxed’ fiber (a) with ∼6 nucleosome/turn interdigitate (b) to form a compact fiber (c). Reproduced from Wu et al. (2016) with permission
What is the structure of the fiber that constrains negative superhelicity? Whether the helical form of the 30-nm fiber is a 1-start helix or solenoid (Finch and Klug 1976; Thoma et al. 1979; Felsenfeld and McGhee 1986) with a single stack of nucleosomes, a 2-start helix with two continuous stacks (Williams et al. 1986; Bordas et al. 1986a, b; Woodcock et al. 1984; Worcel et al. 1981) analogous to DNA, or a multi-start helix (Daban and Bermúdez 1998), or whether fibers with different NRLs differ in topology (Wong et al. 2007) has been extensively debated. There is now compelling experimental evidence that the ‘30-nm’ fiber can adopt a 2-start structure. In addition to early EM images (Woodcock et al. 1984), a 2-start structure is supported by Fourier analysis of images of chromatin fibers (Williams et al. 1986), chemical crosslinking (Dorigo et al. 2004), the crystal structure of a tetranucleosome (Schalch et al. 2005), cryo-EM of native fibers (Bednar et al. 1998), cryo-EM structures of reconstituted fibers containing up to 24 nucleosomes (Song et al. 2014), and cryo-tomographic analysis of native chromatin (Horowitz et al. 1994; Scheffer et al. 2011). Nevertheless, early studies on various native chromatin samples by photochemical dichroism (Sen et al. 1986) and X-ray diffraction (Widom and Klug 1985; Widom et al. 1985) revealed, respectively, a small tilt of the nucleosomal disc relative to the fiber axis and narrow diffraction arcs at 110 Å. Together, these observations suggested a helical structure with a low pitch, more consistent with a 1-start model. More recently, EM and cryo-EM measurements on the dimensions of more compact reconstituted fibers covering a wide range of linker lengths led to the proposal of a 1-start interdigitated structure (Robinson et al. 2006). Unexpectedly, the data revealed a step-wise increase in fiber packing density and diameter. Modeling of these fibers predicted the existence of multi-start fibers with several nucleosome stacks (Robinson and Rhodes 2006; Wong et al. 2007; Depken and Schiessel 2009; Kepper et al. 2008), but did not provide a uniform explanation for the observed step-wise changes in fiber parameters.
These apparent contradictory interpretations can be reconciled by assuming that negative superhelicity drives the interdigitation of the two nucleosome stacks in a 2-start structure to form a single continuous left-handed one-start structure with a low pitch (Fig. 6) (Wu et al. 2016). It is this interdigitation which is responsible for the step-wise changes in fiber parameters. However, the coil d iffers from a solenoid in that the dyad axes of adjacent nucleosomes alternate, whereas in a true solenoid these axes are all orthogonal to the superhelical axis. The structure thus retains the 2-start topology of the DNA while assuming a 1-start morphology. For NRLs in the range of 185–210 bp in the most compact 1-start stack, a nucleosome is flanked on one side by a nucleosome separated from it by 5 nucleosomes in the linear array, and on the other side by a 7-nucleosome separation. These values are consistent with recent cross-linking studies on condensed fibers (Grigoryev et al. 2016).
In its most compact form, this fiber constrains ∼1.6–1.7 superhelical turns/nucleosome (Wu et al. 2016). This result is at variance with the early observation that linker histones do not increase the negative superhelicity constrained by a nucleosome array (Keller et al. 1978). However, in these latter experiments, only Na+ ions were present and, consequently, full compaction would not be attained (Widom 1986).
The NRL itself is another important variable for chromatin fiber structure. In vivo, this can vary from ∼154 bp in Schizosaccharomyces pombe chromatin (Lantermann et al. 2010) to ∼240 bp in the sperm of certain echinoderms (Spadafora et al. 1976; Zalenskaya et al. 1981; Widom et al. 1985), the most widespread naturally occurring NRLs being in the range of 186–208 bp. However, the NRLs reported for in vivo chromatin fibers are average values and do not necessarily imply regular spacing of nucleosomes. The variation in NRL in principle affects the superhelical constraint of the fiber. This is because the superhelical constraint from the coiling of the fiber is proportional to (1-sinγ), where γ is the angle between the linker DNA and the normal to the superhelical axis. As the fiber compacts, γ decreases and hence the fiber becomes more supercoiled (Wu et al. 2016). However, packing constraints suggest that, for fibers with short NRLs (e.g., 167 and 177 bp), the pitch angle is higher (Routh et al. 2008) and, therefore, these fibers in principle constrain less negative superhelicity. Importantly, this same principle implies that, as the fiber untwists, unconstrained negative superhelicity can be released, while conversely increasing compaction results in greater constraint.
In the context of active chromatin, constraint of negative superhelicity by a chromatin fiber provides a mechanism for maintaining compaction. Ahead of an advancing polymerase, positive superhelicity will both decompact the fiber and, in principle, unwrap nucleosomes, while behind it, both nucleosomes and the fiber will be reformed. In bacteria, it is likely, that a similar principle operates. One of the principal NAPs in E. coli and other bacteria for maintaining compaction in an active nucleoid is HU (Hammel et al. 2016), a protein that both binds untwisted DNA and has the potential to form higher-order fibers (Guo and Adhya 2007). The functional effect of this NAP is linked to transcriptional activity (Berger et al. 2010), and the dominant paralogues vary with different stages of the growth cycle such that the dominant form in stationary phase constrains little superhelicity (Bordes et al. 2003). Both these characteristics suggest a coupling between HU activity and DNA superhelicity.
H-NS nucleoprotein complexes
H-NS is an abundant bacterial NAP, but, unlike HU, which is believed to constrain a toroidal DNA structure analogous to that in a nucleosome (Rouvière-Yaniv et al. 1979; Guo and Adhya 2007), H-NS complexes can bridge two DNA duplexes (Dame et al. 2000) and concomitantly stabilize the plectonemic form of negatively supercoiled DNA (Schneider et al. 2001). In vivo, two principal functions have been ascribed to H-NS: as a repressor of certain genes and gene clusters located in A/T-rich regions of the genome (Navarre et al. 2006; Ouafa et al. 2012), and as a domainin, assumed to function by stabilizing barriers between distinct topological domains (Hardy and Cozzarelli 2005). Both functions are compatible with the structures assumed by plectonemically coiled DNA superhelices. Although in a plectoneme H-NS can bridge the two component duplexes, the binding of the protein is nucleated on one strand by an A/T-rich sequence (Bouffartigues et al. 2007; Lang et al. 2007; Dorman 2007). H-NS binding can then spread to adjacent regions of similar base composition, forming a nucleofilament. Although such an unbridged structure is likely bent in the form of a right-handed coil, it would not by itself constrain negative superhelicity. Indeed, such writhing would be more consistent with a small positive constraint, analogous to the fibers formed by DnaA (Dong and Berger 2007).
Experimental data has been interpreted to suggest that nucleofilament formation—a stiff complex—occurs in the absence of a polyvalent cation such as magnesium, while such a cation potentiates plectoneme formation and stabilization (Lim et al. 2014). However, in vitro, ionic composition also strongly affects the plectonemic configuration of supercoiled DNA alone (Bednar et al. 1994; Gebe et al. 1996; Rybenkov et al. 1997). The structure of the plectoneme is more ‘open’ at low concentrations of monovalent cation, while the addition of millimolar concentrations of magnesium, facilitate the close approach of two duplexes in the supercoiled structure (Gebe et al. 1996), possibly by neutralizing the negative charges on the DNA backbone. This is analogous to the eukaryotic 30-nm fiber, where full compaction is only observed in the presence of a polyvalent cation, either magnesium or hexamminecobalt (Widom 1986; Wu et al. 2016). This compaction is destabilized by monovalent ions (Widom 1986). In this case, the likely explanation is that the divalent ions facilitate the close approach of the linker DNAs in the cenete of the fiber.
DNA bridging in a supercoiled DNA environment is not confined to abundant proteins such as H-NS. Both AraC and the lac repressor act as bivalent DNA-binding proteins and stabilize a supercoiled loop (Lee and Schleif 1989; Bellomy et al. 1988). Similarly, the dimeric GalR protein, which wraps DNA, specifically binds to the OE and OI operators of the E. coli galactose operon to form a short loop in the presence of HU protein (Lia et al. 2003). For short loops of this type, the two DNA binding sites are normally on the same face of the double helix such that the distance between them can only be varied by an integral number of double helical turns. The formation of the GalR loop requires both negatively supercoiled DNA and HU (Lia et al. 2003). Simulations of the binding of HU to the GalR and LacR loops indicate that DNA superhelicity can direct the binding of HU to a specific site within the loop and confer an optimal trajectory on the looped DNA for loop closure by the repressor protein (Wei et al. 2014). By untwisting constrained DNA, HU has the potential to alter the average helical repeat in the loop and so enable optimal interactions. Such modulations might also potentially influence the topology of the loop itself (see, e.g., Geanacopoulos et al. 2001).
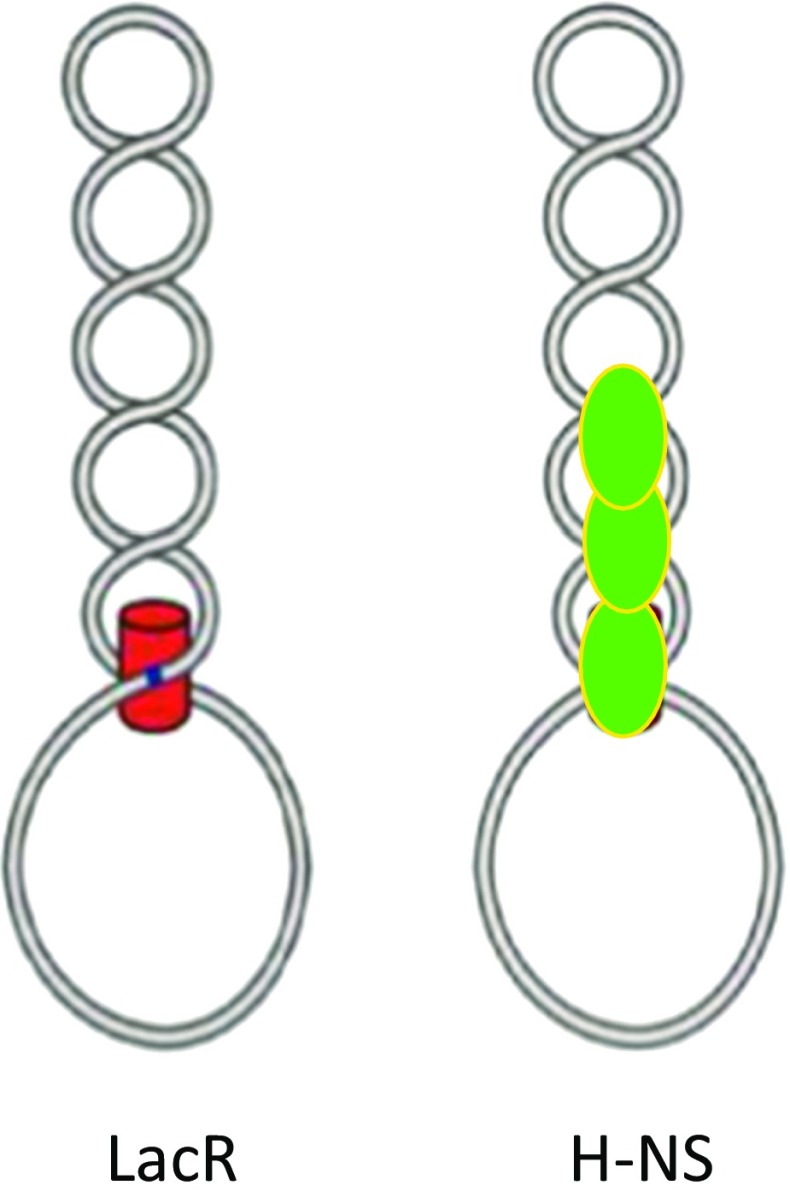
The stabilization of such loops implies that proteins like LacR can act as topological barriers delimiting independent topological domains, a phenomenon which has been experimentally verified in vitro (Leng et al. 2011). The specific bridging of separated sites by LacR and AraC may be compared to the bridging of a plectoneme by H-NS and suggests a mechanism whereby H-NS acts as a domainin by simultaneously securing two distant DNA sequences (Fig. 7).
Fig. 7.
Possible mechanism of delimitation of topological domains by a lacR and b H-NS. Adapted from Leng et al. (2011)
Site-specific recombination complexes
DNA supercoiling impacts all DNA transactions, including DNA recombination (Chi et al. 2006; Kobayashi et al. 2009; Banda et al. 2016). Among recombination systems, the most pervasive impact of DNA superhelicity has been revealed in studies of various site-specific recombination reactions involving formation of higher-order nucleoprotein complexes (Griffith and Nash 1985; Abremski and Hoess 1985; Kanaar et al. 1989; Benjamin et al. 1996; Crisona et al. 1999; Kelly et al. 2006; Trigueros et al. 2009). In vitro, these reactions respond not only to variations in the supercoiling level of DNA substrate (Castell and Halford 1989; Lim and Simon 1992) but also to negative superhelicity accumulated in the wake of translocating RNA polymerase according to the twin-supercoil domain model (Dröge 1993). The response of site-specific recombination to the variable strength of transcription has been observed also in vivo (Booker et al. 2010).
A critical requirement of site-specific recombination reactions is the control of their directionality. Indeed, failure of the selective channeling of particular recombination reactions toward producing fusions, deletions or inversions may be harmful or even detrimental for the cell. These reactions are normally catalyzed by a tetramer of recombinase enzyme binding specific sites organized either as inverted or direct repeats and juxtaposing the partner duplexes in close proximity within the so-called synaptic complex, or synaptosome. Synaptosome formation is often assisted by DNA architectural proteins determining the trajectory of the DNA in the complex which, on its assembly, stores negative superhelicity by trapping DNA crossings (Richet et al. 1988; Kanaar et al. 1989; Kelly et al. 2006). Recombination leads to loss of stored negative superhelicity, the energy of which is assumed to drive the strand exchange. Determination of the resultant ∆Lk, by sharply limiting the acceptable mechanisms of strand exchange, facilitated the identification of the structure of synaptic complexes (Stark et al. 1989; Kanaar et al. 1989; Crisona et al. 1999; Canosa et al. 2003). In that sense, analyses of topological constraints imposed on intramolecular reactions catalyzed by the invertase/resolvase family of recombinases turned out to be most informative.
In the synaptosomes stabilized by invertases and resolvases, two dimers of recombinase protein bind specific DNA sites organized, respectively, as inverted and direct repeats. For effective reaction, the DNA must be negatively supercoiled and the sites must be located in the same topological domain. In the invertasome, the recombinase dimers form a tetramer interacting with accessory DNA architectural protein FIS, whereas in the resolvosome the enzymatically active tetramer is associated with inactive recombinase tetramers and/or DNA architectural proteins bound at the adjacent accessory/regulatory DNA sites (Mouw et al. 2008; Johnson 2015; Rice 2015). Consistent with the notion that DNA supercoiling increases the probability of juxtaposition of three DNA sites at branch points (Vologodskii and Cozzarelli 1996), the invertasome is assembled at a branch point in the negatively supercoiled DNA substrate on juxtaposition of three DNA sites, two recombination sites bound by the invertase tetramer, and the FIS-bound recombinational enhancer sequence (Johnson and Bruist 1989; Dhar et al. 2009). This specificity of structural organization acts as a “topological filter” imposing control on the alignment of recombination sites in the synaptic complex and, thus, in conjunction with stored negative superhelicity, imparting directionality on the reaction (Crisona et al. 1994; Dhar et al. 2009). DNA supercoiling energy plays an important role in this process, since any attempt to properly align recombination sites arranged in improper orientation in the substrate DNA (e.g., in the case of sites organized as direct repeats, instead of inverted repeats in an inversion substrate) would impose an energetic penalty by demanding introduction of an extra loop into the DNA. Indeed, when symmetric recombination sites are used for invertasome assembly (i.e. sites which preclude the distinction between direct and inverted repeats), the recombination reaction generates inversions, rather than deletions, underscoring the role of DNA supercoiling in determining the directionality of reaction (Moskowitz et al. 1991). However, it is noteworthy that, under permissive conditions and with knotted DNA substrates, the unique direction of strand exchange by resolvase has been attributed to the trajectory of DNA in the synaptosome, rather than to global topology (Dröge and Cozzarelli 1989).
DNA superhelicity not only facilitates the assembly of productive synaptic complexes but also the local untwisting of DNA associated with cleavage of recombination sites (Klippel et al. 1993; Benjamin et al. 1996; Lim et al. 1997). Two lines of evidence support this notion. First, the “permissive” invertase mutants, which both loose directionality and also cleave the DNA independent of the FIS–recombinational enhancer complex, show an increased untwisting of the recombination sites (Klippel et al. 1993). Second, a mutation of the invertase binding site has been identified that binds the invertase only if the DNA is supercoiled, whereby, under the influence of supercoiling, this mutation facilitates local untwisting of the recombination site (Bae et al. 2006). These observations together with strict topological requirements of productive invertasome assembly strongly suggest a direct link between the global topology of the synaptic complex and the local geometry of recombination sites.
Although the sequential steps of recombination reaction catalyzed by members of invertase/resolvase family, such as the pairing of recombination sites, assembly of synaptic complexes, cleavage of DNA and, finally, strand exchange, may differ in their dependence on the topology of DNA (Castell and Halford 1989; Lim and Simon 1992; Benjamin et al. 1996), it is likely that, overall, the DNA torsional energy stored in the form of trapped negative crossover nodes in the synaptic complex, in conjunction with chirality of supercoiling and particular DNA trajectory stabilized by architectural proteins, is determinative for the efficiency and directionality of the reaction. This might also apply to integrative recombination catalyzed by the phage λ integrase (Thompson et al. 1988; Crisona et al. 1999), as well as other reactions catalyzed by the members of integrase superfamily of site-specific recombinases (Abremski and Hoess 1985; Dove and Dorman 1994; Corcoran and Dorman 2009). On the other hand, the recombination by C-terminally-modified SSV1 virus integrase from a thermophilic archaeon characterized by positively supercoiled DNA is more efficient with fully relaxed/linear DNA substrates, and is also directionally highly promiscuous in vitro (Muskhelishvili et al. 1993), implicating additional, presently unknown, factors in imparting directionality on the SSV1 integrase-driven recombination in vivo (Schleper et al. 1992).
The role of DNA superhelicity in chromatin dynamics
We have argued that DNA supercoiling in both the eukaryotic nucleus and bacterial nucleoid is a major determinant of chromatin organization (Teves and Henikoff 2014), responsible not only for the structure of nucleoprotein assemblies involved in transcription and recombination but also for facilitating the dynamics of processes mediated by DNA translocases and, thus, for coordinating the genetic activity. For example, DNA supercoiling facilitates both transcription initiation and elongation in a chromatin environment. This relationship is reciprocal, as the DNA translocases themselves have the potential to generate superhelicity by the Liu–Wang mechanism. Crucially, asymmetry in the storage and relaxation of positive and negative supercoils generally results in preferential constraint of negative, but not positive, supercoils in genomic DNA. This stored superhelicity is then available to potentiate gene expression, which is fine-tuned by spatial organization and relative orientation of the transcriptons. DNA superhelicity thus both reflects and drives a dynamic flux of different structural chromatin states such that, ultimately, DNA can affect its own metabolism (Fogg et al. 2012).
Acknowledgments
This work was supported by the Medical Research Council([MRC file reference number MC_U105178783) and in part by Deutsche Forschungsgemeinschaft (DFG MU1549/11-1).
Author contributions
AT and GM wrote the manuscript.
Compliance with ethical standards
Conflict of Interests
Georgi Muskhelishvili declares that he has no conflicts of interest. Andrew Travers declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
* Throughout, the term twist refers to the intrinsic twist of the DNA molecule reflecting its topological geometry in contrast to the surface twist (and its converse, the surface helical repeat) which is a measure of the periodicity of occurrence of, for example, inward-facing minor grooves of wrapped DNA. Writhe is the topological bending equivalent of intrinsic twist. (For full discussions, see Travers and Klug 1987 and Cozzarelli et al. 1990)
This article is part of a Special Issue on ‘DNA supercoiling, protein interactions and genetic function’ edited by Laura Finzi and Wilma Olson.
References
- Abremski K, Hoess R. Phage P1 Cre-loxP site-specific recombination. Effects of DNA supercoiling on catenation and knotting of recombinant products. J Mol Biol. 1985;184:211–220. doi: 10.1016/0022-2836(85)90374-2. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Dehghani H, Rugg-Gunn P, Fussner E, Rossant J, Bazett-Jones DP. Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allemand JF, Bensimon D, Lavery R, Croquette V. Stretched and overwound DNA forms a Pauling-like structure with exposed bases. Proc Natl Acad Sci U S A. 1996;95:14152–14157. doi: 10.1073/pnas.95.24.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amouyal M, Buc H. Topological unwinding of strong and weak promoters by RNA polymerase. A comparison between the lac wild-type and the UV5 sites of Escherichia coli. J Mol Biol. 1987;95:795–808. doi: 10.1016/0022-2836(87)90485-2. [DOI] [PubMed] [Google Scholar]
- Anselmi C, Bocchinfuso G, De Santis P, Savino M, Scipioni A. A theoretical model for the prediction of sequence-dependent nucleosome thermodynamic stability. Biophys J. 2000;79:601–613. doi: 10.1016/S0006-3495(00)76319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auner H, Buckle M, Deufel A, Kutateladze T, Lazarus L, Mavathur R, Muskhelishvili G, Pemberton I, Schneider R, Travers A. Mechanism of transcriptional activation by FIS: role of core promoter structure and DNA topology. J Mol Biol. 2003;331:331–344. doi: 10.1016/S0022-2836(03)00727-7. [DOI] [PubMed] [Google Scholar]
- Azam TA, Iwata A, Nishimura A, Ueda S, Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol. 1999;181:6361–6370. doi: 10.1128/jb.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Yun SH, Sun D, Lim HM, Choi BS. Structural and dynamic basis of a supercoiling-responsive DNA element. Nucleic Acids Res. 2006;34:254–261. doi: 10.1093/nar/gkj428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandina A, Kamashev D, Rouviere-Yaniv J. The bacterial histone-like protein HU specifically recognizes similar structures in all nucleic acids. DNA, RNA, and their hybrids. J Biol Chem. 2002;277:27622–27628. doi: 10.1074/jbc.M201978200. [DOI] [PubMed] [Google Scholar]
- Banda S, Tiwari PB, Darici Y, Tse-Dinh YC. Investigating direct interaction between Escherichia coli topoisomerase I and RecA. Gene. 2016;585:65–70. doi: 10.1016/j.gene.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar J, Furrer P, Stasiak A, Dubochet J, Egelman E, Bates A. The twist, writhe and overall shape of supercoiled DNA change during counterion-induced transition from a loosely to a tightly interwound superhelix. Possible implications for DNA structure in vivo. J Mol Biol. 1994;235:625–637. doi: 10.1006/jmbi.1994.1042. [DOI] [PubMed] [Google Scholar]
- Bednar J, Horowitz RA, Grigoryev SA, Carruthers LM, Hansen JC, Koster AJ, Woodcock CL. Nucleosomes, linker DNA, and linker histone form a unique structural motif that directs the higher-order folding and compaction of chromatin. Proc Natl Acad Sci U S A. 1998;95:14173–14178. doi: 10.1073/pnas.95.24.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomy GR, Mossing MC, Record MT., Jr Physical properties of DNA in vivo as probed by the length dependence of the lac operator looping process. Biochemistry. 1988;27:3900–2906. doi: 10.1021/bi00411a002. [DOI] [PubMed] [Google Scholar]
- Benjamin KR, Abola AP, Kanaar R, Cozzarelli NR. Contributions of supercoiling to Tn3 resolvase and phage Mu Gin site-specific recombination. J Mol Biol. 1996;256:50–65. doi: 10.1006/jmbi.1996.0067. [DOI] [PubMed] [Google Scholar]
- Berger M, Farcas A, Geertz M, Zhelyazkova P, Brix K, Travers A, Muskhelishvili G. Coordination of genomic structure and transcription by the major bacterial nucleoid-associated protein HU. EMBO Rep. 2010;11:59–64. doi: 10.1038/embor.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand-Burggraf E, Schnarr M, Lefevre JF, Daune M. Effect of superhelicity on the transcription from the tet promoter of pBR322. Abortive initiation and unwinding experiments. Nucleic Acids Res. 1984;12:7741–7752. doi: 10.1093/nar/12.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot N, Mavathur R, Geertz M, Travers A, Muskhelishvili G. Homeostatic regulation of supercoiling sensitivity coordinates transcription of the bacterial genome. EMBO Rep. 2006;7:710–715. doi: 10.1038/sj.embor.7400729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker DM, Deng S, Higgins NP. DNA topology of highly transcribed operons in Salmonella typhimurium. Mol Microbiol. 2010;78:1348–1364. doi: 10.1111/j.1365-2958.2010.07394.x. [DOI] [PubMed] [Google Scholar]
- Bordas J, Perez-Grau L, Koch MH, Vega MC, Nave C. The superstructure of chromatin and its condensation mechanism. I. Synchrotron radiation X-ray scattering results. Eur Biophys J. 1986;13:157–173. doi: 10.1007/BF00542560. [DOI] [PubMed] [Google Scholar]
- Bordas J, Perez-Grau L, Koch MH, Vega MC, Nave C. The superstructure of chromatin and its condensation mechanism. II. Theoretical analysis of X-ray scattering patterns and model calculations. Eur Biophys J. 1986;13:175–185. doi: 10.1007/BF00542561. [DOI] [PubMed] [Google Scholar]
- Bordes P, Conter A, Morales V, Bouvier J, Kolb A, Gutierrez C. DNA supercoiling contributes to disconnect sigmaS accumulation from sigmaS-dependent transcription in Escherichia coli. Mol Microbiol. 2003;48:561–571. doi: 10.1046/j.1365-2958.2003.03461.x. [DOI] [PubMed] [Google Scholar]
- Bouffartigues E, Buckle M, Baudaut C, Travers A, Rimsky S. High affinity sites direct the cooperative binding of H-NS to a regulatory element required for transcriptional silencing. Nat Struct Mol Biol. 2007;14:441–448. doi: 10.1038/nsmb1233. [DOI] [PubMed] [Google Scholar]
- Bowater RP, Chen D, Lilley DMJ. Elevated unconstrained supercoiling of plasmid DNA generated by transcription and translation of the tetracycline resistance gene in eubacteria. Biochemistry. 1994;33:9266–9275. doi: 10.1021/bi00197a030. [DOI] [PubMed] [Google Scholar]
- Brewster NK, Johnston GC, Singer RA. A bipartite yeast SSRP1 analog comprised of Pob3 and Nhp6 proteins modulates transcription. Mol Cell Biol. 2001;21:3491–3502. doi: 10.1128/MCB.21.10.3491-3502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle M, Buc H, Travers AA. DNA deformation in nucleoprotein complexes between RNA polymerase, cAMP receptor protein and the lacUV5 promoter probed by singlet oxygen. EMBO J. 1992;11:2619–2625. doi: 10.1002/j.1460-2075.1992.tb05327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden DA, Osheroff N. In vitro evolution of preferred topoisomerase II DNA cleavage sites. J Biol Chem. 1999;274:5227–5235. doi: 10.1074/jbc.274.8.5227. [DOI] [PubMed] [Google Scholar]
- Calladine CR, Hr D, Luisi BF, Travers AA. Understanding DNA. San Diego: Elsevier; 2004. [Google Scholar]
- Canosa I, López G, Rojo F, Boocock MR, Alonso JC. Synapsis and strand exchange in the resolution and DNA inversion reactions catalysed by the beta recombinase. Nucleic Acids Res. 2003;31:1038–1044. doi: 10.1093/nar/gkg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell SE, Halford SE. DNA supercoiling determines the activation energy barrier for site specific recombination by Tn21 resolvase. Nucleic Acids Res. 1989;17:7045–7058. doi: 10.1093/nar/17.17.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Kwon Y, Seong C, Epshtein A, Lam I, Sung P, Klein HL. Yeast recombination factor Rdh54 functionally interacts with the Rad51 recombinase and catalyzes Rad51 removal from DNA. J Biol Chem. 2006;281:26268–79. doi: 10.1074/jbc.M602983200. [DOI] [PubMed] [Google Scholar]
- Cohen BA, Mitra RD, Hughes JD, Church GM. A computational analysis of whole-genome expression data reveals chromosomal domains of gene expression. Nat Genet. 2000;26:183–186. doi: 10.1038/79896. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jamieson ER, Lippard SJ. Enhanced binding of the TATA-binding protein to TATA boxes containing flanking cisplatin 1,2-cross-links. Biochemistry. 2000;39:8259–8265. doi: 10.1021/bi0004495. [DOI] [PubMed] [Google Scholar]
- Corcoran CP, Dorman CJ. DNA relaxation-dependent phase biasing of the fim genetic switch in Escherichia coli depends on the interplay of H-NS, IHF and LRP. Mol Microbiol. 2009;74:1071–1082. doi: 10.1111/j.1365-2958.2009.06919.x. [DOI] [PubMed] [Google Scholar]
- Cozzarelli NR, Boles TC, White JH. Primer on the topology and geometry of DNA supercoiling. In: Cozzarelli NR, Wang JC, editors. DNA topology and its biological effects. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1990. pp. 139–184. [Google Scholar]
- Crisona NJ, Kanaar R, Gonzalez TN, Zechiedrich EL, Klippel A, Cozzarelli NR. Processive recombination by wild-type gin and an enhancer-independent mutant. Insight into the mechanisms of recombination selectivity and strand exchange. J Mol Biol. 1994;243:437–457. doi: 10.1006/jmbi.1994.1671. [DOI] [PubMed] [Google Scholar]
- Crisona NJ, Weinberg RL, Peter BJ, Sumners DW, Cozzarelli NR. The topological mechanism of phage lambda integrase. J Mol Biol. 1999;289:747–775. doi: 10.1006/jmbi.1999.2771. [DOI] [PubMed] [Google Scholar]
- Daban JR, Bermúdez A. Interdigitated solenoid model for compact chromatin fibres. Biochemistry. 1998;3:4299–4304. doi: 10.1021/bi973117h. [DOI] [PubMed] [Google Scholar]
- Dame RT, Wyman C, Goosen N. H-NS mediated compaction of DNA visualised by atomic force microscopy. Nucleic Acids Res. 2000;28:3504–3510. doi: 10.1093/nar/28.18.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depken M, Schiessel H. Nucleosome shape dictates chromatin fibre structure. Biophys J. 2009;96:777–784. doi: 10.1016/j.bpj.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar G, Heiss JK, Johnson RC. Mechanical constraints on Hin subunit rotation imposed by the Fis/enhancer system and DNA supercoiling during site-specific recombination. Mol Cell. 2009;34:746–759. doi: 10.1016/j.molcel.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesch J, Hannan RD, Sanij E. Genome wide mapping of UBF binding-sites in mouse and human cell lines. Genom Data. 2015;3:103–105. doi: 10.1016/j.gdata.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov SI, Russanova VR, Pashev IG. The globular domain of histone H5 is internally located in the chromatin 30 nm fiber: an immunochemical study. EMBO J. 1987;6:2387–2392. doi: 10.1002/j.1460-2075.1987.tb02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong KC, Berger JM. Structural basis for gate-DNA recognition and bending by type IIA topoisomerases. Nature. 2007;450:1201–1205. doi: 10.1038/nature06396. [DOI] [PubMed] [Google Scholar]
- Dorigo B, Schalch T, Kulangara A, Duda S, Schroeder RR, Richmond T. Nucleosome arrays reveal the two-start organization of the chromatin fibre. Science. 2004;306:1571–1573. doi: 10.1126/science.1103124. [DOI] [PubMed] [Google Scholar]
- Dorman CJ. H-NS, the genome sentinel. Nat Rev Microbiol. 2007;5:157–161. doi: 10.1038/nrmicro1598. [DOI] [PubMed] [Google Scholar]
- Dove SL, Dorman CJ. The site-specific recombination system regulating expression of the type 1 fimbrial subunit gene of Escherichia coli is sensitive to changes in DNA supercoiling. Mol Microbiol. 1994;14:975–988. doi: 10.1111/j.1365-2958.1994.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Drew HR, Travers AA. DNA bending and its relation to nucleosome positioning. J Mol Biol. 1985;186:773–790. doi: 10.1016/0022-2836(85)90396-1. [DOI] [PubMed] [Google Scholar]
- Drew HR, Weeks JR, Travers AA. Negative supercoiling induces spontaneous unwinding of a bacterial promoter. EMBO J. 1985;4:1025–1032. doi: 10.1002/j.1460-2075.1985.tb03734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge P. Transcription-driven site-specific DNA recombination in vitro. Proc Natl Acad Sci U S A. 1993;90:2759–2763. doi: 10.1073/pnas.90.7.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge P, Cozzarelli NR. Recombination of knotted substrates by Tn3 resolvase. Proc Natl Acad Sci U S A. 1989;86:6062–6066. doi: 10.1073/pnas.86.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltsov M, MacLellan KM, Maeshima K, Frangakis A, Dubochet J. Analysis of cryo-electron microscopy images does not support the existence of 30-nm chromatin fibers in mitotic chromosomes in situ. Proc Natl Acad Sci U S A. 2009;105:19732–19737. doi: 10.1073/pnas.0810057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G, McGhee JD. Structure of the 30 nm chromatin fibre. Cell. 1986;44:375–377. doi: 10.1016/0092-8674(86)90456-3. [DOI] [PubMed] [Google Scholar]
- Fernández-Sierra M, Shao Q, Fountain C, Finzi L, Dunlap D. E. coli gyrase fails to negatively supercoil diaminopurine-substituted DNA. J Mol Biol. 2015;427:2305–2318. doi: 10.1016/j.jmb.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch JT, Klug A. A solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976;73:1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg JM, Randall GL, Pettitt BM, de Sumners WL, Harris SA, Zechiedrich L. Bullied no more: when and how DNA shoves proteins around. Q Rev Biophys. 2012;45:257–299. doi: 10.1017/S0033583512000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T, Eriksson P, Wittmeyer J, Ginn J, Yu Y, Stillman DJ. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J. 2001;2:3506–3517. doi: 10.1093/emboj/20.13.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkiel-Krispin D, Levin-Zaidman S, Shimoni E, Wolf SG, Wachtel EJ, Arad T, Finkel SE, Kolter R, Minsky A. Regulated phase transitions of bacterial chromatin: a non-enzymatic pathway for generic DNA protection. EMBO J. 2001;20:1184–1191. doi: 10.1093/emboj/20.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussner E, Ching RW, Bazett-Jones DP. Living without 30 nm chromatin fibers. Trends Biochem Sci. 2011;36:1–6. doi: 10.1016/j.tibs.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Geanacopoulos M, Vasmatzis G, Zhurkin VB, Adhya S. Gal repressosome contains an antiparallel DNA loop. Nat Struct Biol. 2001;8:432–436. doi: 10.1038/87595. [DOI] [PubMed] [Google Scholar]
- Gebe JA, Delrow JJ, Heath PJ, Fujimoto BS, Stewart DW, Schurr JM. Effects of Na + and Mg2+ on the structures of supercoiled DNAs: comparison of simulations with experiments. J Mol Biol. 1996;262:105–128. doi: 10.1006/jmbi.1996.0502. [DOI] [PubMed] [Google Scholar]
- Gellert M, Mizuuchi K, O’Dea MH, Nash HA. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlando R, Felsenfeld G. Chromatin structure outside and inside the nucleus. Biopolymers. 2013;99:225–232. doi: 10.1002/bip.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano V, Gerchman SE, Schneider DK, Ramakrishnan V. Histone H1 is located in the interior of the 30 nm fibre. Nature. 1994;368:351–354. doi: 10.1038/368351a0. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Nash HA. Genetic rearrangement of DNA induces knots with a unique topology: implications for the mechanism of synapsis and crossing-over. Proc Natl Acad Sci U S A. 1985;82:3124–3128. doi: 10.1073/pnas.82.10.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev SA, Bascom G, Buckwalter JM, Schubert MB, Woodcock CL, Schlick T. Hierarchical looping of zigzag nucleosome chains in metaphase chromosomes. Proc Natl Acad Sci U S A. 2016;11:1238–1243. doi: 10.1073/pnas.1518280113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Adhya S. Spiral structure of Escherichia coli HUαβ provides foundation for DNA supercoiling. Proc Natl Acad Sci U S A. 2007;104:4309–4314. doi: 10.1073/pnas.0611686104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel M, Amlanjyoti D, Hayes FE, Chen JH, Parpana R, Tang HY, Larabell CA, Tainer JA, Adhya S (2016) HU multimerization shift controls nucleoid compaction. Sci Adv 2:e1600650 [DOI] [PMC free article] [PubMed]
- Hansen JC. Human mitotic chromosome structure: what happened to the 30 nm fibre? EMBO J. 2012;31:1621–1623. doi: 10.1038/emboj.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Ohara O, Takatsuki A, Itoh H, Shimamoto N, Kinosita K., Jr Direct observation of DNA rotation during transcription by Escherichia coli RNA polymerase. Nature. 2001;409:113–115. doi: 10.1038/35051126. [DOI] [PubMed] [Google Scholar]
- Hardy CD, Cozzarelli NR. A genetic selection for supercoiling mutants of Escherichia coli reveals proteins implicated in chromosome structure. Mol Microbiol. 2005;57:1636–1652. doi: 10.1111/j.1365-2958.2005.04799.x. [DOI] [PubMed] [Google Scholar]
- Harris SA, Laughton CA, Liverpool TB. Mapping the phase diagram of the writhe of DNA nanocircles using atomistic molecular dynamics simulations. Nucleic Acids Res. 2008;36:21–29. doi: 10.1093/nar/gkm891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield GW, Benham CJ. DNA topology-mediated control of global gene expression in Escherichia coli. Annu Rev Genet. 2002;36:175–203. doi: 10.1146/annurev.genet.36.032902.111815. [DOI] [PubMed] [Google Scholar]
- Horowitz RA, Agard DA, Sedat JW, Woodcock CL. The three-dimensional architecture of chromatin in situ: electron tomography reveals fibers composed of a continuously variable zig-zag nucleosomal ribbon. J Cell Biol. 1994;12:1–10. doi: 10.1083/jcb.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irobalieva R, Fogg JM, Catanese DJ, Sutthibutpong T, Chen M, Barker AK, Ludtke SJ, Harris SA, Schmid MF, Chiu W, Zechiedrich L. Structural diversity of supercoiled DNA. Nat Commun. 2015;6:8440. doi: 10.1038/ncomms9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson ER, Jacobson MP, Barnes CM, Chow CS, Lippard SJ. Structural and kinetic studies of a cisplatin-modified DNA icosamer binding to HMG1 domain B. J Biol Chem. 1999;274:12346–12354. doi: 10.1074/jbc.274.18.12346. [DOI] [PubMed] [Google Scholar]
- Japaridze A (2015) From polymers to gene regulation: A bottom up approach to the bacterial chromatin. PhD thesis, École Polytechnique Fédérale de Lausanne
- Johnson RC. Site-specific DNA inversion by serine recombinases. Microbiol Spectr. 2015;3:1–36. doi: 10.1128/microbiolspec.MDNA3-0047-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Bruist MF. Intermediates in Hin-mediated DNA inversion: a role for Fis and the recombinational enhancer in the strand exchange reaction. EMBO J. 1989;8:1581–1590. doi: 10.1002/j.1460-2075.1989.tb03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juo ZS, Chiu TK, Leiberman PM, Baikalov I, Berk AJ, Dickerson RE. How proteins recognize the TATA box. J Mol Biol. 1996;261:239–54. doi: 10.1006/jmbi.1996.0456. [DOI] [PubMed] [Google Scholar]
- Kanaar R, van de Putte P, Cozzarelli NR. Gin-mediated recombination of catenated and knotted DNA substrates: implications for the mechanism of interaction between cis-acting sites. Cell. 1989;58:147–159. doi: 10.1016/0092-8674(89)90411-X. [DOI] [PubMed] [Google Scholar]
- Kar S, Choi EJ, Guo F, Dimitriadis EK, Kotova SL, Adhya S. Right-handed DNA supercoiling by an octameric form of histone-like protein HU: modulation of cellular transcription. J Biol Chem. 2006;281:40144–40153. doi: 10.1074/jbc.M605576200. [DOI] [PubMed] [Google Scholar]
- Keller W, Müller U, Eicken I, Wendel I, Zentgraf H. Biochemical and ultrastructural analysis of SV40 chromatin. Cold Spring Harb Symp Quant Biol. 1978;42:227–244. doi: 10.1101/SQB.1978.042.01.025. [DOI] [PubMed] [Google Scholar]
- Kelly A, Conway C, Cróinín T, Smith SG, Dorman CJ. DNA supercoiling and the Lrp protein determine the directionality of fim switch DNA inversion in Escherichia coli K-12. J Bacteriol. 2006;188:5356–5363. doi: 10.1128/JB.00344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepper N, Foethke D, Stehr R, Wedemann G, Rippe K. Nucleosome geometry and internucleosomal interactions control the chromatin fiber conformation. Biophys J. 2008;95:3692–3705. doi: 10.1529/biophysj.107.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Geiger JH, Hahn S, Sigler PB. Crystal structure of a yeast TBP/TATA-box complex. Nature. 1993;365:512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- Kim JL, Nikolov DB, Burley SK. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature. 1993;365:520–527. doi: 10.1038/365520a0. [DOI] [PubMed] [Google Scholar]
- Klippel A, Kanaar R, Kahmann R, Cozzarelli NR. Analysis of strand exchange and DNA binding of enhancer-independent Gin recombinase mutants. EMBO J. 1993;12:1047–1057. doi: 10.1002/j.1460-2075.1993.tb05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Aida M, Nagaoka H, Begum NA, Kitawaki Y, Nakata M, Stanlie A, Doi T, Kato L, Okazaki IM, Shinkura R, Muramatsu M, Kinoshita K, Honjo T. AID-induced decrease in topoisomerase 1 induces DNA structural alteration and DNA cleavage for class switch recombination. Proc Natl Acad Sci U S A. 2009;106:22375–80. doi: 10.1073/pnas.0911879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzine F, Sanford S, Elisha-Feil Z, Levens D. The functional response of upstream DNA to dynamic supercoiling in vivo. Nat Struct Mol Biol. 2008;15:146–154. doi: 10.1038/nsmb.1372. [DOI] [PubMed] [Google Scholar]
- Kouzine F, Gupta A, Baranello L, Wojtowicz D, Ben-Aissa K, Liu J, Przytycka TM, Levens D. Transcription-dependent dynamic supercoiling is a short-range genomic force. Nat Struct Mol Biol. 2013;20:396–403. doi: 10.1038/nsmb.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasilnikov AS, Podtelezhnikov A, Vologodskii A, Mirkin SM. Large-scale effects of transcriptional DNA supercoiling in vivo. J Mol Biol. 1999;292:1149–1160. doi: 10.1006/jmbi.1999.3117. [DOI] [PubMed] [Google Scholar]
- Kruglyak S, Tang H. Regulation of adjacent yeast genes. Trends Genet. 2000;16:109–111. doi: 10.1016/S0168-9525(99)01941-1. [DOI] [PubMed] [Google Scholar]
- Lamond AI. Supercoiling response of a bacterial tRNA gene. EMBO J. 1985;4:501–507. doi: 10.1002/j.1460-2075.1985.tb03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang B, Blot N, Bouffartigues E, Buckle M, Geertz M, Gualerzi CO, Mavathur R, Muskhelishvili G, Pon C, Rimsky S, Stella S, Babu MM, Travers A. High affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 2007;34:6330–6337. doi: 10.1093/nar/gkm712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantermann AB, Straub T, Strålfor A, Yuan GC, Ekwall K, Korber P. Schizosaccharomyces pombe genome-wide nucleosome mapping reveals positioning mechanisms distinct from Saccharomyces cerevisiae. Nat Struct Mol Biol. 2010;17:251–257. doi: 10.1038/nsmb.1741. [DOI] [PubMed] [Google Scholar]
- Lazarus LR, Travers AA. The E. coli FIS protein is not required for the activation of tyrT transcription on simple nutritional upshift. EMBO J. 1993;12:2483–2494. doi: 10.1002/j.1460-2075.1993.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Schleif RF. In vivo DNA loops in araCBAD: size limits and helical repeat. Proc Natl Acad Sci U S A. 1989;86:476–480. doi: 10.1073/pnas.86.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng F, Chen B, Dunlap DD. Dividing a supercoiled DNA molecule into two independent topological domains. Proc Natl Acad Sci U S A. 2011;108:19973–19978. doi: 10.1073/pnas.1109854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia G, Bensimon D, Croquette V, Allemand JF, Dunlap D, Lewis DE, Adhya S, Finzi L. Supercoiling and denaturation in Gal repressor/heat unstable nucleoid protein (HU)-mediated DNA looping. Proc Natl Acad Sci U S A. 2003;100:11373–11377. doi: 10.1073/pnas.2034851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley DMJ. The inverted repeat as a recognizable structural feature in supercoiled DNA molecules. Proc Natl Acad Sci U S A. 1980;77:6468–6472. doi: 10.1073/pnas.77.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HM, Simon MI. The role of negative supercoiling in Hin-mediated site-specific recombination. J Biol Chem. 1992;267:11176–11182. [PubMed] [Google Scholar]
- Lim HM, Lee HJ, Jaxel C, Nadal M. Hin-mediated inversion on positively supercoiled DNA. J Biol Chem. 1997;272:18434–18439. doi: 10.1074/jbc.272.29.18434. [DOI] [PubMed] [Google Scholar]
- Lim CJ, Kenney LJ, Yan J. Single-molecule studies on the mechanical interplay between DNA supercoiling and H-NS DNA architectural properties. Nucleic Acids Res. 2014;42:8369–8378. doi: 10.1093/nar/gku566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge JK, Kazic T, Berg DE. Formation of supercoiling domains in plasmid pBR322. J Bacteriol. 1989;171:2181–2187. doi: 10.1128/jb.171.4.2181-2187.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M, Hillisch A, Payet D, Buttinelli M, Travers A, Diekmann S. DNA bending induced by high mobility group proteins studied by fluorescence resonance energy transfer. Biochemistry. 1999;38:12150–12158. doi: 10.1021/bi990459+. [DOI] [PubMed] [Google Scholar]
- Lynch AS, Wang JC. Anchoring of DNA to the bacterial cytoplasmic membrane through cotranscriptional synthesis of polypeptides encoding membrane proteins or proteins for export: a mechanism of plasmid hypernegative supercoiling in mutants deficient in DNA topoisomerase I. J Bacteriol. 1993;175:1645–1655. doi: 10.1128/jb.175.6.1645-1655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Cook DN, Pon NG, Hearst JE. Efficient anchoring of RNA polymerase in Escherichia coli during coupled transcription-translation of genes encoding integral inner membrane polypeptides. J Biol Chem. 1994;269:15362–15370. [PubMed] [Google Scholar]
- Mace HAF, Pelham HRB, Travers AA. Association of an S1 nuclease-sensitive structure with short direct repeats 5′ of Drosophila heat shock genes. Nature. 1983;304:555–557. doi: 10.1038/304555a0. [DOI] [PubMed] [Google Scholar]
- Maeshima K, Hihara S, Eltsov M. Chromatin structure; does the 30-nm fibre exist in vivo? Curr Opin Struct Biol. 2010;22:291–297. doi: 10.1016/j.ceb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Malarkey CS, Churchill ME. The high mobility group box: the ultimate utility player of a cell. Trends Biochem Sci. 2012;37:553–62. doi: 10.1016/j.tibs.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr C, Geertz M, Hütt MT, Muskhelishvili G. Dissecting the logical types of network control in gene expression profiles. BMC Syst Biol. 2008;2:18. doi: 10.1186/1752-0509-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PB, Struhl K. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol. 2003;23:8323–8333. doi: 10.1128/MCB.23.22.8323-8333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer S, Fritz J, Muskhelishvili G, Travers A. RNA polymerase and an activator form discrete subcomplexes in a transcription initiation complex. EMBO J. 2006;25:3784–3790. doi: 10.1038/sj.emboj.7601261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer S, Fritz J, Muskhelishvili G. A systematic in vitro study of nucleoprotein complexes formed by bacterial nucleoid-associated proteins revealing novel types of DNA organization. J Mol Biol. 2009;387:1261–1276. doi: 10.1016/j.jmb.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Meyer S, Beslon G. Torsion-mediated interaction between adjacent genes. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minyat EE, Khomyakova EB, Petrova MV, Zdobnov EM, Ivanov VI. Experimental evidence for slipped loop DNA, a novel folding type for polynucleotide chain. J Biomol Struct Dyn. 1995;13:523–527. doi: 10.1080/07391102.1995.10508862. [DOI] [PubMed] [Google Scholar]
- Mitchell JS, Harris SA. Thermodynamics of writhe in DNA minicircles from molecular dynamics simulations. Phys Rev Lett. 2013;110:148105. doi: 10.1103/PhysRevLett.110.148105. [DOI] [PubMed] [Google Scholar]
- Moreira JM, Holmberg S. Chromatin-mediated transcriptional regulation by the yeast architectural factors NHP6A and NHP6B. EMBO J. 2000;19:6804–6813. doi: 10.1093/emboj/19.24.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz IP, Heichman KA, Johnson RC. Alignment of recombination sites in Hin-mediated site-specific DNA recombination. Genes Dev. 1991;5:1635–1645. doi: 10.1101/gad.5.9.1635. [DOI] [PubMed] [Google Scholar]
- Moulin L, Rahmouni AR, Boccard F. Topological insulators inhibit diffusion of transcription-induced positive supercoils in the chromosome of escherichia coli. Mol Microbiol. 2005;55:601–610. doi: 10.1111/j.1365-2958.2004.04411.x. [DOI] [PubMed] [Google Scholar]
- Mouw KW, Rowland SJ, Gajjar MM, Boocock MR, Stark WM, Rice PA. Architecture of a serine recombinase-DNA regulatory complex. Mol Cell. 2008;30:145–155. doi: 10.1016/j.molcel.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskhelishvili G, Travers A. Transcription factor as a topological homeostat. Front Biosci. 2003;8:279–285. doi: 10.2741/969. [DOI] [PubMed] [Google Scholar]
- Muskhelishvili G, Travers A. Integration of syntactic and semantic properties of the DNA code reveals chromosomes as thermodynamic machines converting energy into information. Cell Mol Life Sci. 2013;70:4555–4567. doi: 10.1007/s00018-013-1394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskhelishvili G, Palm P, Zillig W. SSV1 encoded site-specific recombination system in Sulfolobus shibatae. Mol Gen Genet. 1993;237:334–342. doi: 10.1007/BF00279436. [DOI] [PubMed] [Google Scholar]
- Muskhelishvili G, Travers AA, Heumann H, Kahmann R. FIS and RNA polymerase holoenzyme form a specific nucleoprotein complex at a stable RNA promoter. EMBO J. 1995;14:1446–1452. doi: 10.1002/j.1460-2075.1995.tb07131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskhelishvili G, Buckle M, Heumann H, Kahmann R, Travers AA. FIS activates sequential steps during transcription initiation at a stable RNA promoter. EMBO J. 1997;16:3655–3665. doi: 10.1093/emboj/16.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton C, Avlonitis N, Corless S, Prendergast JG, Mati IK, Eijk PP, Cockroft SL, Bradley M, Ylstra B, Gilbert N. Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures. Nat Struct Mol Biol. 2013;20:387–395. doi: 10.1038/nsmb.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- Nigatu D, Henkel W, Sobetzko P, Muskhelishvili G. Relationship between digital information and thermodynamic stability in bacterial genomes. J Bioinform Syst Biol. 2016;2018:4. doi: 10.1186/s13637-016-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino Y, Eltsov M, Joti Y, Ito K, Takata H, Takahashi Y, Hihara S, Frangakis AS, Imamoto N, Ishikawa T, Maeshima K. Human mitotic chromosomes consist predominantly of irregularly folded nucleosome fibres without a 30-nm chromatin structure. EMBO J. 2012;31:1644–1653. doi: 10.1038/emboj.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins AL, Olins DE. Spheroid chromatin units (v bodies) Science. 1974;183:330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Oostra BA, van Vliet AJ, Ab G, Gruber M (1981) Involvement of DNA gyrase in the transcription of ribosomal RNA. J Bacteriol 148:782–787 [DOI] [PMC free article] [PubMed]
- Oram M, Travers AA, Howells AJ, Maxwell A, Pato ML. Dissection of the bacteriophage Mu strong gyrase site (SGS): significance of the SGS right arm in Mu biology and DNA gyrase mechanism. J Bacteriol. 2006;188:619–632. doi: 10.1128/JB.188.2.619-632.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G, Wu WH, Lane WS, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises human Spt16 and SSRP1 proteins. Nature. 1999;400:284–288. doi: 10.1038/22350. [DOI] [PubMed] [Google Scholar]
- Ouafa ZA, Reverchon S, Lautier T, Muskhelishvili G, Nasser W. The nucleoid-associated proteins H-NS and FIS modulate the DNA supercoiling response of the pel genes, the major virulence factors in the plant pathogen bacterium Dickeya dadantii. Nucleic Acids Res. 2012;40:4306–4319. doi: 10.1093/nar/gks014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P, Gross-Bellard M, Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975;4:281–300. doi: 10.1016/0092-8674(75)90149-X. [DOI] [PubMed] [Google Scholar]
- Papillon J, Ménétret JF, Batisse C, Hélye R, Schultz P, Potier N, Lamour V. Structural insight into negative DNA supercoiling by DNA gyrase, a bacterial type 2A DNA topoisomerase. Nucleic Acids Res. 2013;41:7815–7827. doi: 10.1093/nar/gkt560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton IK, Muskhelishvili G, Travers AA, Buckle M. FIS modulates the kinetics of successive interactions of RNA polymerase with the core and upstream regions of the tyrT promoter. J Mol Biol. 2002;318:615–613. doi: 10.1016/S0022-2836(02)00142-0. [DOI] [PubMed] [Google Scholar]
- Quinn J, Fyrberg AM, Ganster RW, Schmidt MC, Peterson CL. DNA-binding properties of the yeast SWI/SNF complex. Nature. 1996;379:844–847. doi: 10.1038/379844a0. [DOI] [PubMed] [Google Scholar]
- Recouvreux P, Lavelle C, Barbi M, Condé e Silva N, Le Cam E, Victor JM, Viovy JL. Linker histones incorporation maintains chromatin fiber plasticity. Biophys J. 2011;100:2726–2735. doi: 10.1016/j.bpj.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice PA (2015) Serine resolvases. Microbiol Spectr 3: MDNA3-0045-2014 [DOI] [PMC free article] [PubMed]
- Richet E, Abcarian P, Nash HA. Synapsis of attachment sites during lambda integrative recombination involves capture of a naked DNA by a protein-DNA complex. Cell. 1988;52:9–17. doi: 10.1016/0092-8674(88)90526-0. [DOI] [PubMed] [Google Scholar]
- Richmond TJ, Davey CA. The structure of DNA in the nucleosome core. Nature. 2003;423:145–150. doi: 10.1038/nature01595. [DOI] [PubMed] [Google Scholar]
- Rivetti C, Guthold M, Bustamante C. Wrapping of DNA around the E.coli RNA polymerase open promoter complex. EMBO J. 1999;18:4464–4475. doi: 10.1093/emboj/18.16.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJJ, Rhodes D. Structure of the ‘30 nm’ chromatin fibre: a key role for the linker histone. Curr Opin Struct Biol. 2006;16:336–343. doi: 10.1016/j.sbi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Robinson PJJ, Fairall L, Huynh VAT, Rhodes D. EM measurements define the dimensions of the “30-nm” chromatin fibre: Evidence for a compact, interdigitated structure. Proc Natl Acad Sci U S A. 2006;103:6506–6511. doi: 10.1073/pnas.0601212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman M, Aviv M, Glaser G, Muskhelishvili G. Promoter protection by a transcription factor acting as a local topological homeostat. EMBO Rep. 2002;3:355–360. doi: 10.1093/embo-reports/kvf067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh A, Sandin S, Rhodes D. Nucleosome repeat length and linker histone stoichiometry determine chromatin fiber structure. Proc Natl Acad Sci U S A. 2008;105:8872–8877. doi: 10.1073/pnas.0802336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J, Yaniv M, Germond JE. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979;17:265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Rybenkov VV, Vologodskii AV, Cozzarelli NR. The effect of ionic conditions on DNA helical repeat, effective diameter and free energy of supercoiling. Nucleic Acids Res. 1997;25:1412–1418. doi: 10.1093/nar/25.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador ML, Klein U, Bogorad L. Endogenous fluctuations of DNA topology in the chloroplast of Chlamydomonas reinhardtii. Mol Cell Biol. 1998;18:7235–7242. doi: 10.1128/MCB.18.12.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaLucia J., Jr A unified view of polymer, dumbbell and oligonucleotide nearest-neighbor thermodynamics. Proc Natl Acad Sci U S A. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch T, Duda S, Sargent DF, Richmond TJ. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature. 2005;436:138–141. doi: 10.1038/nature03686. [DOI] [PubMed] [Google Scholar]
- Scheffer MP, Eltsov M, Frangakis AS. Evidence for short-range helical order in the 30-nm chromatin fibres of erythrocyte nuclei. Proc Natl Acad Sci U S A. 2011;108:16992–16997. doi: 10.1073/pnas.1108268108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleper C, Kubo K, Zillig W. The particle SSV1 from the extremely thermophilic archaeon Sulfolobus is a virus: demonstration of infectivity and of transfection with viral DNA. Proc Natl Acad Sci U S A. 1992;89:7645–7649. doi: 10.1073/pnas.89.16.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Travers A, Muskhelishvili G. FIS modulates growth-phase dependent topological transitions of DNA in E. coli. Mol Microbiol. 1997;26:519–530. doi: 10.1046/j.1365-2958.1997.5951971.x. [DOI] [PubMed] [Google Scholar]
- Schneider R, Travers A, Kutateladze T, Muskhelishvili G. A DNA architectural protein couples cellular physiology and DNA topology in Escherichia coli. Mol Microbiol. 1999;34:953–964. doi: 10.1046/j.1365-2958.1999.01656.x. [DOI] [PubMed] [Google Scholar]
- Schneider R, Travers A, Muskhelishvili G. The expression of the Escherichia coli fis gene is strongly dependent on the superhelical density of DNA. Mol Microbiol. 2000;38:167–176. doi: 10.1046/j.1365-2958.2000.02129.x. [DOI] [PubMed] [Google Scholar]
- Schneider R, Lurz R, Tolksdorf C, Lüder G, Travers A, Muskhelishvili G. An architectural role of the Escherichia coli chromatin protein FIS in organising DNA. Nucleic Acids Res. 2001;29:5107–5114. doi: 10.1093/nar/29.24.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam S, Koirala D, Yu Z, Mao H. Quantficiation of topological coupling DNA superhelicity and G-quadruplex formation. J Am Chem Soc. 2014;136:13967–13970. doi: 10.1021/ja5064394. [DOI] [PubMed] [Google Scholar]
- Semon M, Duret L. Evolutionary origin and maintenance of coexpressed gene clusters in mammals. Mol Biol Evol. 2006;23:1715–1723. doi: 10.1093/molbev/msl034. [DOI] [PubMed] [Google Scholar]
- Sen D, Mitra S, Crothers DM. Higher order structure of chromatin: Evidence from photochemically detected linear dichroism. Biochemistry. 1986;25:3441–3447. doi: 10.1021/bi00359a052. [DOI] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. Bell Syst Technol J. 1948;27:379–423623656. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- Sheinin MY, Li M, Soltani M, Luger K, Wang MD. Torque modulates nucleosome stability and facilitates H2A/H2B dimer loss. Nat Commun. 2013;4:2579. doi: 10.1038/ncomms3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton CK, Klysik J, Stirdivant SM, Wells RD. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982;299:312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Sobetzko P. Transcription-coupled DNA supercoiling dictates the chromosomal arrangement of bacterial genes. Nucleic Acids Res. 2016;44:1514–1524. doi: 10.1093/nar/gkw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobetzko P, Travers A, Muskhelishvili G. Gene order and chromosome dynamics coordinate spatiotemporal gene expression during the bacterial growth cycle. Proc Natl Acad Sci U S A. 2012;109:E49–E50. doi: 10.1073/pnas.1108229109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobetzko P, Glinkowska M, Travers A, Muskhelishvili G. DNA thermodynamic stability and supercoil dynamics determine the gene expression program during the bacterial growth cycle. Mol BioSyst. 2013;9:1643–1651. doi: 10.1039/c3mb25515h. [DOI] [PubMed] [Google Scholar]
- Song F, Chen P, Sun D, Wang M, Dong L, Liang D, Xu RM, Zhu P, Li G. Cryo-EM study of the chromatin fibre reveals a double helix twisted by tetranucleosomal units. Science. 2014;344:376–380. doi: 10.1126/science.1251413. [DOI] [PubMed] [Google Scholar]
- Spadafora C, Bellard M, Compton J, Chambon P. The DNA repeat lengths from sea urchin sperm and gastrule cells are markedly different. FEBS Lett. 1976;69:281–285. doi: 10.1016/0014-5793(76)80704-1. [DOI] [PubMed] [Google Scholar]
- Stark WM, Sherratt DJ, Boocock MR. Site-specific recombination by Tn3 resolvase: topological changes in the forward and reverse reactions. Cell. 1989;58:779–790. doi: 10.1016/0092-8674(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Stella S, Cascio D, Johnson RC. The shape of the DNA minor groove directs binding by the DNA-bending protein Fis. Genes Dev. 2010;24:814–26. doi: 10.1101/gad.1900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirdivant SM, Crossland LD, Bogorad L. DNA supercoiling affects transcription of two maize chloroplast genes differently. Proc Natl Acad Sci U S A. 1985;82:4886–4890. doi: 10.1073/pnas.82.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick TR, Allemand JF, Bensimon D, Croquette V. Behavior of supercoiled DNA. Biophys J. 1998;74:2016–2028. doi: 10.1016/S0006-3495(98)77908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Hurley LH. The importance of negative superhelicity in inducing the formation of G-quadruplex and i-motif structures in the c-Myc promoter: implications for drug targeting and control of gene expression. J Med Chem. 2009;52:2863–2874. doi: 10.1021/jm900055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves SS, Henikoff S. DNA torsion as a feedback mediator of transcription and chromatin dynamics. Nucleus. 2014;5:211–218. doi: 10.4161/nucl.29086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F, Koller T, Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JO, Travers AA. HMG1 and 2 and related “architectural” DNA-binding proteins. Trends Biochem Sci. 2001;27:168–175. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Snyder UK, Landy A. Helical-repeat dependence of integrative recombination of bacteriophage lambda: role of the P1 and H1 protein binding sites. Proc Natl Acad Sci U S A. 1988;85:6323–6327. doi: 10.1073/pnas.85.17.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers AA, Klug A. The bending of DNA in nucleosomes and its wider implications. Philos Trans R Soc Lond B. 1987;317:537–561. doi: 10.1098/rstb.1987.0080. [DOI] [PubMed] [Google Scholar]
- Travers A, Muskhelishvili G (1998) DNA microloops and microdomains: a general mechanism for transcription activation by torsional transmission. J Mol Biol 279:1027–1043 [DOI] [PubMed]
- Travers A, Muskhelishvili G. DNA structure and function. FEBS J. 2015;282:2279–95. doi: 10.1111/febs.13307. [DOI] [PubMed] [Google Scholar]
- Travers AA, Muskhelishvili G, Thompson JMT. DNA information; from digital code to analogue structure. Philos Trans R Soc Lond A. 2012;370:2960–2986. doi: 10.1098/rsta.2011.0231. [DOI] [PubMed] [Google Scholar]
- Trigueros S, Tran T, Sorto N, Newmark J, Colloms SD, Sherratt DJ, Tolmasky ME. mwr Xer site-specific recombination is hypersensitive to DNA supercoiling. Nucleic Acids Res. 2009;37:3580–3587. doi: 10.1093/nar/gkp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Timsit Y. Differential stability of chiral DNA crossovers mediated by divalent cations. Nucl Acid Res. 2010;38:4163–4172. doi: 10.1093/nar/gkq150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan A, Ahmed K, Even-Faitelson L, Lleres D, Bazett-Jones DP, Lamond AI. Modulation of higher order chromatin conformation in mammalian cell nuclei can be mediated by polyamines and divalent cations. PLoS ONE. 2013;8:e67689. doi: 10.1371/journal.pone.0067689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vologodskii AV, Cozzarelli NR (1996) Effect ofsupercoiling on the juxtaposition and relative orientationof DNA sites. Biophys J 70:2548–2556 [DOI] [PMC free article] [PubMed]
- Voloshin ON, Veselkov AG, Belotserkovskii BP, Danilevskaya ON, Pavlova MN, Dobrynin VN, Frank-Kamenetskii MD. An eclectic DNA structure adopted by human telomeric sequence under superhelical stress and low pH. J Biomol Struct Dyn. 1992;9:643–652. doi: 10.1080/07391102.1992.10507945. [DOI] [PubMed] [Google Scholar]
- Wall MK, Mitchenall LA, Maxwell A. Arabidopsis thaliana DNA gyrase is targeted to chloroplasts and mitochondria. Proc Natl Acad Sci U S A. 2004;101:7821–7826. doi: 10.1073/pnas.0400836101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Liu LF. DNA replication: topological aspects and the roles of DNA topoisomerases. In: Cozzarelli NR, Wang JC, editors. DNA topology and its biological effects. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1990. pp. 321–340. [Google Scholar]
- Wang GZ, Lercher MJ, Hurst LD. Transcriptional coupling of neighboring genes and gene expression noise: evidence that gene orientation and noncoding transcripts are modulators of noise. Genome Biol Evol. 2011;3:320–331. doi: 10.1093/gbe/evr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Czapla L, Grosner MA, Swigon D, Olson WK. DNA topology confers sequence specificity to nonspecific architectural proteins. Proc Natl Acad Sci U S A. 2014;111:16742–16747. doi: 10.1073/pnas.1405016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom J. Physicochemical studies of the folding of the 100 Å nucleosome filament into the 300 Å filament. Cation dependence. J Mol Biol. 1986;190:411–424. doi: 10.1016/0022-2836(86)90012-4. [DOI] [PubMed] [Google Scholar]
- Widom J, Klug A. Structure of the 300 Å chromatin filament: X-ray diffraction from oriented samples. Cell. 1985;43:207–213. doi: 10.1016/0092-8674(85)90025-X. [DOI] [PubMed] [Google Scholar]
- Widom J, Finch JT, Thomas JO. Higher-order structure of long repeat chromatin. EMBO J. 1985;4:3198–3194. doi: 10.1002/j.1460-2075.1985.tb04064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Bowles DJ. Coexpression of neighboring genes in the genome of Arabidopsis thaliana. Genome Res. 2004;14:1060–1067. doi: 10.1101/gr.2131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SP, Athey BD, Muglia LJ, Schappe RS, Gough AH, Langmore JP. Chromatin fibres are left-handed double helices with diameter and mass per unit length that depend on linker length. Biophys J. 1986;49:233–248. doi: 10.1016/S0006-3495(86)83637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H, Victor JM, Mozziconacci J. An all-atom model of the chromatin fibre containing linker histones reveals a versatile structure tuned by the nucleosome repeat length. PLoS ONE. 2007;2 doi: 10.1371/journal.pone.0000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock C, Frado LL, Ratner JB. The higher-order structure of chromatin: evidence for a helical ribbon arrangement. J Cell Biol. 1984;99:42–52. doi: 10.1083/jcb.99.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A, Stogatz S, Riley D. Structure of chromatin and the linking number of DNA. Proc Natl Acad Sci U S A. 1981;78:1461–1465. doi: 10.1073/pnas.78.3.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, McGeehan JE, Travers A. A metastable structure for the compact 30 nm chromatin fibre. FEBS Lett. 2016 doi: 10.1002/1873-3468.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Wang MD, Svoboda K, Landick R, Block SM, Gelles J. Transcription against an applied force. Science. 1995;270:1653–1657. doi: 10.1126/science.270.5242.1653. [DOI] [PubMed] [Google Scholar]
- Zalenskaya IA, Pospelov VA, Zalensky AO, Vorob’ev VI. Nucleosomal structure of sea urchin and starfish sperm chromatin. Histone H2B is possibly involved in determining the length of linker DNA. Nucleic Acids Res. 1981;9:473–487. doi: 10.1093/nar/9.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanovic Y, Goulet I, Revet B, Le Bret M, Prunell A. Chromatin reconstitution on small DNA rings. II. DNA supercoiling on the nucleosome. J Mol Biol. 1988;200:267–290. doi: 10.1016/0022-2836(88)90239-2. [DOI] [PubMed] [Google Scholar]
- Zivanovic Y, Duband-Goulet I, Schultz P, Stofer E, Oudet P, Prunell A. Chromatin reconstitution on small DNA rings. III. Histone H5 dependence of DNA supercoiling in the nucleosome. J Mol Biol. 1990;214:479–495. doi: 10.1016/0022-2836(90)90195-R. [DOI] [PubMed] [Google Scholar]