Abstract
Photodynamic therapy (PDT) combines a photosensitiser, light and molecular oxygen to induce oxidative stress that can be used to kill pathogens, cancer cells and other highly proliferative cells. There is a growing number of clinically approved photosensitisers and applications of PDT, whose main advantages include the possibility of selective targeting, localised action and stimulation of the immune responses. Further improvements and broader use of PDT could be accomplished by designing new photosensitisers with increased selectivity and bioavailability. Porphyrin-based photosensitisers with amphiphilic properties, bearing one or more positive charges, are an effective tool in PDT against cancers, microbial infections and, most recently, autoimmune skin disorders. The aim of the review is to present some of the recent examples of the applications and research that employ this specific group of photosensitisers. Furthermore, we will highlight the link between their structural characteristics and PDT efficiency, which will be helpful as guidelines for rational design and evaluation of new PSs.
Keywords: Photodynamic therapy, Photosensitiser, Porphyrin, Cancer, Infection
Introduction
In comparison to the conventional anticancer therapies such as surgery, chemotherapy and radiotherapy, photodynamic therapy (PDT) has several advantages. It is largely non-invasive, and site-specific, and thus can be used when conventional therapy is ineffective and/or not advised (Allison and Moghissi 2013a). Photosensitiser (PS) is usually non-toxic in the absence of light, and is passively accumulated and retained in the tumour tissue (Vaidya et al. 2006). The mechanism that conferes this therapeutically advantageous feature is known as enhanced permeability and retention effect (EPR) (Maeda et al. 2016). Once a PS is accumulated in the tumour, it is activated with local application of light of the appropriate wavelength, which leads to the formation of the cytotoxic singlet oxygen (1O2) that can kill tumour cells. Singlet oxygen diffusion is usually limited to within the cell due to its short half-life (Skovsen et al. 2005), so upon photoactivation only those cells that accumulated PS are killed, with minimal collateral damage to the surrounding healthy tissue. PDT also usually spares the surrounding connective tissue, which is very important when the tumour is in a delicate region and the organ structure must be preserved (Allison and Moghissi 2013b).
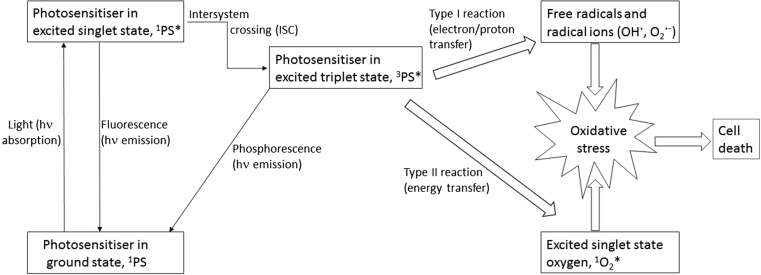
In PDT, illumination brings the PS to the excited triplet state via intersystem crossing (ISC), and this energy is then transferred to the molecular oxygen, generating the singlet oxygen (1O2 *) (Dolmans et al. 2003). The singlet oxygen generation is thought to be the primary mechanism by which PDT damages tumour cells, and is known as the type II process. The electron/energy transfer can also result in the formation of other reactive oxygen species (ROS) such as superoxide anion and hydroxyl radicals, which is known as the type I process (Fig. 1) (Dolmans et al. 2003). It is often difficult to establish how much each of these processes contributes to the overall cell damage, but it is generally accepted that the singlet oxygen is the most important factor in the targeted killing of tumour cells in sufficiently oxygenated tumours, whereas the type I process is more important in hypoxic conditions (Sibata et al. 2000; Ding et al. 2011). In addition to the direct ROS-mediated damage, two additional mechanisms contribute to tumour cell elimination: vascular damage and induction of immune responses. Solid tumours, for instance, are sensitive to all of these PDT-induced anticancer mechanisms, and, moreover, their synergistic effects have been reported (Dolmans et al. 2003).
Fig. 1.
Scheme of the photophysical and photochemical processes involved in PDT
Curiously, although the principle of PDT was discovered more than 100 years ago, after PS-treated microorganisms died upon exposure to sunlight (Ackroyd et al. 2001), only recently has it been developed as a potential antimicrobial therapy. Like cancer cells, pathogens can be eliminated by the direct killing via oxidative stress and there is some evidence that this also enhances the PDT-mediated immune response to certain bacteria (Tanaka et al. 2012). The development of drug resistance is a common problem in many antiviral treatments and chemotheraphies, and antibiotic resistance is an imminent global threat to public health (WHO 2016; Housman et al. 2014), so new treatment modalities for these diseases are widely sought after. Photodynamic antimicrobial chemotherapy (PACT) is a possible remedy as it proved to be effective against methicillin-resistant Staphylococcus aureus (MRSA) and other multi-resistant bacteria (Maisch 2015). Moreover, there is no evidence for the development of resistance to PACT, and such resistance is considered highly improbable, because the oxidative stress in PACT is initiated by a PS that is usually present at multiple cellular sites (Tavares et al. 2010; Maisch 2015). Similarly, PDT attacks tumours by causing oxidative damage to different cellular components, and even though there are some examples of resistance to PDT, evidence of re-sensitisation and synergy in chemoresistant tumour cell lines support the notion that PDT could play an important role in overcoming cancer drug resistance (Spring et al. 2015).
Despite having several advantages over conventional therapies, the use of PDT in cancer is so far limited to superficial or endoscope-accesible tumours, therefore primarily those situated in the skin, esophagous, stomach, bile duct, bladder, and lung (Wang et al. 2001; Allison et al. 2004; van Straten et al. 2017). Moreover, even though numerous studies have demonstrated that PS can be used against essentially all types of microorganisms, PDT is infrequently used as antimicrobial treatment, except in limited scope in dermatology and dentistry (Kharkwal et al. 2011). This is in part caused by still suboptimal selectivity of PS for targeted cells. This problem is thus far being mitigated by topical PS administration, which in turn severely limits the applicability of PDT. Therefore, more effective and selective PS are much sought after. In this review, we argue for the advantages of a specific group of porphyrin-based cationic photosensitisers with amphiphilic properties, and elaborate why their chemical properties could be beneficial in PDT against cancer cells, microorganisms and possibly inflammatory/autoimmune diseases. Emphasis will be given to discussing amphiphilic porphyrin-based compounds as small molecule PS drugs, bearing various numbers of positive charges and lipophilic moieties, intended for passive targeting approaches in PDT. Cationic porphyrins that are used for conjugation to large biomolecules, and their amphiphilic conjugates, will only be mentioned in the context of active targeting in cancer therapy. The directions for the future tailor-made PS will also be discussed.
The development of photosensitisers for clinical use
The fluorescence and the tumour tissue accumulation of haematoporphyrin derivatives (HpD) were described at the beginning of the twentieth century (Mitton and Ackroyd 2008). HpD are obtained by isolating the blood-derived heme, which is the prosthetic group of haemoglobin, myoglobin and cytochromes, and by its acid hydrolysis. Further research and purification of HpD led to the preparation of the first registered photosensitiser, Photofrin®, at the end of the twentieth century. Controversy about the efficacy and safety of the “first generation” PS is ongoing because its exact composition is still unknown (Ackroyd et al. 2001). A slight improvement was made with Photofrin II, which has a higher content of active components (Berg et al. 2005). However, the main drawbacks of all first-generation photosensitisers are: (in addition to) unknown composition, insufficient accumulation in the malignant tissue, and concomitant (prolonged) photosensitivity of healthy tissues (Ackroyd et al. 2001). Furthermore, porphyrins are mostly absorbing light in the region of 400–420 nm (Soret band), with weaker absorptions at higher wavelengths (Q bands). This is inconvenient because the red light can penetrate deeper into the tissue, thus making the PS with stronger red light absorption more valuable in PDT (Stolik et al. 2000). Several improvements came with the advent of the second-generation photosensitisers, which are chemically pure compounds, obtained either by de novo synthesis or by modification of the known natural compounds. Some of them, such as chlorin and bacteriochlorin derivatives, also exhibit an improved absorption in the red-light region (Berg et al. 2005). However, many photosensitisers of the second generation are still not selective enough to reduce or prevent the undesirable side-effects associated with generalised skin photosensitivity (Ackroyd et al. 2001; Josefsen and Boyle 2008).
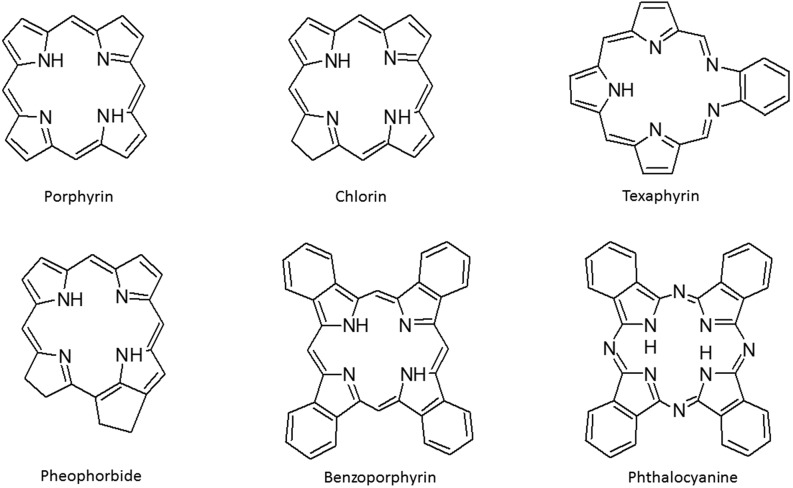
Photosensitisers currently approved for clinical use are mostly porphyrin-related, and, apart from HpD (Photofrin®), are mostly second generation, such as benzoporphyrin derivative monoacid ring A (Visudyne®), meso-tetra(hydroxyphenyl)chlorin (Foscan®), lutetium texaphyrin (Antrin®), mono-L-aspartyl chlorin e6 (LS11), aluminium phthalocyanine (Photosens®), 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a (Photochlor) and 1,5-aminolevulinic acid and its derivatives as precursors of protoporphyrin IX (Levulan®, Metvix®) (Allison et al. 2004; van Straten et al. 2017). A significant number of photosensitisers are currently being tested in numerous clinical trials for the treatment of different types of tumours, age-related macular degeneration and various skin disorders (Wilson and Patterson 2008). Apart from porphyrin-based sensitisers (the basic structures of the most common PSs are presented in Fig. 2), there are some non-porphyrin compounds under investigation in clinical trials, such as hypericin (an anthraquinone), methylene blue (a phenothiazine), rose bengal (a xanthene), curcumin (a curcuminoid) and merocyanine 540 (a cyanine), but these are at the moment less promising, possibly due to a lack of knowledge about their pharmacokinetic properties (Ormond and Freeman 2013).
Fig. 2.
Basic structure of porphyrin and the most common porphyrin-type photosensistisers
The efficacy and safety of a PS could be substantially improved by careful PS design and synthesis. The ideal PS should be highly selective for the target tissue (i.e. tumours in PDT or microbes in PACT), and having fast accumulation and efficient retention in the target tissue, but should be rapidly cleared from the body to avoid systemic toxicity and skin photosensitivity. It also has to be prepared by a reliable and cost-effective synthesis and has to be a stable, pure compound that is not toxic in the absence of light, but is efficient in generating singlet oxygen or other ROS upon red light activation. The generally accepted requirements that one has to take into account at the beginning of a search for a new PS also include easy administration and distribution (Vicente 2001; Pushpan et al. 2002; Ethirajan et al. 2011; Yoon et al. 2013; Mehraban and Freeman 2015). Porphyrin-type compounds (Fig. 2), both synthetic and modified natural products, have almost ideal physical and chemical properties for therapeutic purposes because they are mostly non-toxic without irradiation, but efficiently absorb light in the visible, and some of them even in the near infra-red area, and can generate singlet oxygen. In addition, they have an ability to release fluorescence, which also makes them attractive for diagnostic purposes, and with potential application in theranostics (=diagnostics + targeted therapy) and fluorescence-guided tumour dissection (Allison 2016; Vicente 2001; Josefsen and Boyle 2012).
Most PSs already have so-called passive selectivity for tumours, but their targeting in cancer therapy can be improved into “active targeting” by conjugation to biological macromolecules such as monoclonal antibodies (further discussed below), homing peptides and sugars; these conjugates are known as third-generation PSs (Ethirajan et al. 2011; Josefsen and Boyle 2012). The latest development in PDT research is the possibility of using nanomaterials as PS carriers, and, furthermore, delivering PS together with an imaging agent via nanocarriers for selective and targeted PDT (Wang et al. 2004; Josefsen and Boyle 2012).
Mechanism of PS action in PDT
PDT is thought to exert antitumor effects by three main mechanisms: (1) direct ROS-mediated cell cytotoxicity, (2) vascular destruction, and (3) stimulation of immune responses (Sharma et al. 2012). ROS are highly reactive and exert toxicity mainly by damaging lipids, and to a lesser extent proteins and nucleic acids, thus leading to the disintegration of cell and organelle membranes. Such damage triggers direct apoptotic and/or necrotic tumour cell death. PDT can also induce autophagy, which sometimes also triggers death, but the role of autophagy in PDT is not yet fully understood and it seems ambiguous, promoting both cell survival and death (Reiners et al. 2010; Inguscio et al. 2012). A second important PDT target is the tumour vasculature. Activated PS can directly damage the existing vessels and/or inhibit the formation of new ones (Olivo et al. 2010). The balance between these two executive mechanisms of PDT depends not only on the nature of the PS but also on the choice of the drug light interval (DLI), which is the period between the time of drug administration and the time of irradiation. In the case of direct tumour cell targeting, the DLI is usually much longer than in the case of vascular targeting, to allow PS accumulation in the tumor cells (Chen et al. 2006). The PDT that preferentially targets blood vessels is known as vascular-targeted photodynamic (VTP) therapy. For instance, a second-generation sensitiser, palladium bactereopheophorobide (TOOKAD®), was specifically developed for soluble VTP therapy (Azzouzi et al. 2013). Finally, PDT can also elicit immune responses, which can sometimes be long-lasting and prevent tumour recurrence (Allison and Moghissi 2013a, b). Importantly, by providing this vaccination effect, PDT has a notable advantage over the conventional anticancer therapies in which potentially immunogenic tumour tissue is excized and/or the protective immune responses are suppressed by chemotherapy (Brackett and Gollnick 2011). The choice of PS for a specific application will thus be based on matching its preferential mechanism of action to the tumour type. For example, heavily vasculated tumours might be good targets for PS with the dominant VTP effect. It has been suggested, though, that sometimes the combination strategy which acts by all of these mechanisms is the most effective, as exemplified in bulky solid tumours (Huang et al. 2008).
Targeting considerations
Even when applied systemically, most PSs passively accumulate in tumours. Some of the parameters that positively correlate with such accumulation are: high metabolic activity, tumour neoangiogenesis coupled with vascular leakiness and impaired lymphoid drainage, upregulation of LDL expression, decreased pH, and presence of macrophages (Sharma et al. 2012). However, for some PSs, the dose achieved by passive accumulation is insufficient for therapeutic effect. An attempt to increase the specificity was made with the third-generation PSs, which are bio-conjugated compounds, designed to achieve high-level targeted therapy (Smith et al. 2011). This so-called active targeting is accomplished by conjugating PSs to monoclonal antibodies, tumour-homing and cell-penetrating peptides. Such conjugates can be prepared through synthesis of porphyrins and the related compounds bearing amine-reactive functional groups to ensure stable covalent bonding with biomolecules, and cationic moieties within porphyrins structure can facilitate conjugation by improving their water solubility (Giuntini et al. 2011). Several water-soluble cationic diphenyl- and tetraphenyl-porphyrins with an isothiocyanate group were synthesised and successfully conjugated to a range of monoclonal antibodies (anti-EpCAM, anti-CD146, anti-CD104, anti-CD326). All these bioconjugates had the same binding properties in vitro as the unconjugated antibodies, and they were more phototoxic than porphyrins alone, while in vivo they suppressed growth of a colon cancer model in significantly lower doses compared to Photofrin® (Malatesti et al. 2006; Smith et al. 2011). Furthermore, some of the porphyrins and the same methodology were also used for the successful conjugation to single-chain variable fragment (scFv) antibody fragments, and described in one of the first reports on scFv conjugates for PDT (Staneloudi et al. 2007).
The third-generation PS have been successfully used to increase the vascular targeting in a mouse squamous cell carcinoma and glioma models (Palumbo et al. 2011; Acker et al. 2016). These photoimmunoconjugates were constructed from 5-[4-(succinimide-N-oxycarbonyl)phenyl]-10,15,20-tris-(4-N-methylpyridimiumyl)porphyrin trichloride linked to small immune proteins (SIP), i.e. antibody fragments against fibronectin, a marker of angiogenesis. As such, they selectively localised in the tumour vasculature (Palumbo et al. 2011). However, some of the observed drawbacks of the active targeting by conjugation to monoclonal antibodies were indicated by less successful tumour penetration and activity in vivo (Ethirajan et al. 2011), and loading of PS that can be problematic in two almost opposite ways. The non-covalent bonding between the antibody and the photosensitiser has been shown to compromise binding properties of the antibody, while the covalent binding approach to forming conjugates often results in a minimal loading, which can be impractical for PDT applications (Milgrom 2008).
Remaining challenges in both active and passive targeting intensified the search for nanomaterials, such as liposomes, polymeric, and gold and silica nanoparticles, that may combine both types of targeting, increase passive targeting of PS through the EPR effect or deliver otherwise water-insoluble PS (Hong et al. 2016). An effective nano-PDT system was constructed from mesoporus silica nanocarriers that were loaded with protoporphyrin IX and an imaging agent (FITC), then derivatised with folate on the phospholipid-capped nanocarriers for selective targeting (Teng et al. 2013). Nanomaterials can also be loaded with immunostimulatory agents, together with a PS, to enhance PDT-induced immune responses (Yang et al. 2016). However, systemic toxicity associated with nanomaterials is still an issue that needs to be fully addressed to ensure safe and successful clinical translation (Hong et al. 2016).
Finally, targeting in PDT has to take into account the propensity of PSs for associating to LDL or other serum lipoproteins or proteins that can serve as their delivery vehicles, as well as their final subcellular PS localisation (Allison and Moghissi 2013a). The latter is of considerable importance because the short half-life of PSs ensures that the maximum ROS-mediated damage in PDT is achieved at a precise subcellular localisation (van Straten et al. 2017). Some PSs exhibit preferential localisation in the ER, mitochondria, plasma membrane, lysosomes, or nucleic acids. The latter is especially important in antimicrobial and anticancer PDT (Li et al. 1997; Castano et al. 2004). The physicochemical properties that determine the preferential subcellular location are complex, and are discussed at length in the next section. The evidence so far suggests that targeting mitochondria is favourable for most PDT applications, because such PSs rapidly cause mitochondrial depolarisation and elicit effective photodamage and consequently trigger apoptosis (Morgan and Oseroff 2001). A positive charge in the structure of a PS can be used to target mitochondria, and in comparison to neutral and anionic PSs, cationic forms of the PSs are more readily attracted by mitochondria (Morgan and Oseroff 2001). However, mitochondrial targeting can be challenging, as PSs can redistribute during photoactivation. PS lipophilicity and binding to serum proteins and lipoproteins may also influence intracellular localisation. Overall, the rational PS design could provide more selective targeting, which will perhaps in some cases overcome the need for conjugation to antibodies or other targeting molecules. From a practical point of view, this would be beneficial because passive PSs are cheaper, easier to prepare, administer, and distribute.
Amphiphilic cationic porphyrin-based photosensistisers
Structure–activity relationships
Recently, a quantitative structure–activity relationship (QSAR) model was developed using the multiple linear regression analysis (MLRA) technique to correlate the structural features of 36 poprhyrin-based PSs with their PDT activity, and it showed a strong influence of descriptors such as electrostatic and steric properties on the PDT activity. The model confirmed some of the compounds as active PSs, and was also able to discover some potential PSs, but additionally some compounds that were flagged theoretically as active PSs by this model did not show good PDT activities in experimental conditions, indicating the need for further improvements of the model (Frimayanti et al. 2011). Nevertheless, the development of such models is expected to help the search for new and better PSs, especially in the so-called passive drug approach. As would be expected, there is a good correlation between the generation of 1O2 and PDT efficiency; however, the balance between hydrophilic and hydrophobic characters of the compound and the overall lipophilicity has been shown to have a high influence on PDT activity, but a more precise correlation between all variables and PDT efficiency needs to be established to elucidate the structure of an ideal PS (Frimayanti et al. 2011). Several examples of structure–activity relationships (SAR) of porphyrin-based PSs for PDT have indicated that the most efficient photosensitisers could be those with amphiphilic properties (Graham et al. 2003; Ethirajan et al. 2011).
Types of porphyrin-based cationic amphiphilic PSs
Porphine, a tetrapyrrole core of all porphyrins, is hydrophobic. Amphiphilic porphyrins, synthetic and natural compounds (e,g. heme) have both hydrophilic (polar head) and hydrophobic (tail) moieties. Hydrophilic moieties derive from charged or uncharged polar head groups, and additional hydrophobic moieties most often from hydrocarbon (alkyl) chains (Pisarek et al. 2014). Cationic amphiphilic porphyrin-based PSs obtained completely by synthesis are mostly meso-substituted porphyrins with different symmetry patterns, free or in complex with metal (Caminos et al. 2006; Lazzeri and Durantini 2003). There are somewhat fewer examples of different cationic amphiphilic phthalocyanines, mostly metalated with zinc(II) (Dummin et al. 1997; Kussovski et al. 2009), but also with other metals such as silicon (Allen et al. 2001; Mantareva et al. 2013), then synthetic chlorins (Costa et al. 2012a), bacteriochlorins (Sharma et al. 2013; Yakubovskaya et al. 2014) and isobacteriochlorins (Mesquita et al. 2014). The great advantage of phthalocyanines, chlorins and (iso)bacteriochlorins as PSs is better absorption of red light, which facilitates treatments of deeper infections and deep-seated tumours, but some of the frequent drawbacks often associated with these compounds include a propensity for aggregation and insufficient solubility in water (Mantareva et al. 2013; Sharma et al. 2013). Recently, porphycene, which is a structural isomer of porphyrin, is receiving more attention as a PS for PACT (Ragàs et al. 2010). Cationic amphiphilic PSs can also be prepared by modification of natural porphyrin-related compunds such as the preparation of derivatives of pyropheophorbide-a (Stamati et al. 2010). Interestingly, there are only a few examples of cationic derivatives of chlorin e6 (Ce6), and these were surprisingly insoluble in water despite their hydrophilic moieties (Tarabukina et al. 2015). On the other hand, there are more examples of Ce6 being loaded in cationic liposomes as nanocarriers for both PDT against tumours (Shim et al. 2011) and for PACT against Candida albicans (Yang et al. 2013).
Metalated PSs
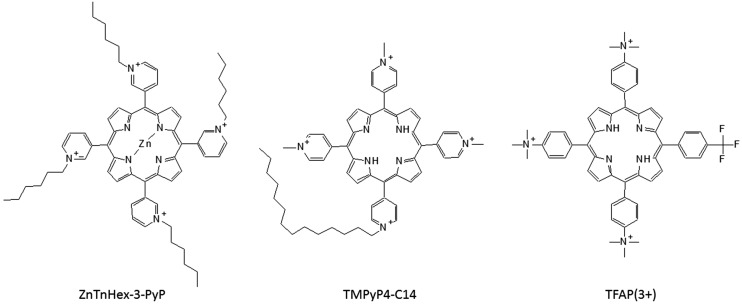
Metal ion is known to change photophysical properties of the porphyrins and related macrocycles, and thus it can be expected to influence photodynamic properties (Lazzeri and Durantini 2003). When preparing new PSs by core metalation, one should keep in mind that generally diamagnetic metals have a long triplet lifetime, while paramagnetic materials exhibit a shorter liftime, and thus higher singlet oxygen quantum yield could be expected with diamagnetic metals. This fact seems mostly relevant for phthalocyanines and texaphyrins that are better PSs when existing as diamagnetic complexes rather than in paramagnetic or free base forms. However, the most efficient porphyrin PSs are metal-free (Josefsen and Boyle 2008). Amongst metalloporphyrins, those with manganese(II) are interesting as contrast agents for magnetic resonance imaging (MRI) (Ali and van Lier 1999). Mn(III) N-alkylpyridylporphyrins are known superoxide dismutase (SOD) mimics, and comparison between the ortho, meta and para isomers, as well as the length of the alkyl chain, confirmed the importance of the lipophilicity on this activity (Kos et al. 2009). Interestingly, the same group of compounds, upon change of the metal centre from Mn(III) to Zn(II), such as zinc (II) meso-tetrakis(N-n-hexylpyridinium-3-yl)porphyrin (ZnTnHex-3-PyP) (Fig. 3) showed potential as PS instead of acting as SOD mimic, and are now being studied in PACT (Alenezi et al. 2017). Even though the most efficient porphyrin PSs are metal-free, possible interactions with metal ions in the organism should be taken into an account. For instance, it has been suggested that all free base PSs can interact with copper, and this may contribute to dark cytotoxicity and phototoxicity of the tumour (Al-Omari 2013).
Fig. 3.
Structures of some cationic amphiphilic porphyrins: zinc(II) meso-tetrakis(N-n-hexylpyridinium-3-yl)porphyrin (ZnTnHex-3-PyP); 5,10,15-tris(N-methyl-4-pyridyl)-20-(N-tetradecyl-4-pyridyl)porphyrin (TMPyP4-C14); 5-(4-trifluoromethylphenyl)-10,15,20-tris(4-trimethylammoniumphenyl)porphyrin (TFAP(3+))
Synthesis of amphiphilic PSs
The synthesis of the most common cationic amphiphilic porphyrins was reviewed recently by Pisarek et al. (2014). The largest fraction of cationic amphiphilic porphyrins are 2-, 3- or 4-pyridinium salts, obtained by alkylation of 2-, 3- or 4-pyridylporphyrins with alkyl halides of different chain lengths. Depending on the number of the pyridyl groups on the porphyrin, the number of cations can range from 1 to 4, and there are two isomers of the porphyrin with two pyridinium groups (cis or AABB and trans or ABAB). Lipophilicity of all these compounds can easily be increased by increasing the number of carbons in the alkyl chains and/or decreasing the number of cations. Notably, monocationic porphyrins are usually not water-soluble, while cis isomers are less hydrophobic than trans isomers and considered better amphiphiles, thanks to the well-defined head/tail structure (Pisarek et al. 2014). There are also ammonium and phosphonium salts as cationic amphiphiles, but phosphonium groups are more lipophilic and were considered less favourable for PS structure due to self-aggregation because of the interactions of highly polarisable phosphonium cation with the highly polarisable porphyrin π-surface (Jin et al. 1997). However, the triphenylphosphonium group in the structure of a PS has recently been getting more attention as delocalised lipophilic cations (DLC), indicated for selective cancer mitochondria targeting (Modica-Napolitano and Aprille 2001). Apart the introduction of a long(er) alkyl chain, lipophilicity of a molecule can be increased with a trifluoromethyl group (Böhm et al. 2004), and a series of cationic amphiphilic porphyrins with one or more CF3 groups, such as 5-(4-trifluorophenyl)-10,15,20-tris(4-trimethylammoniumphenyl) porphyrin (TFAP(3+)) (Fig. 3) have been prepared, mostly for PACT applications (Lazzeri and Durantini 2003; Caminos et al. 2006).
PS distribution
The overall lipophilicity and charge determine pharmacokinetic and pharmacodynamic PS properties, and are important for drug distribution and the intracellular localisation. Therefore, amphiphilic structures are attractive for PDT because the hydrophilic part facilitates drug administration by making it water-soluble, while the hydrophobic part facilitates cell entry and accumulation (Ethirajan et al. 2011). The interaction of PS with serum proteins also impacts its distribution, and several groups have investigated the possibility of using serum proteins as PS carriers. Hydrophobic PSs are more likely to bind to the low-density lipoproteins (LDL), while more hydrophilic ones bind albumin fractions (Bonneau and Vever-Bizet 2008). For example, it was found that bacteriochlorophyll derivatives with improved amphiphilic properties had an increased affinity to serum albumin, and this was shown to be beneficial for the vascular targeted PDT (Brandis et al. 2005). Asymmetric and amphiphilic PSs can be associated with both albumin and high-density lipoproteins (HDL) (Bonneau and Vever-Bizet 2008). Cationic tetrapyridinium porphyrin makes a stable complex with the serum albumin, which then displays intensive fluorescence (Lebedeva et al. 2013). The tumour environment is known to be acidic, and it can shift PSs preference from albumin to LDL, which, in turn, is connected to better tumour retention (Bonneau and Vever-Bizet 2008). This mechanism is not yet completely understood, thus new investigations are needed to achieve more effective PDT by finding an optimal combination of the structure of the PS and the irradiation time, as well as the dose of the applied light, since all these factors are interconnected in a complex manner (Bonneau and Vever-Bizet 2008).
The relevance of positive charge(s) on targeting and solubility
Cationic PSs can efficiently target Gram(−) bacteria, even without pre-treatment with a metal chelator such as ethylenediaminetetraacetic acid (EDTA), which otherwise has to be applied when anionic PSs are used in order to remove divalent cations and to enhance the permeability of the outer wall (Jori et al. 2006; Gsponer et al. 2015). This ability of cationic PSs stems from electrostatic interactions with the negatively charged cell envelope (Liu et al. 2015), and it has been suggested that, with a higher number of positive charges, but also adequate length of alkyl chains, such compounds should be a good choice for clinical PDT of microbial infections caused by Gram(+) and Gram(−) bacteria, yeasts, fungi, mycoplasmas and pathogenic protozoa (Jori et al. 2006). The PDT activity of cationic porphyrin derivatives with one, two and three positive charges, deriving from the same number of 4-(trimethylammonium)phenyl groups on the periphery of the macrocycle, were studied against Escherichia coli. The SAR for photoinactivation of E. coli indicated the porphyrin with the highest number of charges as the most promising PS (Spesia et al. 2005). On the other hand, a high number of cationic charges is characteristic of the water-soluble zinc phthalocyanine with 16 quaternised imidazolyl moieties on the periphery, and this PS was presented with low dark toxicity to non-cancerous cells and high anticancer PDT (Makhseed et al. 2013). Important to note, the high number of positive charges was introduced to enhance water solubility and avoid aggregation typical for phthalocyanines and other PSs that are too lipophilic. It is well known that self-aggregation of a PS is generally a drawback in PDT since it reduces the 1O2 formation (DeRosa and Crutchley 2002). Self-aggregation tendencies of cationic amphiphilic porphyrins have been extensively studied, and it seems that the most important factors are electronic and structural effects that influence self-aggregation in a complex manner (Kano et al. 2000). The length of the alkyl chain has a strong impact on aggregation, and pyridinium porphyrins with longer alkyl chains (C8, C12 and C18) have been shown to form micelles (Dancil et al. 1997). Nevertheless, aggregation is generally not so problematic for cationic amphiphilic porphyrins because they usually start to aggregate in concentrations higher than 10−5 M, while a good PS is expected to be PDT-efficient in concentrations < 10−6 M (Malatesti et al. 2016; Simões et al. 2016).
Lipophilicity and cellular uptake
The octanol–water partitioning coefficient, logP, is a parameter for hydrophobicity and has proved to be useful for the prediction of the ability of a PS to enter into biomembranes or bind to liposomes, and to study cellular uptake of PSs (Ben-Dror et al. 2006). Lipophilicity was shown to be important for better cellular uptake and consequently stronger anticancer activity in PDT (Rapozzi et al. 2014), as well as microbial photoinactivation in PACT (Mantareva et al. 2013). The amphiphilic and completely water-soluble porphyrin with tri-cationic pyridinium-3-yl groups and a very lipophilic 4-octadecanamidophenyl group at the fourth meso-position was used in PDT on HeLa cell line, and it exhibited significantly higher phototoxicity than the hydrophilic counterpart bearing a 4-acetamidophenyl group instead of the one with a long alkyl chain. These two cationic porphyrins had almost identical, photophysical properties, but different logP and hydrophilic–lipophilic balance (HLB), and subsequently very different PDT activity, thus confirming the importance of the lipophilicity on the cellular uptake and overall PDT efficiency (Malatesti et al. 2016). Four cationic Zn(II) phthalocyanines with different lengths of the alkyl side-chain ranging from (methyl)-pyridyloxy to (dodecyl)-pyridyloxy were used for the photoinactivation of Aeromonas hydrophila, and the study showed that both the accumulation and the PDT efficacy increases with the length of the alkyl chain (Kussovski et al. 2009). Metallophthalocyanines with different charges, hydrophobicity (four or eight N-alkylpyridyloxy groups) and metal ions [Al(III), Ga(III), In(III), Si(IV) and Ge(IV)] were investigated in PACT, and those cationic PSs were shown to be more promising than anionic ones against all the studied pathogenic bacteria and fungi, including fungal biofilms. The cationic metallophthalocyanines with longer alkyl chains also showed better uptake in the studied microbial cells (Mantareva et al. 2013). Similarly, the relevance of logP and phototoxicities of 16 different meso-porphyrins were investigated on HaCaT keratinocytes. In comparison, among anionic, neutral with polar groups and neutral hydrophobic porphyrins, only cationic meso-(1-methyl-4-pyridinium)porphyrins were shown to be PDT-efficient and were proposed to be used as PSs for psoriasis treatment. Amongst the cationic PSs, the most efficient were those with two and more charges, especially the dicationic cis-isomer, and those with methylpyridinium groups directly attached to the meso carbon of the porphyrin ring (Slomp et al. 2017). Finally, two new derivatives of pyropheophorbide-a (PPa) have been synthesised and their PDT activity on SKOV3 ovarian cancer cells studied. The results showed increased cellular uptake of both new derivatives in comparison to PPa, but different localisation, and it was suggested that hydrophobic and hydrophilic groups are the most effective when they are on opposite sides (Stamati et al. 2010).
Hydrophobic PSs need to be loaded on nanocarriers, such as liposomes, for their delivery to the targeted cells, but a by-product of this mode of delivery is that they also seem to target the plasma membrane which results in necrosis, which is a less desirable mode of cell death for some PDT applications. Neutral lipophilic porphyrins with long alkyl chains (C16) were conjugated to cationic liposomes, used as carriers to enable the PSs to enter the studied cancer cells [Me45, Hct116 and B16(F10)] by endocytosis. After 4 and 12 h, PSs were localised in the plasma membrane and in peripheral regions of the cytoplasm, and more necrotic cells in comparison to apoptotic were observed after longer time periods, and especially when a higher light dose was used (Kramer-Marek et al. 2006). Conversely, targeting mitochondria in PDT is preferable to targeting plasma membrane and lysosomes, because it was shown to induce apoptosis rapidly, both in vivo and in vitro (Morgan and Oseroff 2001; Almeida et al. 2004; Plaetzer et al. 2005; Bonneau and Vever-Bizet 2008).
Mitochondrial targeting
Tumour cells have different mitochondria than healthy cells, and their role is very important in cell survival and death. The mitochondrial internal membrane is negatively charged, and cationic PSs are more likely to be attracted to mitochondria through electrostatic interactions than neutral and anionic PSs (Morgan and Oseroff 2001). Four cationic meso-tetraphenylporphyrins, two with triphenylphosphonium and two with triethylammonium ion at the end of the alkoxy chain, in either the meta or para position of one meso-phenyl group, were all proved by confocal fluorescence microscopy to target mitochondria in a human breast cancer cell line (MCF-7 cells), despite the difference in lipophilicity (Lei et al. 2010). Cationic phthalocyanine, 2,9,16,23-tetrakis (hexyldimethylammonio)zinc(II)phthalocyanine tetramethylsulphate (ZnPcA6), was shown to be accumulated in the inner mitochondrial membrane of HeLa cells. Furthermore, this cationic lipophilic ZnPcA6 was more efficient than the analogue with shorter alkyl side-chains (ZnPcA1), the ether analogue (ZnPcE6) and the anionic phthalocyanine (ZnPcS) (Dummin et al. 1997). More specifically, cytochrome C oxidase (COX) in mitochondria of living HeLa cells was identified as the binding site for the cationic lipophilic Pt(II)-tetramethylpyridylporphyrin as PS (Börsch 2010. Yet another example describes conjugation of a core-modified porphyrin (CMP-OH) to delocalised lipophilic cations (DLC), claimed for targeting mitochondria, rhodamine B and one or two triphenyl phosphonium cations (tPP), to obtain three DLC-porphyrin conjugates, CMP-Rh, CMP-(tPP) and CMP-(tPP)(2), respectively. All three cationic conjugates were significantly more PDT-efficient in mouse colon cancer cells (Colon 26), because of improved cellular uptake in comparison to unconjugated CMP-OH, but, based on fluorescence microscopy, selective mitochondria-targeting in human breast cancer cells (MCF-7) has been suggested only for CMP-Rh. A conjugate with two tPP groups was somewhat less efficient in targeting mitochondria, and the conjugate with only one tPP group was not evident in mitochondria, but both conjugates were more phototoxic than CMP-Rh, especially CMP-(tPP) which showed the highest cellular uptake (Rajaputra et al. 2012). Surprisingly, mitochondrial localisation could not be proven for a series of derivatives of 5,10,15,20-tetrakis-(4-N-methylpyridyl)-porphine (TMPyP4), with alkyl chains of various lengths instead of a methyl group, despite bearing positive charges. Instead, they were shown to localise in lysosomes of human HT1080 fibrosarcoma cells, and there was no evidence of intracellular redistribution of the PS during PDT which probably resulted in necrosis. Lipophilicity was named in this case as a main factor that influenced the cellular uptake (by endocytosis), distribution and localisation (in lysosomes), and resulted in higher PDT efficiency of the PSs with longer alkyl chains (Ricchelli et al. 2005).
The number and position of charges vs. HLB in targeting, cellular uptake and localisation
Clearly, the mere presence of a positive charge in the structure of a PS does not confer mitochondrial targeting, and several studies have shown that both the number and position of charges could be relevant for intracellular localisation and subsequent PDT efficiency of a PS. Five cationic amphiphilic porphyrins with different numbers and positions of charges were prepared by changing the numbers and positions of the 1-methylpyridinium-4-yl (cationic polar head) and pentafluorophenyl (hydrophobic) groups, and their ability for photoinactivation of E. coli was studied. Monocationic porphyrin was not sufficiently water-soluble and consequently not promising as a PS, while the dicationic cis isomer was a better PS than the trans isomer, and more lipophilic tricationic porphyrin was shown to be a better PS than hydrophilic porphyrin with four positive charges (Simões et al. 2016). It has been previously suggested that cationic PSs with symmetrically positioned charges are more likely to be taken into cells through endocytosis and to target lysosomes, while those with asymmetrical charges are more likely to penetrate the plasma membrane by diffusion and to target mitochondria (Kessel et al. 2003). A more systematic study of the binding of cationic meso-porphyrins to cellular membranes has been reported (Engelman et al. 2007). Investigation of interactions of 3- and 4-N-methylpyridinium porphyrins, having one to four positive charges, with liposomes, mitochondria and erythrocytes, revealed that the binding to liposomes or mitochondria is inversely proportional to the polarity, and thus it can be predicted by the logP values of the PSs, apart from the case of the amphiphilic dicationic cis-isomers that were presented with the highest binding constants. Dicationic cis-isomers, even though they have lower logP values than trans-isomers, were suggested to have a more optimal structure, to penetrate deeper into membranes than all the other porphyrins that seem to bind the membranes more superficially. The electrostatic effect derived from the mitochondrial transmembrane potential was estimated to contribute to up to 15% of the binding constants (Engelman et al. 2007). Furthermore, molecular dynamics simulations that were employed to model binding between a series of cationic meso-(N-methyl-4-pyridinium)phenylporphyrins and phospholipid membranes supported such experimental evidence, and confirmed the importance of the number and position of the charges as well as hydrophobicity–hydrophilicity balance, and their effect on the cellular uptake, intracellular localisation and PDT efficiency of a PS (Cordeiro et al. 2012). In a similar study, hydrophobic and electrostatic interactions were found to be responsible for the association of a series of meso-substituted cationic porphyrins with liposomes. More hydrophilic tri(N-methyl-4-pyridyl)monophenylporphine chloride and tetra(N-methyl-4-pyridyl)porphine chloride were shown to be located closer to the polar heads of liposomes and had higher quantum yields of 1O2, while more hydrophobic mono(N-methyl-4-pyridyl)triphenylporphine chloride and cis(N-methyl-4-pyridyl)diphenylporphine chloride were indicated in the lipidic phase and had lower quantum yields of 1O2. This study pointed out that too high affinity of the porphyrin for the liposomal nanocarrier can be as problematic as too low, because it can hamper the release of the PS (Angeli et al. 2008). On the other hand, less efficient binding of a PS to cellular membranes could be an advantage in applications such as the treatment of blood infections where PS should have low affinity for blood cells and mich higher affinity for pathogen microbial cells (Engelman et al. 2007).
Finally, although not belonging strictly in this group of compounds, but worth mentioning, are porphysomes, liposome-like structures formed by self-assembly of the porphyrin-lipids. Porphyrin-lipid can be prepared by conjugation of a porphyrin molecule with one or more phospholipids, and this important and recent discovery is paving the way for many biological, diagnostic and therapeutic applications, including the preparation of activatable PSs for PDT (Huynh and Zheng 2014).
Photodynamic antimicrobial activity
The progress of antimicrobial PDT has been considerably slower than that for anticancer PDT, a situation which can at least in part be attributed to the discovery of highly effective antimicrobial compounds, such as antibiotics (penicillin, tetracycline, etc.) and antivirals (nucleoside analogs, etc.) (Wainwright et al. 2017). However, although very safe and effective, the use of target-specific drugs (e.g. specifically against cell-wall, virus polymerase or protease) has inevitably led to the emergence of resistant clones, thus becoming a global threat (Bacon et al. 2003; Coen and Whitley 2011; Cortez et al. 2011; Memoli et al. 2011). Due to ROS-mediated targeting of microbial membranes, membrane proteins, DNA, etc., of various pathogens, PACT represents a broad-spectrum multitargeted approach with very limited, if any, chances for the development of resistant bacteria or viruses (Jori et al. 2006, Tavares et al. 2010; Costa et al. 2012b; Dosselli et al. 2012; Maisch 2015). PACT has been applied successfully to inactivate bacteria, fungi, protozoans and parasites, especially in topical applications such as skin wounds (burns and diabetic foot ulcers) or oral infections (periodontitis and root canal infections) (Tim 2015). The success of antibacterial PDT depends upon more rapid PS uptake in bacteria in comparison to host cells (Wainwright et al. 2017). Another advantage of PDT over antibiotics in antibacterial treatment is that PDT kills faster, and can target bacteria in biofilms and bacterial spores (Jori et al. 2006). There are recent extensive reviews on antibacterial PDT (Sperandio et al. 2013; Chang et al. 2016; Hamblin 2016; Liang et al. 2016; Rodrigues et al. 2016a), and thus here we will focus on the equally relevant, but less developed, area of antiviral PDT. We will particularly emphasise the molecular mechanisms of inhibition and focus on the antiviral use of porphyrin-based cationic amphiphilic PSs.
From the initial observation that methylene blue in the presence of light irreversibly inhibits T4 bacteriophage (Perdrau and Todd 1933), through to the pioneering work of Wallis and Melnick on a number of different viruses and photoactive compounds, paved the road for the development of modern PACT (Wallis and Melnick 1963; Wallis et al. 1963; Wallis et al. 1967; Gerba 1977; Melnick and Wallis 1977), antiviral application of PACT has improved immensely due to innovations of efficient PSs, especially cationic amphiphilic photosensitizers. Currently, most efforts in the area of antiviral PACT focus on three areas: (1) Treatment of human papilloma virus (HPV)-induced lesions and warts (e.g. respiratory papillomatosis). However, although the etiology of the HPV lesions is linked to the virus, to our knowledge the PACT application has not been studied in the context of virus infection per se, but rather as a treatment of a virus-induced tumor, aiming to induce cell death and trigger immune responses in the affected area (reviewed in Lieder et al. 2014; Stern et al. 2012; Tao et al. 2014); (2) Inhibition of viruses in blood and blood products. The transmission of bloodborne pathogens by transfusion, despite thorough screening, remains a serious problem; and it is particularly important in light of emerging infections (e.g. ZIKA and West Nile viruses) and in underdeveloped countries that lack screening or established inactivation procedures (Bihl et al. 2007); (3) Treatment of active herpes simplex virus 1 (HSV-1) and 2 (HSV-2) infections (e.g. herpes keratitis, herpes labialis, genital herpes).
Molecular targets of antiviral PACT
Viruses are the most diverse biological entity, and light-activated compounds found to be effective for some viruses might be ineffective for others. Current understanding of the exact mechanisms of inactivation is rather limited, and in most studies novel compounds have not been thoroughly investigated, or are limited to the assessment of cytotoxicity and the reduction in virus yield. Most studies have found a higher susceptibility of enveloped than non-enveloped viruses to PACT, suggesting that the virus envelope is the major target for PSs (Horowitz et al. 1991; North et al. 1992; Smetana et al. 1994; Ke et al. 2014; reviewed in Costa et al. 2012b). Using electron microscopy, a massive destruction of virus envelopes was detected after treatment with phthalocyanines containing metal ligands, leading to a significant decrease of virus infectivity of several enveloped viruses [Varicella zoster virus (VZV), HSV-1 and HSV-2], whereas non-enveloped adenovirus was largely resistant to the treatment (Smetana et al. 1994). Nevertheless, several studies have presented evidence that non-enveloped viruses are also sensitive to direct PACT inactivation (Kasturi and Platz 1992; Gaspard et al. 1995; Costa et al. 2008, 2010; Zupan et al. 2008), which might be explained by photoactivated protein crosslinking that leads to direct damage or loss of proteins, and finally the loss of infectivity (Lenard et al. 1993; Smetana et al. 1997; Orosz et al. 2013). At physiological conditions, non-enveloped viruses have negatively charged capsids and enveloped viruses harbour negatively charged glycoproteins on their surface, and are thus likely directly targeted by cationic porphyrins via electrostatic interactions, like previously described for Gram(−) bacteria (Karlin and Brendel 1988; Michen and Graule 2010; Liu et al. 2015). Nucleic acids can also be directly targeted by both cationic porphyrins and phthalocyanines, and PDT can induce photocleavage of both RNA and DNA viruses (Abe and Wagner 1995; Moor et al. 1997; Mettath et al. 1999; Zupan et al. 2005; Lopez Zeballos et al. 2014). Nonetheless, to what extent such intercalation and cleavage contributes to virus inhibition in comparison to other possible mechanisms is not clear (Zupan et al. 2005). Moreover, in addition to direct effect of PACT on virus structure and/or genetic material, intrinsic antiviral cellular mechanisms triggered by PACT are largely unknown (Gaspard et al. 1995).
Antiviral PACT and phthalocyanines
Phthalocyanines have been extensively studied as potent antiviral agents, in particular for inactivation of viruses in blood and blood-based products (serum, plasma, platelets, etc.) (Horowitz et al. 1991; Rywkin et al. 1992, 1994, 1995; Gaspard et al. 1995; Remichkova et al. 2016; Rodrigues et al. 2016b). In most of these studies, a significant decrease in virus infectivity was observed with a variety of different phthalocyanine derivatives. However, the underlying molecular mechanisms are largely unknown. For example, the early work by Horowitz et al. using aluminum phthalocyanine derivatives (AIPc; AIPc-chloride, AIPc-disulfonate, AIPc-tetrasulfonate) showed an efficient inactivation of both cell-associated and cell-free enveloped vesicular stomatitis virus (VSV) and human immunodeficiency virus 1 (HIV-1), but not non-enveloped encephalomyocarditis virus (EMCV) in whole blood, with minimal damage to red blood cells (RBC) (Horowitz et al. 1991; Smetana et al. 1994; Ke et al. 2014). Whereas this study suggested that the virus envelope was the major target for inactivation, Reminchkova et al. observed equally efficient inactivation of cell-free enveloped HSV-1 and vaccinia virus (VV) with cationic and anionic phthalocyanine Zn(II) complexes-PDT, but found rather striking differences between these two compounds on the enveloped bovine viral diarrhea virus (BVBV) (Remichkova et al. 2016). Both compounds were largely ineffective in inhibiting enveloped Newcastle disease virus (NDV), and non-enveloped Coxsakie virus B1 and human adenovirus type 5 (ADV5). One could argue that, although it has been generally accepted that efficacy of PACT depends on a metal ligand (Ben-hur et al. 1992; Horowitz et al. 1991), at this point it is not possible to predict its activity based solely on the structure of a compound and the structure of a virus (enveloped/non-enveloped). The molecular basis for these differences is not known. Regarding the molecular action of phthalocyanines on target molecules, it is important to mention that addition of vitamin E or mannitol, antioxidants and ROS scavengers for oxygen species generated by type I mechanism can prevent damage to RBC, but the inactivation of viruses will not be affected (Rywkin et al. 1992, 1994). On the contrary, quenchers of singlet oxygen, such as L-histidine or sodium azide, protect from the PDT inactivation in several studies (reviewed in Costa et al. 2012b). Taken together, these results indicate that free radicals have very little effect on virus inhibition and suggest the importance of singlet oxygen in virus inactivation by phthalocyanine derivatives. However, a lower and variable rate of the photoinactivation of non-enveloped T7 bacteriophages (convenient models to study important human pathogens like hepatitis A virus, HAV) and HSV-1 virus in the presence of ROS scavengers also have been observed (O’Brien et al. 1992; Egyeki et al. 2003).
Antiviral PACT activity on cell-associated virus and virus replication have been observed in many studies, a finding that, in part, can be attributed to direct inactivation of viruses within the cell, but also to an inhibition of viruses by intrinsic antiviral mechanisms triggered by PACT. For instance, in addition to direct activity on cell-free virus, prior treatment of cells with phthalocyanines, at concentrations that do not provoke significant cytotoxicity, will generate non-permissive conditions for virus replication (Horowitz et al. 1991; Smetana et al. 1994, 1998). Moreover, interestingly, cells infected with HSV-1, HSV-2 and VZV are gradually resistant to delayed treatment with cationic and amphiphilic phthalocyanines (up to 30–60 min after the addition of a virus), whereas amphiphilic dyes have prolonged activity (up to 3 h) probably due to their ability to penetrate cell membranes more readily (Smetana et al. 1994). The antiviral activity can be explained by photoactivated damage to endosomes early in the infection, along with damage to envelope proteins (Smetana et al. 1998). On the other hand, cells treated prior to infection with different phthalocyanine derivatives, including CuPcS4, ZnPc(3-OPy)4 and ZnPc(3-MeO-Py)4, were completely refractory to non-enveloped Rhinovirus type 5(RV5) at non-toxic concentrations, and at the same time the compound was ineffective against a cell-free virus, which might indicate an undetermined intrinsic antiviral mechanism (Gaspard et al. 1995). Obviously, more thorough analysis of cellular proteome and virus gene expression is needed to address and reveal the exact molecular mechanisms responsible for such inhibition.
Antiviral PACT and porphyrin-based cationic amphiphilic PSs
Porphyrin-based cationic amphiphilic PS are promising but poorly explored antiviral agents. The first report of antiviral PACT using cationic meso-tetrakis (N-methyl-4-pyridiniumyl) porphyrin (TMPyP4) and anionic tetrakis (4-sulfonatophenil) porphyrin (TPPS4) showed an efficient inactivation of non-enveloped MS2 bacteriophage and HAV in PBS solution and plasma. Importantly, none of these compounds were mutagenic, and the activity of cationic compound was much better with regards to the time of light exposure required to achieve significant virus inactivation, and also its activity in the absence light (Casteel et al. 2004). The efficiency of TMPyP4 encouraged efforts to develop more effective cationic amphiphilic PS with increased lipophilicity, such as amphiphilic meso-alkylated tetrakis (N-(n-octly)-4-pyridiniumyl) porphyrin (TOcPyP4) and tetrakis (N-(n-butyl)-4-pyridiniumyl) porphyrin (TBuPyP4) and tetrakis (N-(n-hexadecyl)-4-pyridiniumyl) porphyrin (THdPyP4), which showed similar activity against HAV and MS2. Photodynamic activity of such PS was, to a certain degree, less efficient in plasma than PBS, which can be explained by affinity to albumin and other plasma proteins that increases with an increase in hydrophobicity of the porphyrin side chain (Casteel et al. 2004). Similar to the case for pthalocyanines, the molecular mechanism by which cationic porphyrines inhibit viruses has not received sufficient attention. Their main mechanism appears to be through singlet oxygen (Costa et al. 2013), and their antiviral efficacy strongly depends on the charge and the substituents in the meso-position (Costa et al. 2008). Silva et al. have tested several cationic β-vinyl substituted meso-tetraphenolyporphyrins at non-cytotoxic concentrations and efficiently inhibited cell-free HSV-1 (>99%), but, interestingly, the authors noted that compounds with strikingly similar chemical structures showed dramatically different inhibitory activity on HSV-1 (Silva et al. 2005). Another recent study showed that neutral glycoporphyrins having unprotected hydoxy groups and galactopyranoside can effectively inhibit infectivity of HSV-1 and HSV-2, which was even comparable to acyclovir and foscarnet at non-toxic cell concentrations (Tome et al. 2005, 2007). Surprisingly, in contrast to phtalocyanines which were active only if infected cells were treated early during the infection (Smetana et al. 1994), inhibition of intercellular virus was possible even 16 h after infection (Tome et al. 2005, 2007). Taken together, the presented studies clearly demonstrate a vast potential of PS in antiviral therapies. However, to evaluate any novel PS, it is imperative to compare it to the compounds that are already in use, such as acyclovir, foscarnet and Visudyne, and Foscan.
PDT and the immune system
PDT-mediated immune responses in cancer
Activation of the immune system is an important effector arm of PDT. Ample evidence shows that both the innate and adaptive immune systems participate in PDT-induced tumour elimination (Nowis et al. 2005; Castano et al., 2006). The immune response is activated by PDT-induced tumour cell death or damage, which triggers the release of various damage-associated molecular patterns (DAMPs) including heat shock protein 70 (HSP70), calreticulin (CRT), high-mobility group box 1 (HMDB1), and ATP (Korbelik 2006; Maeding et al. 2016; Tanaka et al. 2016). A particularly immunogenic type of cell death is necrosis, where a lot of DAMPs are released, but apoptotic and stressed cells contribute as well, especially if the extent of damage is large and/or rapidly peaks upon PDT application (Korbelik 2006). DAMPs activate the complement system and the resident innate immune cells such as macrophages, dendritic cells and mastocytes, which in turn orchestrate acute inflammation and recruit other innate immune subsets such as neutrophils and natural killer (NK) cells (Cecic et al. 2001; Korbelik 2006; Kousis et al. 2007). Several processes that follow promote tumour cell death; these include the activation of macrophages and neutrophils to promote apoptosis and/or necrosis by release of proinflammatory cytokines (such as IL-1β, TNF and IL-6), the complement system-induced damage to endothelial cells, and direct recognition and elimination of tumour cells by NK cells. Such PDT-induced inflammation can have a vaccination effect, i.e. it can elicit adaptive immune responses because activated dendritic cells increase the uptake and presentation of tumour antigens to T cells. The latter was perhaps most impressively demonstrated in experiments in which local PDT triggered the development of specific anti-tumoural CD8 effector and memory T cells that not only prevented local tumour recurrence but also eliminated distant metastases (Kabingu et al. 2007; Kleinovink et al. 2016). Notably, mice with severe combined immunodeficiency, lacking T and B cells, were incapable of eliminating metastases, and this was reversed by the adoptive transfer of CD8, but not CD4, T cells, suggesting that CD8 T cells are indispensable for PDT-triggered long-lasting anti-tumoural immunity. PDT also increases the expression of MHC-related stress molecules on tumour cells, thus making them the direct targets of NK-mediated cell cytotoxicity (Park et al. 2011; Belicha-Villanueva et al. 2012). In addition, PDT was also reported to eliminate suppressor CD4 T cells, which are often found in tumours, and associated with poor prognosis (Castano et al. 2006). Therefore, PDT can simultaneously stimulate various anti-tumour immune mechanisms, such as acute inflammation, antigen uptake and presentation, generation of CD8 effector and memory T cells, suppressor CD4 T cell elimination, and NK activation to exert a powerful vaccination effect and provide long-term tumour remission.
The immune responses to porphyrin-based cationic amphiphilic PS have thus far not been tested in animal cancer models. However, several reports have shown their beneficial features in vitro: low dark toxicity, better selectivity for malignant cells, and higher efficiency compared to their hydrophilic PS analogues (Tovmasyan et al. 2014; Malatesti et al. 2016). They are known to have favourable pharmacokinetic properties (Henderson et al. 1997), and were found to be promising in in vitro and in vivo cancer models (Faudale et al. 2012; Rapozzi et al. 2014). Importantly, because of their efficient accumulation in mitochondria, they are expected to trigger immunogenic death. Of note is the fact that they are not significantly accumulated in the nucleus and hence do not cause extensive DNA damage (Rosenkranz et al. 2000; Tovmasyan et al. 2014), and are therefore not expected to be mutagenic themselves, which is an important feature for anticancer agents.
PDT-mediated immune responses in non-malignant applications
In contrast to the well-described beneficial effects of the immune system activation in anticancer PDT, the immune responses in non-malignant applications are less understood. In fact, both immunosupressive and immunostimulatory effects of PDT have been reported (Nowis et al. 2005; Mroz and Hamblin 2011; Kim et al. 2015). In theory, the same vaccination effect described for anticancer PDT should facilitate adaptive immune responses to microbes, but, to the best of our knowledge, this has not yet been experimentally addressed. Nevertheless, in a murine MRSA-mediated arthritis model, Photofrin-mediated PDT stimulated strong neutrophil infiltration, which positively correlated with bacterial killing (Tanaka et al. 2012). Neutrophil infiltration could also be triggered by pretreatment, suggesting that the proinflammatory effect of PDT can be separated from its direct effect on microbial killing. Interestingly, PS treatment during the infection optimally worked only within a narrow therapeutic window, with neutrophil infiltration being suppressed at higher doses. The latter example very vividly depicts the dual role that PDT exerts on the immune system: it can either be stimulatory or immunosuppressive (Nowis et al. 2005; Mroz and Hamblin 2011; Kim et al. 2015). The doses required for immunosuppression are lower than those in anticancer PDT, and the immunosuppressive mechanisms are diverse, and include direct toxicity toward immune cells, and mitigation of their activation (Ratkay et al. 2000). Direct toxicity is similar to that in cancer cells, because activated immune cells are metabolically extremely active, and thus preferentially accumulate PSs, and undergo apoptosis (Ratkay et al. 2000; Jiang et al. 2002). For example, compared to resting T cells, activated T cells incorporated several-fold more silicon phthalocyanine (Pc) 4, especially in the mitochondria, and were more sensitive to PDT-induced apoptosis (Soler et al. 2016). Indirect mechanisms are in place too. For example, Ce6-PDT had a strong anti-inflamatory effect during in vitro and in vivo infection models with bacteria found in acne lesions (Propionibacterium acnes), primarily by suppressing proinflamatory NF-kB and MAPK signaling pathways (Wang et al. 2017). In this case at least, no obvious direct antimicrobial effect was observed, since responses to live and heat-killed bacteria were suppressed to the same extent. Similarly, an immunosuppressive effect of a topically applied cationic PS, 5,10-diphenyl-15,20- di(N-methylpyridinium-4-yl)porphyrin was recently reported in a mouse psoriasis model (Carrenho et al. 2015). Such PDT diminished several inflammatory indicators, including oedema, proinflammatory cytokine secretion and neutrophil infiltration, and led to reduced keratinocyte proliferation. Therefore, in psoriasis, as well as in various other chronic inflammatory diseases, PDT exerts direct immunosuppressive effects (reviewed in Mroz and Hamblin 2011; Reinhard et al. 2015). It must be emphasized that a recent meta-analysis on psoriasis showed lower efficiency of ALA-PDT compared to the classical phototherapy (Almutawa et al. 2015). It remains to be seen if this is an ALA-specific effect and that more optimized PS, such as perhaps the aforementioned cationic PS, would be more effective.
In summary, the prerequisites for efficient adaptive immune responses are immunogenic tumors and sufficient damage to induce adaptive response. Lesser damage, as well as a direct PDT effect on the immune cells, can sometimes lead to tolerance and/or immunosuppression. For anticancer PDT, this means that combination therapies, designed for eliciting stronger immune responses, will be more effective in tumour elimination.
Combination strategies in PDT
Some PS and/or PDT protocols do not cause (efficient) immunogenic tumour cell death, and, moreover, malignant tumours are notorious for escaping immune response (Schreiber et al. 2011), thus limiting the success of PDT. For this reason, several combination therapies aimed at increasing tumour immunogenicity and/or boosting the ongoing immune responses are being tested. For example, immunisation with peptides from tumour antigens improved survival after Bremachlorin-PDT of aggressive mouse cancer models, and led to eradication of distal metastases and protection to tumour rechallenge (Kleinovink et al. 2016). Similar results were found in mouse models of colon and lung carcinoma when Photofrin® was combined with a granulocyte colony-stimulating factor (G-CSF), which stimulated innate immunity and antigen presentation (Goląb et al. 2000). Combining PDT with low-dose chemotherapy led to a decrease in suppressor CD4 T cells and cured 70% of a mouse metastatic sarcoma model (Castano et al. 2008). The use of combination strategies is also aimed at achieving higher impact at lower doses of each drug, thus avoiding the usual serious side effects, such as those linked with chemotherapy. A synergistic effect in killing cancer cells was found by combining two photosensitisers in PDT, zinc(II)-phthalocyanine and meso-tetrakis(4-N-methylpyridyl)porphine (Acedo et al. 2014).
Although one of the first requirements for a good PS is to be non-toxic without light, some recent successful examples show considerable toxicity (Faudale et al. 2012). Certain photosensitisers were shown to be multifunctional therapeutic anticancer agents by having effects other than ROS-generation. For example, some cationic porphyrins can be employed in a cancer treatment without light activation. Di-, tri-, and tetra-cationic tetraaryl-porphyrins that were identified as inhibitors of the activity of the growth factor receptor tyrosine kinase were proposed for the role of inhibitiors of angiogenesis or neovascularisation. In particular, one non-symmetric porphyrin, with three positively charged pyridyl units, efficiently inhibited the activation of the basic fibroblast growth factor (bFGF) and its binding to the FGF receptor (Yayon et al. 2004). Also, cationic pyridinium porphyrins can directly target nucleic acids (Zhao et al. 2008). The most recent example shows that TMPyP4 binds to G-quadruplex (G4) DNA resulting in the inhibition of telomerase activity (Wei et al. 2009). However, TMPyP4 is not the best candidate in anticancer treatment, because it lacks selectivity and has high affinity for all the types of nucleic acids. Thus, different derivatives are now being explored for G4-nucleic acid binding, such as the one with propyl groups instead of methyl (TPrPyP4) (Wei et al. 2009), and the tricationic porphyrin, tris(1-methyl-4-pyridinio)porphyrin, bearing the carboxyalkyl linker (TMPyP3+) (Ryazanova et al. 2016). Nice examples of selective G4-targeting were reported for amphiphilic cationic porphyrin tri-meso(N-methyl-4-pyridyl), meso(N-tetradecyl-4-pyridyl)porphine (TMPyP4-C14) (Fig. 3), which were successful in oncogenic KRAS pancreatic cancer cells and melanoma mouse models (Faudale et al. 2012; Rapozzi et al. 2014).
Pathogenic bacteria can be sensitised to antibiotic therapy by PSs (Barra et al. 2015). Tetracationic porphyrin TMPyP4 was used on Pseudomonas aeruginosa biofilms, and caused direct cell killing as well as detachment of the biofilm after light activation. The enhanced killing of the cells when TMPyP was used in the combination with tobramycin indicates a light-independent mechanism, and it has been suggested that the two mechanisms together could be employed for the treatment of biofilms and infections not easily reachable by light (Collins et al. 2010).
A novel method in cancer therapy, the so-called photochemical internalization (PCI), uses PSs, preferably amphiphilic porphyrins, to enhance the activity of macromolecules used as cytotoxic agents by delivering them to the desired location. Photoactivation of the amphiphilic PSs, which are located in the membranes of the endocytic vesicles, leads to membrane damage and subsequent release of macromolecules into the cytosol. Cell death is a result of the presence of these cytotoxic macromolecules within the cell rather than PDT (Berg et al. 2010). One of the recent successful examples of this methodology includes a porphyrin–β-cyclodextrin conjugate (mTHPP-βCD) that was used to facilitate the release of the chemotherapeutic drug tamoxifen into the cytosol of breast cancer cells (Theodossiou et al. 2015).
It is already common in clinical practice to customize anticancer therapy to best fit both the cancer type and the patient’s overall condition. PDT has aforementioned advantages over other therapies, but is not equally effective with all types of tumours, and it has certain limitations concerning the depth of light penetration and the availability of oxygen in the tumour tissue. Therefore, the combination of other types of therapies and PDT, together with different diagnostic techniques, could be the most successful approach in treating cancer (Celli et al. 2010).
Dosimetry in PDT research and applications
Despite various advantages of PDT compared to current therapies of cancer and microbial infections, and many other possible applications outside medicine, the research area of PDT is inevitably interdisciplinary, and the resulting complexity in a study of any of its aspects could explain the somewhat slow progress. The efficiency of PDT depends on three main factors: light, drug and oxygen. However, the biologic diversity of targeted tumours or infected tissues, which differ in location, bulkiness, heterogeneity, etc., inevitably makes PDT one of the most complex iatrogenic challenges. Not to mention that, if counting on an immune-mediated PDT, one has to navigate between the Scylla and Charybdis of immunostimulatory and immunosuppressive effects, and find an optimal therapeutic window for an individual application. The relationship between the concentrations of the PS, the incubation time, available molecular oxygen, and the intensity (light fluence) and duration of irradiation (gives the overall dose of light) is nonlinear, and all these parameters depend on each other dynamically. Therefore, it is very difficult to measure all the parameters in real time and subsequently calculate the appropriate therapeutic doses (Huang et al. 2008). Photosensitisers of the same type can have different effects on different cell types; this makes the evaluation of their efficiency even more complicated. The difficulty in comparing published results is already present at the level of in vitro studies, because in most of them different combinations of the above-mentioned parameters are used. Evidently, the in vivo animal models and patient studies are even more complex. A recent tutorial provides instructions on what steps to take to complete in vitro characterisation of any new PS for both PDT and PACT, and offers a beacon guiding investigators towards a more unified approach (Kiesslich et al. 2013).
There are four classes of PDT dosimetry that can be studied: explicit dosimetry, implicit dosimetry, biophysical/biological tissue response monitoring and direct dosimetry (Jarvi et al. 2006). Direct dosimetry is mostly concerned with monitoring the singlet oxygen, which is formed as a result of PDT, by the measurement of phosphorescence at 1270 nm. Although direct dosimetry has the advantage that it measures the final parameter responsible for direct cell killing, it is often impractical and it is still very expensive (Jarvi et al. 2006). Implicit dosimetry consists in following PDT indirectly by measuring photobleaching of the PS. This can be done by the measurement of the decrease in fluorescence of the PS, or the absorption of the product of photobleaching (Jarvi et al. 2012). The main advantage of implicit dosimetry is that photobleaching depends on all three PDT factors (light, drug, oxygen) and, among the two mentioned ways of measuring, a decrease in fluorescence is especially attractive because it is a very sensitive technique, relatively affordable and easy to measure (Jarvi et al. 2012). It cannot be applied on all PSs, especially on those that are complex mixtures such as Photofrin, or endogenous protoporhyrin IX that is induced by 5-aminolevulinic acid (5-ALA), but it is more applicable with photosensitisers that are synthetic and pure compounds (Jarvi et al. 2012). Recently, two new cationic porphyrins, mesoimidazolium-substituted porphyrin derivative (ImP) and pyridinium-substituted porphyrin derivative (PyP), were synthesised for PACT against periodontal Gram(+) and Gram(−) pathogenic bacteria, and their photophysical properties were evaluated in this context. The decrease in their fluorescence was measured over time and under PDT irradiation: PyP exhibited fast photobleaching, with much higher photostability demonstrated by ImP. Based on its rapid bleaching, PyP was proposed for clinical trials, and fluorescence monitoring was suggested for implicit dosimetry during PDT (Prasanth et al. 2012).
Finally, a mode of cell death depends not only on a PDT dose (Plaetzer et al. 2002) but also on cell type (Wyld et al. 2001. Therefore, dosimetry investigations in different cell types may provide evidence to facilitate the choice of the appropriate application for a certain PS. Tricationic 5-phenyl-10,15,20-tris(N-methyl-4-pyridyl)porphyrin chloride (TriP4) was more effective in photodynamic inactivation of S. aureus than human dermal fibroblasts, thus optimal dosing of this PS and light could provide selective PACT for the treatment of burn wound infections (Lambrechts et al. 2005). In general, PS more ideal for PACT should kill the target microbial cell after rapid uptake and short irradiation, in concentrations non-toxic for mammalian cells (Alenezi et al. 2017). Hence, extensive research of dosimetry is crucial in finding an optimal application of any new PS with proved PDT efficiency.
Conclusions
Amphiphilicity of a PS is important for its efficiency in PDT: its hydrophilicity will facilitate its distribution, and lipophilicity its cellular uptake. As such, PS can passively accumulate in target tissues, often without the need for an active targeting approach via antibodies. Amphiphilic cationic PSs seem to preferentially target mitochondria in metabolically active cancer and/or immune cells and Gram(−) bacteria, thus making them potentially attractive in anticancer, immunosupressive and antimicrobial treatments, respectively. This is not straightforward, and the search for an ideal PS based on its amphiphilic properties requires establishing the optimal ratio between the hydrophilic and hydrophobic regions, and taking into account both the number and the position of charges. The cationic amphiphilic porphyrin-based PSs have various advantages over neutral and anionic PSs. However, a challenge remains in determining the structural components in a PS that could be important in any highly selective targeting of cancer cells over non-cancer, or micro-organisms over mammalian cells. All of this has been difficult so far, mostly because the protocols for PS assessment are not standardised, thus making the published studies hard to compare. It seems that there should be more systematic research with (Q)SAR studies included, which could signal the most important structural features. The models obtained should be then tested in experimental conditions to generate new data. In experimental PDT studies, it would be useful to follow suggested standardised protocols (Kiesslich et al. 2013), and to study as many parameters as possible (drug dose, light intensity, light dose, drug-dose interval, molecular oxgen concentrations, etc.).
In recent years, cationic amphiphilic PSs have been extensively tested in antimicrobial PDT against various pathogens, especially in dermatology and dentistry, while they were somewhat less present in anticancer PDT research. High numbers of publications have evaluated cationic meso-tetraarylporphyins as PSs for various PDT applications, and have indicated that the structures with the higher number of positive charges, but also with certain lipophilicity, asymmetrically distributed, such as in dicationic cis-isomers, seem to be the most efficient. What seems to be lacking, especially in PACT studies, are mechanistic analyses that could provide more information on targeting at a molecular level. On the other hand, further dosimetry studies are needed to find optimal applications, and to maximise the PDT effect in targeted cells while minimising the off-target effects. Finally, an emerging area of PDTs worth investigating are combination therapies where a PS can be used as a multifunctional drug, or in combination with other anticancer, antimicrobial and immunosuppressive agents, to induce synergistic or additive effects, and overcome the limitations of monotherapies. With all the challenges ahead, it is our belief that standardisation in upcoming PDT research is of utmost significance, and is as important or even more important as the discovery of new compounds.
Acknowledgements
NM, IM and IJ acknowledge funding by the University of Rijeka, grants #13.11.1.2.03, 13.11.1.2.08 and 13.11.1.2.09, respectively; IM acknowledges funding by Croatian science foundation grant #7459, and IJ by FP7-PEOPLE-2013-CIG-EU grant #618587. The authors also acknowledge the equipment purchased within the project “Research Infrastructure for Campus-based Laboratories at University of Rijeka”, financed by European Regional Development Fund (ERDF).
Compliance with ethical standards
Conflicts of interest
Nela Malatesti declares that she has no conflicts of interest. Ivana Munitic declares that she has no conflicts of interest. Igor Jurak declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Abe H, Wagner SJ (1995) Analysis of viral DNA, protein and envelope damage after methylene blue, phthalocyanine derivative or merocyanine 540 photosensitization. Photochem Photobiol 61:402–409 [DOI] [PubMed]
- Acedo P, Stockert JC, Cañete M, Villanueva A. Two combined photosensitizers: a goal for more effective photodynamic therapy of cancer. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker G, Palumbo A, Neri D, Vajkoczy P, Czabanka M. F8-SIP mediated targeted photodynamic therapy leads to microvascular dysfunction and reduced glioma growth. J Neuro-Oncol. 2016;129:33–38. doi: 10.1007/s11060-016-2143-8. [DOI] [PubMed] [Google Scholar]
- Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656–669. doi: 10.1562/0031-8655(2001)074<0656:THOPAP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Alenezi K, Tovmasyan A, Batinic-Haberle I, Benov LT. Optimizing Zn porphyrin-based photosensitizers for efficient antibacterial photodynamic therapy. Photodiagn Photodyn Ther. 2017;17:154–159. doi: 10.1016/j.pdpdt.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Ali H, van Lier JE. Metal complexes as photo- and radiosensitizers. Chem Rev. 1999;99:2379–2450. doi: 10.1021/cr980439y. [DOI] [PubMed] [Google Scholar]
- Allen CM, Sharman WM, Van Lier JE. Current status of phthalocyanines in the photodynamic therapy of cancer. J Pept Res. 2001;5:161–169. [Google Scholar]
- Allison RR (2016) Fluorescence guided resection (FGR): A primer for oncology. Photodiagnosis Photodyn Ther 13:73–80 [DOI] [PubMed]
- Allison RR, Moghissi K. Oncologic photodynamic therapy: clinical strategies that modulate mechanisms of action. Photodiagn Photodyn Ther. 2013;10:331–341. doi: 10.1016/j.pdpdt.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Allison RR, Moghissi K. Photodynamic therapy (PDT): PDT mechanism. Clin Endocrinol. 2013;46:24–29. doi: 10.5946/ce.2013.46.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison RR, Downie GH, Cuenca R, Hu X-H, Childs CJH, Sibata CH. Photosensitizers in clinical PDT. Photodiagn Photodyn Ther. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- Almeida RD, Manadas BJ, Carvalho AP, Duarte CB. Intracellular signaling mechanisms in photodynamic therapy. Biochim Biophys Acta. 2004;1704:59–86. doi: 10.1016/j.bbcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Almutawa F, Thalib L, Hekman D, Sun Q, Hamzavi I, Lim HW (2015) Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed 31:5-–14 [DOI] [PubMed]
- Al-Omari S (2013) Toward a molecular understanding of the photosensitizer-copper interaction for tumour destruction. Biophys Rev 5:305 [DOI] [PMC free article] [PubMed]
- Angeli NG, Lagorio MG, San Román EA, Dicelio LE. Meso-substituted cationic porphyrins of biological interest. Photophysical and physicochemical properties in solution and bound to liposomes. Photochem Photobiol. 2008;72:49–56. doi: 10.1562/0031-8655(2000)072<0049:mscpob>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Azzouzi AR, Barret E, Moore CM, Villers A, Allen C, Scherz A, Muir G, de Wildt M, Barber NJ, Lebdai S, Emberton M. TOOKAD(®) soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013;112:766–774. doi: 10.1111/bju.12265. [DOI] [PubMed] [Google Scholar]
- Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D (2003) Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev 16:114–128 [DOI] [PMC free article] [PubMed]
- Barra F, Roscetto E, Soriano AA, Vollaro A, Postiglione I, Pierantoni GM, Palumbo G, Catania MR. Photodynamic and antibiotic therapy in combination to fight biofilms and resistant surface bacterial infections. Int J Mol Sci. 2015;16:20417–20430. doi: 10.3390/ijms160920417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belicha-Villanueva A, Riddell J, Bangia N, Gollnick SO. The effect of photodynamic therapy on tumor cell expression of major histocompatibility complex (MHC) class I and MHC class I-related molecules. Lasers Surg Med. 2012;44:60–68. doi: 10.1002/lsm.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dror S, Bronshtein I, Wiehe A, Röder B, Senge MO, Ehrenberg B. On the correlation between hydrophobicity, liposome binding and cellular uptake of porphyrin sensitizers. Photochem Photobiol. 2006;82:695–701. doi: 10.1562/2005-09-01-RA-669. [DOI] [PubMed] [Google Scholar]
- Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter BØ, Angellpetersen E, Warloe T, Frandsen N, Høgset A. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc. 2005;218:133–147. doi: 10.1111/j.1365-2818.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Berg K, Weyergang A, Prasmickaite L, Bonsted A, Høgset A, Strand MT, Wagner E, Selbo PK. Photochemical internalization (PCI): a technology for drug delivery. Methods Mol Biol. 2010;635:133–145. doi: 10.1007/978-1-60761-697-9_10. [DOI] [PubMed] [Google Scholar]
- Bihl F, Castelli D, Marincola F, Dodd RY, Brander C (2007) Transfusion-transmitted infections. J Transl Med 5:25 [DOI] [PMC free article] [PubMed]
- Böhm H-J, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, Obst-Sander U, Stahl M. Fluorine in medicinal chemistry. ChemBioChem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- Bonneau S, Vever-Bizet C. Tetrapyrrole photosensitisers, determinants of subcellular localisation and mechanism processes in therapeutic approaches. Expert Opin Ther Pat. 2008;18:1011–1025. doi: 10.1517/13543776.18.9.1011. [DOI] [Google Scholar]
- Börsch M. Targeting cytochrome C oxidase in mitochondria with Pt(II)-porphyrins for photodynamic therapy. Optical methods for tumor treatment and detection: mechanisms and techniques in photodynamic therapy XIX, proc. SPIE. 2010;7551:1–11. [Google Scholar]
- Brackett CM, Gollnick SO. Photodynamic therapy enhancement of anti-tumour immunity. Photochem Photobiol Sci. 2011;10:649–652. doi: 10.1039/c0pp00354a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandis A, Mazor O, Neumark E, Rosenbach-Belkin V, Salomon Y, Scherz A. Novel water-soluble bacteriochlorophyll derivatives for vascular-targeted photodynamic therapy: synthesis, solubility, phototoxicity and the effect of serum proteins. Photochem Photobiol. 2005;81:983–993. doi: 10.1562/2004-12-01-RA-389R1.1. [DOI] [PubMed] [Google Scholar]
- Caminos DA, Spesia MB, Durantini EN. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N, N, N-trimethylammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem Photobiol Sci. 2006;5:56–65. doi: 10.1039/B513511G. [DOI] [PubMed] [Google Scholar]
- Carrenho LZ, Moreira CG, Vandresen CC, Gomes JR, Gonçalves AG, Barreira SM, Noseda MD, Duarte ME, Ducatti DR, Dietrich M, Paludo K, Cabrini DA, Otuki MF. Investigation of anti-inflammatory and anti-proliferative activities promoted by photoactivated cationic porphyrin. Photodiagn Photodyn Ther. 2015;12:444–458. doi: 10.1016/j.pdpdt.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther 1:279–293 [DOI] [PMC free article] [PubMed]
- Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci U S A. 2008;105:5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteel MJ, Jayaraj K, Gold A, Ball LM, Sobsey MD (2004) Photoinactivation of hepatitis A virus by synthetic porphyrins. Photochem Photobiol 80:294–300 [DOI] [PubMed]
- Cecic I, Parkins CS, Korbelik M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem Photobiol. 2001;74:712–720. doi: 10.1562/0031-8655(2001)074<0712:IOSNRI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KP, Kolli BK, New Light G (2016) New "light" for one-world approach toward safe and effective control of animal diseases and insect vectors from leishmaniac perspectives. Parasit Vectors 9:396 [DOI] [PMC free article] [PubMed]
- Chen B, Pogue BW, Hoopes PJ, Hasan T. Vascular and cellular targeting for photodynamic therapy. Crit Rev Eukaryot Gene Expr. 2006;16:279–305. doi: 10.1615/CritRevEukarGeneExpr.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- Coen DM, Whitley RJ (2011) Antiviral drugs and antiviral drug resistance. Curr Opin Virol 1:545–547 [DOI] [PMC free article] [PubMed]
- Collins TL, Markus EA, Hassett DJ, Robinson JB. The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms. Curr Microbiol. 2010;61:411–416. doi: 10.1007/s00284-010-9629-y. [DOI] [PubMed] [Google Scholar]
- Cordeiro RM, Miotto R, Baptista MS. Photodynamic efficiency of cationic meso-porphyrins at lipid bilayers: insights from molecular dynamics simulations. J Phys Chem B. 2012;116:14618–14627. doi: 10.1021/jp308179h. [DOI] [PubMed] [Google Scholar]
- Cortez KJ, Maldarelli F (2011) Clinical management of HIV drug resistance. Viruses 3:347–378 [DOI] [PMC free article] [PubMed]
- Costa L et al. (2008) Sewage bacteriophage photoinactivation by cationic porphyrins: a study of charge effect. Photochem Photobiol Sci 7:415–422 [DOI] [PubMed]
- Costa L et al. (2010) Sewage bacteriophage inactivation by cationic porphyrins: influence of light parameters. Photochem Photobiol Sci 9:1126–1133 [DOI] [PubMed]
- Costa L et al. (2011) Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of aPDT. Antiviral Res 91:278–282 [DOI] [PubMed]
- Costa DC, Gomes MC, Faustino MA, Neves MG, Cunha A, Cavaleiro JA, Almeida A, Tomé JP. Comparative photodynamic inactivation of antibiotic resistant bacteria by first and second generation cationic photosensitizers. Photochem Photobiol Sci. 2012;11:1905–1913. doi: 10.1039/c2pp25113b. [DOI] [PubMed] [Google Scholar]
- Costa L, Faustino MA, Neves MG, Cunha A, Almeida A (2012b) Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 4:1034–1074 [DOI] [PMC free article] [PubMed]
- Costa L et al. (2013) Involvement of type I and type II mechanisms on the photoinactivation of non-enveloped DNA and RNA bacteriophages. J Photochem Photobiol B 120:10–16 [DOI] [PubMed]
- Dancil K-PS, Hilario LF, Khoury RG, Mai KU, Nguyen CK, Weddle KS, Shachter AM. Synthesis and aggregation of cationic porphyrins. J Heterocycl Chem. 1997;34:749–755. doi: 10.1002/jhet.5570340308. [DOI] [Google Scholar]
- DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coord Chem Rev. 2002;233(234):351–371. doi: 10.1016/S0010-8545(02)00034-6. [DOI] [Google Scholar]
- Ding H, Yu H, Dong Y, Tian R, Huang G, Boothman DA, Sumer BD, Gao J. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276–280. doi: 10.1016/j.jconrel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Dosselli R, Millioni R, Puricelli L, Tessari P, Arrigoni G, Franchin C, Segalla A, Teardo E, and Reddi E (2012). Molecular targets of antimicrobial photodynamic therapy identified by a proteomic approach. J Proteomics 77, 329–343 [DOI] [PubMed]
- Dummin H, Cernay T, Zimmermann HW. Selective photosensitization of mitochondria in HeLa cells by cationic Zn(II)phthalocyanines with lipophilic side-chains. J Photochem Photobiol B. 1997;37:219–229. doi: 10.1016/S1011-1344(96)07416-7. [DOI] [PubMed] [Google Scholar]
- Egyeki M, Turoczy G, Majer Z, Toth K, Fekete A, Maillard P, Csik G (2003) Photosensitized inactivation of T7 phage as surrogate of non-enveloped DNA viruses: efficiency and mechanism of action. Biochim Biophys Acta 1624:115–124 [DOI] [PubMed]
- Engelmann FM, Mayer I, Gabrielli DS, Toma HE, Kowaltowski AJ, Araki K, Baptista MS. Interaction of cationic meso-porphyrins with liposomes, mitochondria and erythrocytes. J Bioenerg Biomembr. 2007;39:175–185. doi: 10.1007/s10863-007-9075-0. [DOI] [PubMed] [Google Scholar]
- Ethirajan M, Chen Y, Joshi P, Pandey RK. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem Soc Rev. 2011;40:340–362. doi: 10.1039/B915149B. [DOI] [PubMed] [Google Scholar]
- Faudale M, Cogoi S, Xodo LE. Photoactivated cationic alkyl-substituted porphyrin binding to g4-RNA in the 5’-UTR of KRAS oncogene represses translation. Chem Commun. 2012;48:874–876. doi: 10.1039/C1CC15850C. [DOI] [PubMed] [Google Scholar]
- Frimayanti N, Yam ML, Lee HB, Othman R, Zain SM, Rahman NA. Validation of quantitative structure-activity relationship (QSAR) model for photosensitizer activity prediction. Int J Mol Sci. 2011;12:8626–8644. doi: 10.3390/ijms12128626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard S, Tempete C, Werner GH (1995) Studies on photoinactivation by various phthalocyanines of a free or replicating non-enveloped virus. J Photochem Photobiol B 31:159–162 [DOI] [PubMed]
- Gerba CP, Wallis C, Melnick JL (1977) Application of photodynamic oxidation to the disinfection of tapwater, seawater, and sewage contaminated with poliovirus. Photochem Photobiol 26:499–504 [DOI] [PubMed]
- Giuntini F, Alonso CMA, Boyle RW. Synthetic approaches for the conjugation of porphyrins and related macrocycles to peptides and proteins. Photochem Photobiol Sci. 2011;10:759–791. doi: 10.1039/c0pp00366b. [DOI] [PubMed] [Google Scholar]
- Goląb J, Wilczyński G, Zagoźdźon R, Stokłosa T, Dąbrowska A, Rybczyńska J, Wąsik M, Machaj E, Ołdak T, Kozar K, Kamiński R, Giermasz A, Czajka A, Lasek W, Feleszko W, Jakóbisiak M. Potentiation of the anti-tumour effects of photofrin®-based photodynamic therapy by localized treatment with G-CSF. Br J Cancer. 2000;82:1485–1491. doi: 10.1054/bjoc.1999.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Li G, Chen Y, Morgan J, Oseroff A, Dougherty TJ, Pandey RK. Structure–activity relationship of New octaethylporphyrin-based benzochlorins as photosensitizers for photodynamic therapy. Photochem Photobiol. 2003;77:561–566. doi: 10.1562/0031-8655(2003)077<0561:sronob>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gsponer NS, Spesia MB, Durantini EN. Effects of divalent cations, EDTA and chitosan on the uptake and photoinactivation of Escherichia coli mediated by cationic and anionic porphyrins. Photodiagn Photodyn Ther. 2015;12:67–75. doi: 10.1016/j.pdpdt.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Hamblin M. Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Curr Opin Microbiol. 2016;33:67–73. doi: 10.1016/j.mib.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BW, Bellnier DA, Greco WR, Sharma A, Pandey RK, Vaughan LA, Weishaupt KR, Dougherty TJ (1997) An in vivo quantitative structure-activity relationship for a congeneric series of pyropheophorbide derivatives as photosensitizers for photodynamic therapy. Cancer Res 57:4000–4007 [PubMed]
- Hong EJ, Choi DG, Shim MS. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm Sin B. 2016;6:297–307. doi: 10.1016/j.apsb.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B, Williams B, Rywkin S, Prince AM, Pascual D, Geacintov N, Valinsky J (1991) Inactivation of viruses in blood with aluminum phthalocyanine derivatives. Transfusion 31:102–108 [DOI] [PubMed]
- Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancer. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Xu H, Meyers AD, Musani AI, Wang L, Tagg R, Barqawi AB, Chen YK. Photodynamic therapy for treatment of solid tumors – potential and technical challenges. Technol Cancer Res Treat. 2008;7:309–320. doi: 10.1177/153303460800700405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh E, Zheng G. Porphysome nanotechnology: a paradigm shift in lipid-based supramolecular structures. Nano Today. 2014;9:212–222. doi: 10.1016/j.nantod.2014.04.012. [DOI] [Google Scholar]
- Inguscio V, Panzarini E, Dini L. Autophagy contributes to the death/survival balance in cancer photo dynamic therapy. Cells. 2012;1:464–491. doi: 10.3390/cells1030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvi MT, Niedre MJ, Patterson MS, Wilson BC. Singlet oxygen luminescence dosimetry (SOLD) for photodynamic therapy: current status, challenges and future prospect. Photochem Photobiol. 2006;82:1198–1210. doi: 10.1562/2006-05-03-IR-891. [DOI] [PubMed] [Google Scholar]
- Jarvi MT, Patterson MS, Wilson BC. Insights into photodynamic therapy dosimetry: simultaneous singlet oxygen luminescence and photosensitizer photobleaching measurements. Biophys J. 2012;102:661–671. doi: 10.1016/j.bpj.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Granville DJ, North JR, Richter AM, Hunt DW (2002) Selective action of the photosensitizer QLT0074 on activated human T lymphocytes. Photochem Photobiol 76:224–231 [DOI] [PubMed]
- Jin R-H, Aoki S, Shima KJ. Phosphoniumyl cationic porphyrins Self-aggregation origin from π-π and cation-π interactions. J Chem Soc Faraday Trans. 1997;93:3945–3953. doi: 10.1039/a703965d. [DOI] [Google Scholar]
- Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- Josefsen LB, Boyle RW. Photodynamic therapy and the development of metal-based photosensitisers. Metal-Based Drugs. 2008;2008:276109. doi: 10.1155/2008/276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsen LB, Boyle RW. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics. 2012;2:916–966. doi: 10.7150/thno.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 2007;96:1839–1848. doi: 10.1038/sj.bjc.6603792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano K, Fukuda K, Wakami H, Nishiyabu R, Pasternack RF. Factors influencing self-aggregation tendencies of cationic porphyrins in aqueous solution. J Am Chem Soc. 2000;122:7494–74502. doi: 10.1021/ja000738g. [DOI] [Google Scholar]
- Karlin S, Brendel V (1988) Charge configurations in viral proteins. Proc Natl Acad Sci U S A 85:9396–9400 [DOI] [PMC free article] [PubMed]
- Kasturi C, Platz MS (1992) Inactivation of lambda phage with 658 nm light using a DNA binding porphyrin sensitizer. Photochem Photobiol 56:427–429 [DOI] [PubMed]
- Ke MR et al. (2014) Photodynamic inactivation of bacteria and viruses using two monosubstituted zinc(II) phthalocyanines. Eur J Med Chem 84:278–283 [DOI] [PubMed]
- Kessel D, Luguya R, Vicente MGH. Localization and photodynamic efficacy of two cationic porphyrins varying in charge distributions. Photochem Photobiol. 2003;78:431–435. doi: 10.1562/0031-8655(2003)078<0431:LAPEOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43:755–767. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesslich T, Gollmer A, Maisch T, Berneburg M, Plaetzer K. A comprehensive tutorial on in vitro characterization of New photosensitizers for photodynamic antitumor therapy and photodynamic inactivation of microorganisms. Biomed Res Int. 2013;2013:1–17. doi: 10.1155/2013/840417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Jung HY, Park HJ. Topical PDT in the treatment of benign skin diseases: principles and new applications. Int J Mol Sci. 2015;16:23259–23278. doi: 10.3390/ijms161023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinovink JW, van Driel PB, Snoeks TJ, Prokopi N, Fransen MF, Cruz LJ, Mezzanotte L, Chan A, Löwik CW, Ossendorp F. Combination of photodynamic therapy and specific immunotherapy efficiently eradicates established tumors. Clin Cancer Res. 2016;22:1459–1468. doi: 10.1158/1078-0432.CCR-15-0515. [DOI] [PubMed] [Google Scholar]
- Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38:500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- Kos I, Rebouças JS, DeFreitas-Silva G, Salvemini D, Vujaskovic Z, Dewhirst MW, Spasojević I, Batinić-Haberle I. Lipophilicity of potent porphyrin-based antioxidants: comparison of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins. Free Radic Biol Med. 2009;47:72–78. doi: 10.1016/j.freeradbiomed.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007;67:10501–10510. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Marek G, Serpa C, Szurko A, Widel M, Sochanik A, Snietura M, Kus P, Nunes RM, Arnaut LG, Ratuszna A. Spectroscopic properties and photodynamic effects of new lipophilic porphyrin derivatives: efficacy, localisation and cell death pathways. J Photochem Photobiol B. 2006;84:1–14. doi: 10.1016/j.jphotobiol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kussovski V, Mantareva V, Angelov I, Orozova P, Wöhrle D, Schnurpfeil G, Borisova E, Avramov L. Photodynamic inactivation of Aeromonas hydrophila by cationic phthalocyanines with different hydrophobicity. FEMS Microbiol Lett. 2009;294:133–140. doi: 10.1111/j.1574-6968.2009.01555.x. [DOI] [PubMed] [Google Scholar]
- Lambrechts SA, Schwartz KR, Aalders MC, Dankert JB. Photodynamic inactivation of fibroblasts by a cationic porphyrin. Lasers Med Sci. 2005;20:62–67. doi: 10.1007/s10103-005-0338-x. [DOI] [PubMed] [Google Scholar]
- Lazzeri D, Durantini EN. Synthesis of meso-substituted cationic porphyrins as potential photodynamic agents. ARKIVOC. 2003;10:227–239. [Google Scholar]
- Lebedeva N, Malkova E, Syrbu S, Gubarev Y, Nikitin D. Investigation of interactions between cationic and anionic porphyrins and BSA in aqueous media. Int J Biochem Biophys. 2013;2:13–18. [Google Scholar]
- Lei W, Xie J, Hou Y, Jiang G, Zhang H, Wang P, Wang X, Zhang B. Mitochondria-targeting properties and photodynamic activities of porphyrin derivatives bearing cationic pendant. J Photochem Photobiol B. 2010;98:167–171. doi: 10.1016/j.jphotobiol.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Lenard J, Rabson A, Vanderoef R (1993) Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: inhibition of fusion and syncytia formation. Proc Natl Acad Sci U S A 90:158–162 [DOI] [PMC free article] [PubMed]
- Li H, Fedorova OS, Grachev AN, Trumble WR, Bohach GA, Czuchajowski L (1997) A series of meso-tris (N-methyl-pyridiniumyl)-(4-alkylamidophenyl) porphyrins: synthesis, interaction with DNA and antibacterial activity. Biochim Biophys Acta 1354:252–260 [DOI] [PubMed]
- Liang YI, Lu LM, Chen Y, Lin YK. Photodynamic therapy as an antifungal treatment. Exp Ther Med. 2016;12:23–27. doi: 10.3892/etm.2016.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieder A, Khan MK, Lippert BM (2014) Photodynamic therapy for recurrent respiratory papillomatosis. Cochrane Database Syst Rev:CD009810 [DOI] [PMC free article] [PubMed]
- Liu Y, Qin R, Zaat SAJ, Breukink E, Heger M. Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. J Clin Transl Res. 2015;1:140–167. [PMC free article] [PubMed] [Google Scholar]
- Lopez Zeballos NC, Gauna GA, Garcia Vior MC, Awruch J, Dicelio LE (2014) Interaction of cationic phthalocyanines with DNA. Importance of the structure of the substituents. J Photochem Photobiol B 136:29–33 [DOI] [PubMed]
- Maeda H, Tsukigawa K, Fang J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: next-generation chemotherapeutics and photodynamic therapy-problems, solutions, and prospects. Microcirculation. 2016;23:173–182. doi: 10.1111/micc.12228. [DOI] [PubMed] [Google Scholar]
- Maeding N, Verwanger T, Krammer B. Boosting tumor-specific immunity using PDT. Cancer. 2016;8:91. doi: 10.3390/cancers8100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem Photobiol Sci. 2015;14:1518–1526. doi: 10.1039/C5PP00037H. [DOI] [PubMed] [Google Scholar]
- Makhseed S, Machacek M, Alfadly W, Tuhl A, Vinodh M, Simunek T, Novakova V, Kubat P, Rudolf E, Zimcik P. Water-soluble non-aggregating zinc phthalocyanine and in vitro studies for photodynamic therapy. Chem Commun. 2013;49:11149–11151. doi: 10.1039/c3cc44609c. [DOI] [PubMed] [Google Scholar]
- Malatesti N, Smith K, Savoie H, Greenman J, Boyle RW. Synthesis and in vitro investigation of cationic 5, 15-diphenyl porphyrin-monoclonal antibody conjugates as targeted photodynamic sensitisers. Int J Oncol. 2006;28:1561–1569. [PubMed] [Google Scholar]
- Malatesti N, Harej A, Kraljević SP, Lončarić M, Zorc H, Wittine K, Anđelković U, Josić D. Synthesis, characterisation and in vitro investigation of photodynamic activity of 5-(4- octadecanamidophenyl)-10, 15, 20-tris(N- methylpyridinium-3-yl)porphyrin trichloride on HeLa cells using low light fluence rate. Photodiagn Photodyn Ther. 2016;15:115–126. doi: 10.1016/j.pdpdt.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Mantareva VN, Angelov I, Wöhrle D, Borisova E, Kussovski V. Metallophthalocyanines for antimicrobial photodynamic therapy: an overview of our experience. J Porphyrins Phthalocyanines. 2013;17:399–416. doi: 10.1142/S1088424613300024. [DOI] [Google Scholar]
- Mehraban N, Freeman HS. Development in PDT sensitizers for increased selectivity and singlet oxygen production. Materials. 2015;8:4421–4456. doi: 10.3390/ma8074421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick JL, Wallis C (1977) Photodynamic inactivation of herpes simplex virus: a status report. Ann N Y Acad Sci 284:171–181 [DOI] [PubMed]
- Memoli MJ, Davis AS, Proudfoot K, Chertow DS, Hrabal RJ, Bristol T, Taubenberger JK (2011) Multidrug-resistant 2009 pandemic influenza A(H1N1) viruses maintain fitness and transmissibility in ferrets. J Infect Dis 203:348–357 [DOI] [PMC free article] [PubMed]
- Mesquita MQ, Menezes JCJMDS, Neves MGPMS, Tomé AC, Cavaleiro JAS, Cunha Â, Almeida A, Hackbarth S, Röder B, Faustino MAF. Photodynamic inactivation of bioluminescent Escherichia coli by neutral and cationic pyrrolidine-fused chlorins and isobacteriochlorins. Bioorg Med Chem Lett. 2014;24:808–812. doi: 10.1016/j.bmcl.2013.12.097. [DOI] [PubMed] [Google Scholar]
- Mettath S, Munson BR, Pandey RK (1999) DNA interaction and photocleavage properties of porphyrins containing cationic substituents at the peripheral position. Bioconjug Chem 10:94–102 [DOI] [PubMed]
- Milgrom LR. Toward recombinant antibody-fragment targeted photodynamic therapy. Sci Prog. 2008;91:241–262. doi: 10.3184/003685008X361415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michen B, Graule T (2010) Isoelectric points of viruses. J Appl Microbiol 109:388–397 [DOI] [PubMed]
- Mitton D, Ackroyd R. A brief overview of photodynamic therapy in Europe. Photodiagn Photodyn Ther. 2008;5:103–111. doi: 10.1016/j.pdpdt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano JS, Aprille JR. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev. 2001;49:63–70. doi: 10.1016/S0169-409X(01)00125-9. [DOI] [PubMed] [Google Scholar]
- Moor AC, Wagenaars-van Gompel AE, Brand A, Dubbelman MA, VanSteveninck J (1997) Primary targets for photoinactivation of vesicular stomatitis virus by AIPcS4 or Pc4 and red light. Photochem Photobiol 65:465–470 [DOI] [PubMed]
- Morgan J, Oseroff AR. Mitochondria-based photodynamic anti-cancer therapy. Adv Drug Deliv Rev. 2001;49:71–86. doi: 10.1016/S0169-409X(01)00126-0. [DOI] [PubMed] [Google Scholar]
- Mroz P, Hamblin MR. The immunosuppressive side of PDT. Photochem Photobiol Sci. 2011;10:751–758. doi: 10.1039/c0pp00345j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North J, Freeman S, Overbaugh J, Levy J, Lansman R (1992) Photodynamic inactivation of retrovirus by benzoporphyrin derivative: a feline leukemia virus model. Transfusion 32:121–128 [DOI] [PubMed]
- Nowis D, Stokłosa T, Legat M, Issat T, Jakóbisiak M, Gołąb J (2005) The influence of photodynamic therapy on the immune response. Photodiagnosis Photodyn Ther 2:283–298 [DOI] [PubMed]
- O’Brien JM, Gaffney DK, Wang TP, Sieber F (1992) Merocyanine 540-sensitized photoinactivation of enveloped viruses in blood products: site and mechanism of phototoxicity. Blood 80:277–285 [PubMed]
- Olivo M, Bhuvaneswari R, Lucky SS, Dendukuri N, Thong PSP. Targeted therapy of cancer using photodynamic therapy in combination with multi-faceted anti-tumor modalities. Pharmaceuticals. 2010;3:1507–1529. doi: 10.3390/ph3051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond AB, Freeman HS. Dye sensitizers for photodynamic therapy. Materials. 2013;6:817–840. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz A et al. (2013) Binding of new cationic porphyrin-tetrapeptide conjugates to nucleoprotein complexes. Biophys Chem 177-178:14–23 [DOI] [PubMed]
- Palumbo A, Hauler F, Dziunycz P, Schwager K, Soltermann A, Pretto F, Alonso C, Hofbauer GF, Boyle RW, Neri D. A chemically modified antibody mediates complete eradication of tumours by selective disruption of tumour blood vessels. Br J Cancer. 2011;104:1106–1115. doi: 10.1038/bjc.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MJ, Bae JH, Chung JS, Kim SH, Kang CD. Induction of NKG2D ligands and increased sensitivity of tumor cells to NK cell-mediated cytotoxicity by hematoporphyrin-based photodynamic therapy. Immunol Investig. 2011;40:367–382. doi: 10.3109/08820139.2010.551435. [DOI] [PubMed] [Google Scholar]
- Perdrau JR, Todd C (1933) The photodynamic action of methylene blue of certian viruses. Proc R Soc Lond B 112:288–298
- Pisarek S, Maximova K, Gryko D (2014) Strategies toward the synthesis of amphiphilic porphyrins. Tetrahedron 70: 6685–6715
- Plaetzer K, Kiesslich T, Krammer B, Hammerl P. Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT. Photochem Photobiol Sci. 2002;1:172–177. doi: 10.1039/b108816e. [DOI] [PubMed] [Google Scholar]
- Plaetzer K, Kiesslich T, Oberdanner KB, Krammer B. Apoptosis following photodynamic tumor therapy: induction, mechanisms and detection. Curr Pharm Des. 2005;11:1151–1165. doi: 10.2174/1381612053507648. [DOI] [PubMed] [Google Scholar]
- Prasanth CS, Karunakaran SC, Paul AK, Kussovski V, Mantareva V, Ramaiah D, Selvaraj L, Angelov I, Avramov L, Nandakumar K, Subhash N. Antimicrobial photodynamic efficiency of novel cationic porphyrins towards periodontal gram-positive and gram-negative pathogenic bacteria. Photochem Photobiol. 2014;90:628–640. doi: 10.1111/php.12198. [DOI] [PubMed] [Google Scholar]
- Pushpan SK, Venkatraman S, Anand VG, Sankar J, Parmeswaran D, Ganesan S, Chandrashekar TK. Porphyrins in photodynamic therapy - a search for ideal photosensitizers. Curr Med Chem Anticancer Agents. 2002;2:187–207. doi: 10.2174/1568011023354137. [DOI] [PubMed] [Google Scholar]
- Ragàs X, Sánchez-García D, Ruiz-González R, Dai T, Agut M, Hamblin MR, Nonell S. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. J Med Chem. 2010;53:7796–7803. doi: 10.1021/jm1009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaputra P, Nkepang G, Watley R, You Y. Synthesis and in vitro biological evaluation of lipophilic cation conjugated photosensitizers for targeting mitochondria. Bioorg Med Chem. 2012;21:379–387. doi: 10.1016/j.bmc.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Rapozzi V, Zorzet S, Zacchigna M, Della Pietra E, Cogoi S, Xodo LE. Anticancer activity of cationic porphyrins in melanoma tumour-bearing mice and mechanistic in vitro studies. Mol Cancer. 2014;13:75–92. doi: 10.1186/1476-4598-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratkay LG, Waterfield JD, Hunt DW (2000) Photodynamic therapy in immune (non-oncological) disorders: focus on benzoporphyrin derivatives. BioDrugs 14:127–135 [DOI] [PubMed]
- Reiners JJ, Agostinis P, Berg K, Oleinick NL, Kessel D (2010) Assessing autophagy in the context of photodynamic therapy. Autophagy 6:7–18 [DOI] [PMC free article] [PubMed]
- Reinhard A, Sandborn WJ, Melhem H, Bolotine L, Chamaillard M, Peyrin-Biroulet L (2015) Photodynamic therapy as a new treatment modality for inflammatory and infectious conditions. Expert Rev Clin Immunol 11:637–657 [DOI] [PubMed]
- Remichkova M et al. (2016) Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes. Z Naturforsch C doi:10.1515/znc-2016-0119 [DOI] [PubMed]
- Ricchelli F, Franchi L, Miotto G, Borsetto L, Gobbo S, Nikolov P, Bommer JC, Reddi E. Meso-substituted tetra-cationic porphyrins photosensitize the death of human fibrosarcoma cells via lysosomal targeting. Int J Biochem Cell Biol. 2005;37:306–319. doi: 10.1016/j.biocel.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Rodrigues CF, Silva S, Azeredo J, Henriques M (2016a) Candida glabrata’s recurrent infections: biofilm formation during Amphotericin B treatment Lett Appl Microbiol 63:77–81 [DOI] [PubMed]
- Rodrigues ME, Silva S, Azeredo J, Henriques M (2016b) Novel strategies to fight Candida species infection. Crit Rev Microbiol 42:594–606 [DOI] [PubMed]
- Rosenkranz AA, Jans DA, Sobolev AS (2000) Targeted intracellular delivery of photosensitizers to enhance photodynamic efficiency. Immunol Cell Biol 78:452–464 [DOI] [PubMed]
- Ryazanova O, Zozulya V, Voloshin I, Glamazda A, Dubey I, Dubey L, Karachevtsev V. Interaction of a tricationic meso-substituted porphyrin with guanine-containing polyribonucleotides of various structures. Methods Appl Fluoresc. 2016;4:034005. doi: 10.1088/2050-6120/4/3/034005. [DOI] [PubMed] [Google Scholar]
- Rywkin S, Lenny L, Goldstein J, Geacintov NE, Margolis-Nunno H, Horowitz B (1992) Importance of type I and type II mechanisms in the photodynamic inactivation of viruses in blood with aluminum phthalocyanine derivatives. Photochem Photobiol 56:463–469 [DOI] [PubMed]
- Rywkin S et al. (1994) New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates. Photochem Photobiol 60:165–170 [DOI] [PubMed]
- Rywkin S, Ben-Hur E, Reid ME, Oyen R, Ralph H, Horowitz B (1995) Selective protection against IgG binding to red cells treated with phthalocyanines and red light for virus inactivation. Transfusion 35:414–420 [DOI] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Mroz P, Dai T, Huang Y-Y, Denis TGS, Hamblin MR. Photodynamic therapy for cancer and for infections: what is the difference? Isr J Chem. 2012;52:691–705. doi: 10.1002/ijch.201100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Krayer M, Sperandio FF, Huang L, Huang Y-Y, Holten D, Lindsey JS, Hamblin MR. Synthesis and evaluation of cationic bacteriochlorin amphiphiles with effective in vitro photodynamic activity against cancer cells at low nanomolar concentration. J. Porphyrins Phthalocyanines. 2013;17:73–85. doi: 10.1142/S108842461250126X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim G, Lee S, Kim YB, Kim CW, Oh YK. Enhanced tumor localization and retention of chlorin e6 in cationic nanolipoplexes potentiate the tumor ablation effects of photodynamic therapy. Nanotechnology. 2011;22:365101. doi: 10.1088/0957-4484/22/36/365101. [DOI] [PubMed] [Google Scholar]
- Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ. Photodynamic therapy: a new concept in medical treatment. Braz J Med Biol Res. 2000;33:869–880. doi: 10.1590/S0100-879X2000000800002. [DOI] [PubMed] [Google Scholar]
- Silva EM et al. (2005) Synthesis of cationic beta-vinyl substituted meso-tetraphenylporphyrins and their in vitro activity against herpes simplex virus type 1. Bioorg Med Chem Lett 15:3333–3337 [DOI] [PubMed]
- Simões C, Gomesa MC, Neves MGPMS, Cunha Â, Tomé JPC, Tomé AC, Cavaleiro JAS, Almeida A, Faustino MAF. Photodynamic inactivation of Escherichia coli with cationic meso-tetraarylporphyrins – the charge number and charge distribution effects. Catal Today. 2016;266:197–204. doi: 10.1016/j.cattod.2015.07.031. [DOI] [Google Scholar]
- Skovsen E, Snyder JW, Lambert JD, Ogilby PR. Lifetime and diffusion of singlet oxygen in a cell. J Phys Chem B. 2005;109:8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- Slomp AM, Barreira SM, Carrenho LZ, Vandresen CC, Zattoni IF, Ló SM, Dallagnol JC, Ducatti DR, Orsato A, Duarte ME, Noseda MD, Otuki MF, Gonçalves AG. Photodynamic effect of meso-(aryl)porphyrins and meso-(1-methyl-4-pyridinium)porphyrins on HaCaT keratinocytes. Bioorg Med Chem Lett. 2017;27:156–161. doi: 10.1016/j.bmcl.2016.11.094. [DOI] [PubMed] [Google Scholar]
- Smetana Z, Mendelson E, Manor J, van Lier JE, Ben-Hur E, Salzberg S, Malik Z (1994) Photodynamic inactivation of herpes viruses with phthalocyanine derivatives. J Photochem Photobiol B 22:37–43 [DOI] [PubMed]
- Smetana Z, Malik Z, Orenstein A, Mendelson E, Ben-Hur E (1997) Treatment of viral infections with 5-aminolevulinic acid and light. Lasers Surg Med 21:351–358 [DOI] [PubMed]
- Smetana Z, Ben-Hur E, Mendelson E, Salzberg S, Wagner P, Malik Z (1998) Herpes simplex virus proteins are damaged following photodynamic inactivation with phthalocyanines. J Photochem Photobiol B 44:77–83 [DOI] [PubMed]
- Smith K, Malatesti N, Cauchon N, Hunting D, Lecomte R, van Lier J, Greenman J, Boyle RW. Mono- and tri-cationic porphyrin–monoclonal antibody conjugates: photodynamic activity and mechanism of action. Immunology. 2011;132:256–265. doi: 10.1111/j.1365-2567.2010.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler DC, Ohtola J, Sugiyama H, Rodriguez ME, Han L, Oleinick NL, Lam M, Baron ED, Cooper KD, McCormick TS (2016) Activated T cells exhibit increased uptake of silicon phthalocyanine Pc 4 and increased susceptibility to Pc 4-photodynamic therapy-mediated cell death. Photochem Photobiol Sci 15:822–831 [DOI] [PMC free article] [PubMed]
- Sperandio FF, Huang YY, Hamblin MR. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov. 2013;8:108–120. doi: 10.2174/1574891X113089990012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spesia MB, Lazzeri D, Pascual L, Rovera M, Durantini EN. Photoinactivation of Escherichia coli using porphyrin derivatives with different number of cationic charges. FEMS Immunol Med Microbiol. 2005;44:289–295. doi: 10.1016/j.femsim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Spring BQ, Rizvi I, Xu N, Hasan T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem Photobiol Sci. 2015;14:1476–1491. doi: 10.1039/C4PP00495G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamati I, Kuimova MK, Lion M, Yahioglu G, Phillips D, Deonarain MP. Novel photosensitisers derived from pyropheophorbide-a: uptake by cells and photodynamic efficiency in vitro. Photochem Photobiol Sci. 2010;9:1033–1041. doi: 10.1039/c0pp00038h. [DOI] [PubMed] [Google Scholar]
- Staneloudi C, Smith KA, Hudson R, Malatesti N, Savoie H, Boyle RW, Greenman J. Development and characterization of novel photosensitizer: scFv conjugates for use in photodynamic therapy of cancer. Immunology. 2007;120:512–517. doi: 10.1111/j.1365-2567.2006.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern PL et al. (2012) Therapy of human papillomavirus-related disease. Vaccine 30 Suppl 5:F71–82 [DOI] [PMC free article] [PubMed]
- Stolik S, Delgado JA, Pérez A, Anasagasti L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J Photochem Photobiol B. 2000;57:90–93. doi: 10.1016/S1011-1344(00)00082-8. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Mroz P, Dai T, Huang L, Morimoto Y, Kinoshita M, Yoshihara Y, Nemoto K, Shinomiya N, Seki S, Hamblin MR. Photodynamic therapy Can induce a protective innate immune response against murine bacterial arthritis via neutrophil accumulation. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0039823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kataoka H, Yano S, Sawada T, Akashi H, Inoue M, Suzuki S, Inagaki Y, Hayashi N, Nishie H, Shimura T, Mizoshita T, Mori Y, Kubota E, Tanida S, Takahashi S, Joh T. Immunogenic cell death due to a new photodynamic therapy (PDT) with glycoconjugated chlorin (G-chlorin) Oncotarget. 2016;7:47242–47251. doi: 10.18632/oncotarget.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao XH, Guan Y, Shao D, Xue W, Ye FS, Wang M, He MH (2014) Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia: a systemic review. Photodiagnosis Photodyn Ther 11:104–112 [DOI] [PubMed]
- Tarabukina IS, Startseva OM, Patov SA, Belykh DV. Novel dicationic chlorin e6 derivatives. Macroheterocycles. 2015;8:168–176. doi: 10.6060/mhc150456b. [DOI] [Google Scholar]
- Tavares A, Carvalho CMB, Faustino MA, Neves MGPMS, Tomé JPC, Tomé AC, Cavaleiro JAS, Cunha A, Gomes NCM, Alves E, Almeida A. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng IT, Chang YJ, Wang LS, Lu HY, Wu LC, Yang CM, Chiu CC, Yang CH, Hsu SL, Ho JA. Phospholipid-functionalized mesoporous silica nanocarriers for selective photodynamic therapy of cancer. Biomaterials. 2013;34:7462–7470. doi: 10.1016/j.biomaterials.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Theodossiou TA, Gonçalves AR, Yannakopoulou K, Skarpen E, Berg K. Photochemical internalization of tamoxifens transported by a “Trojan-horse” nanoconjugate into breast-cancer cell lines. Angew Chem Int Ed. 2015;54:4885–4889. doi: 10.1002/anie.201500183. [DOI] [PubMed] [Google Scholar]
- Tim M (2015) Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J Photochem Photobiol B 150:2–10 [DOI] [PubMed]
- Tome JP et al. (2005) Synthesis of glycoporphyrin derivatives and their antiviral activity against herpes simplex virus types 1 and 2. Bioorg Med Chem 13:3878–3888 [DOI] [PubMed]
- Tome JP et al. (2007) Synthesis of neutral and cationic tripyridylporphyrin-D-galactose conjugates and the photoinactivation of HSV-1. Bioorg Med Chem 15:4705–4713 [DOI] [PubMed]
- Tovmasyan A, Babayan N, Poghosyan D, Margaryan K, Harutyunyan B, Grigoryan R, Sarkisyan N, Spasojevic I, Mamyan S, Sahakyan L, Aroutiounian R, Ghazaryan R, Gasparyan G (2014) Novel amphiphilic cationic porphyrin and its Ag(II) complex as potential anticancer agents. J Inorg Biochem 140:94–103 [DOI] [PMC free article] [PubMed]
- Vaidya A, Sun Y, Ke T, Jeong EK, Lu ZR. Contrast enhanced MRI-guided photodynamic therapy for site-specific cancer treatment. Magn Reson Med. 2006;56:761–767. doi: 10.1002/mrm.21009. [DOI] [PubMed] [Google Scholar]
- van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ (2017) Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers (Basel) 9:19 [DOI] [PMC free article] [PubMed]
- Vicente MGH. Porphyrin-based sensitizers in the detection and treatment of cancer: recent progress. Curr Med Chem Anticancer Agents. 2001;1:175–194. doi: 10.2174/1568011013354769. [DOI] [PubMed] [Google Scholar]
- Wachowska M, Gabrysiak M, Muchowicz A, Bednarek W, Barankiewicz J, Rygiel T, Boon L, Mroz P, Hamblin MR, Golab J. 5-Aza-2’-deoxycytidine potentiates antitumour immune response induced by photodynamic therapy. Eur J Cancer. 2014;50:1370–1381. doi: 10.1016/j.ejca.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP, Hamblin MR (2017) Photoantimicrobials-are we afraid of the light. Lancet Infect Dis 17:e49–e55 [DOI] [PMC free article] [PubMed]
- Wallis C, Melnick JL (1963) Photodynamic inactivation of poliovirus. Virology 21:332–341 [DOI] [PubMed]
- Wallis C, Sakurada N, Melnick JL (1963) Influenza vaccine prepared by photodynamic inactivation of virus. J Immunol 91:677–682 [PubMed]
- Wallis C, Scheiris C, Melnick JL (1967) Photodynamically inactivated vaccines prepared by growing viruses in cells containing neutral red. J Immunol 99:1134–1139 [PubMed]
- Wang I, Bendsoe N, Klinteberg CA, Enejder AM, Andersson-Engels S, Svanberg S, Svanberg K (2001) Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol 144:832–840 [DOI] [PubMed]
- Wang S, Gao R, Zhou F, Selke M. Nanomaterials and singlet oxgen photosensitizers: potential applications in photodynamic therapy. J Mater Chem. 2004;14:487–493. doi: 10.1039/b311429e. [DOI] [Google Scholar]
- Wang YY, Ryu AR, Jin S, Jeon YM, Lee MY (2017) Chlorin e6-Mediated Photodynamic Therapy Suppresses P. acnes-Induced Inflammatory Response via NFκB and MAPKs Signaling Pathway. PLoS ONE 12:e0170599 [DOI] [PMC free article] [PubMed]
- Wei C, Jia G, Zhou J, Han G, Li C. Evidence for the binding mode of porphyrins to G-quadruplex DNA. Phys Chem Chem Phys. 2009;11:4025–4032. doi: 10.1039/b901027k. [DOI] [PubMed] [Google Scholar]
- Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53:R61–R109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- World Health Organization Media Centre (2016) Antimicrobial resistance, Fact sheet. http://www.who.int/mediacentre/factsheets/fs194/en. Accessed 06 Feb 2017
- Wyld L, Reed MWR, Brown NJ (2001) Differential cell death response to photodynamic therapy is dependent on dose and cell type. Br J Cancer. 841384–1386 [DOI] [PMC free article] [PubMed]
- Yakubovskaya RI, Plotnikova EA, Plyutinskaya AD, Morozova NB, Chissov VI, Makarova EA, Dudkin SV, Lukyanets EA, Vorozhtsov GN. Photophysical properties and in vitro and in vivo photoinduced antitumor activity of cationic salts of meso-tetrakis(N-alkyl-3-pyridyl)bacteriochlorins. J Photochem Photobiol B. 2014;130:109–114. doi: 10.1016/j.jphotobiol.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Yang Y-T, Chien H-F, Chang P-H, Chen C-T. Photodynamic inactivation of chlorin e6-loaded CTAB-liposomes against Candida albicans. Lasers Surg Med. 2013;45:175–185. doi: 10.1002/lsm.22124. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hu Y, Wang H. Targeting antitumor immune response for enhancing the efficacy of photodynamic therapy of cancer: recent advances and future perspectives. Oxidative Med Cell Longev. 2016;2016:1–11. doi: 10.1155/2016/5274084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A, Aviezer D, Gross Z, Tikva P. Pharmaceuticals compositions comprising porphyrins and some novel porphyrin derivatives. United States Patent. 2004;6(730):666 B1. [Google Scholar]
- Yoon I, Li JZ, Shim YK. Advance in photosensitizers and light delivery for photodynamic therapy. Clin Endocrinol. 2013;46:7–23. doi: 10.5946/ce.2013.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Xu LC, Huang JW, Zheng KC, Fu B, Yu HC, Ji LN. Tricationic pyridium porphyrins appending different peripheral substituents: experimental and DFT studies on their interactions with DNA. Biophys Chem. 2008;135:102–109. doi: 10.1016/j.bpc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Zupan K, Herenyi L, Toth K, Egyeki M, Csik G (2005) Binding of cationic porphyrin to isolated DNA and nucleoprotein complex: quantitative analysis of binding forms under various experimental conditions. Biochemistry 44:15000–15006 [DOI] [PubMed]
- Zupan K, Egyeki M, Toth K, Fekete A, Herenyi L, Modos K, Csik G (2008) Comparison of the efficiency and the specificity of DNA-bound and free cationic porphyrin in photodynamic virus inactivation. J Photochem Photobiol B 90:105–112 [DOI] [PubMed]