Abstract
Icariin is a prenylated flavonol glycoside derived from the Chinese herb Epimedium sagittatum. This study investigated the mechanism by which icariin prevents H2O2-induced apoptosis in rat nucleus pulposus (NP) cells. NP cells were isolated from the rat intervertebral disc and they were divided into five groups after 3 passages: (A) blank control; (B) 200 μM H2O2; (C) 200 μM H2O2 + 20 μM icariin; (D) 20 μM icariin + 200 μM H2O2 + 25 μM LY294002; (E) 200 μM H2O2 + 25 μM LY294002. LY294002 is a selective inhibitor of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway. NP cell viability, apoptosis rate, intracellular reactive oxygen species levels, and the expression of AKT, p-AKT, p53, Bcl-2, Bax, caspase-3 were estimated. The results show that, compared with the control group, H2O2 significantly increased NP cell apoptosis and the level of intracellular ROS. Icariin pretreatment significantly decreased H2O2-induced apoptosis and intracellular ROS and upregulated p-Akt and BCL-2 and downregulated caspase-3 and Bax. LY294002 abolished the protective effects of icariin. Our results show that icariin can attenuate H2O2-induced apoptosis in rat nucleus pulposus cells and PI3K/AKT pathway is at least partly included in this protection effect.
1. Introduction
Low back pain (LBP) is a frequent musculoskeletal disorder worldwide that affects approximately 70% of the adult population sometime in their lives, and most result in musculoskeletal disability. LBP can cause an enormous economic burden every year. The societal cost (converted into 2008 prices) of back pain is estimated at £12.3 billion in the United Kingdom (£1.6 billion for direct healthcare resources, £1.6 billion related to informal care, and indirect costs of £9.1 billion through loss of productivity due to morbidity) and €16.5–50 billion in Germany [1–7]. Therefore, LBP is a major disease that severely impacts human health and results in an enormous strain on limited medical resources [6].
Intervertebral disc (IVD) degeneration is considered to be one of the main reasons for LBP [8]. Nucleus pulposus (NP) cells are of critical importance in maintaining the biomechanical properties of IVDs. A decreased number of NP cells and changes in the extracellular matrix composition are early pathologic signs of IVD degeneration. Moreover, reversal of IVD degeneration is almost impossible due to the poor self-repairing ability of NP tissues.
Icariin is one the most frequently prescribed medicinal herbs in traditional Chinese medicine. Qianggu Capsule, which contains icariin, is used to treat postmenopausal and posttraumatic osteoporosis. An increasing number of articles have reported that icariin possesses multiple biological activities, such as improvement of drug resistance to chemotherapy [9], tumor-suppression, and antioxidation. Additionally, we find that icariin has an initiative pathophysiological effect on the reversal of IVD degeneration. We speculate that this results from a protective effect of the icariin on NP cells. Icariin can prevent apoptosis and oxidative stress in several cell models. Because the selective phosphoinositide 3-kinase (PI3K) inhibitor, LY294002, can reverse this effect; the protection seen with icariin appears to occur through activation of the PI3K/Akt signaling pathway [10], implying that icariin activates this pathway.
In the current study, H2O2 was used to induce apoptosis of NP cell with or without pretreatment with icariin. Our findings suggest that H2O2 induces apoptosis in NP cells and icariin prevents it through the PI3K/AKT signaling pathway. Our results provide an initiative method for the treatment of IVD degeneration disease.
2. Materials and Methods
2.1. General Supplies
Instruments, reagents, and experimental animals were provided by the animal center of Tongji Medical College and Huazhong University of Science and Technology. H2O2 was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Icariin (purity ≥ 98%) was purchased from Nanjing Zelang Pharmaceutical Technology (Nanjing, China). Fetal bovine serum was purchased from Thermo Fisher. F12-Dulbecco's modified Eagle medium was purchased from HyClone (Logan, UT, USA). Cell counting kit-8 (CCK8) was purchased from Kaiji Bioengineering Institute (Jiangsu, China). LY294002 was purchased from Sigma-Aldrich (St. Louis, MO, USA). The reactive oxygen species (ROS) detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The Annexin V-FITC/propidium iodide detection kit was purchased from Nanjing KeyGen Biotech (Nanjing, China). β-Actin, Bcl-2, bax, caspase-3, phospho(p)-Akt, rabbit monoclonal antibodies, and the p53, Akt mouse monoclonal antibody, were purchased from Abcam (Cambridge, UK). Goat anti-rabbit and goat anti-mouse IgG were purchased from Proteintech (Wuhan, China). Microplate Reader was purchased from Thermo. Inverted fluorescence microscope was from Olympus, Japan.
2.2. Culture and Synchronization of the NP Cells and the Detection of Cell Density and Morphology
The density and morphology of NP cells under different treatments were observed and photographed with an inverted phase-contrast microscope. NP cells were isolated using about 200 g NP tissue of rats. Briefly, NP tissue was aseptically removed in a Petri dish containing 0.25% (w/v) type II collagenase and cut into pieces 0.1 mm × 0.1 mm. Then samples were digested with 0.25% (w/v) type II collagenase for 15–20 min and serum was used to stop the reaction. After centrifugation at 1200 rpm for 7 min, the supernatant was discarded and the pellet was resuspended in F12-Dulbecco's modified Eagle medium supplemented with 20% fetal bovine serum, 100 U/mL penicillin, and 100 mg/L streptomycin. Cell cultures were maintained at 37°C and 5% CO2. Medium was changed 3–5 days later when the cells had been attached and then changed every other day. When NP cells reached approximately 80% confluence, each primary culture was subcultured at a 1 : 3 ratio with a 0.25% (w/v) trypsin solution.
2.3. Experimental Protocols
First cells were tested for the ability of icariin to activate the PI3K/AKT pathway. Kinetics of the phosphorylation of AKT were estimated by Western blot analysis at 0 h, 1 h, 2 h, 3 h, 4 h, and 5 h. Other cells were randomly separated into five groups with at least three replicates: (A) blank control; (B) 200 μM H2O2; (C) 20 μM icariin + 200 μM H2O2; (D) 20 μM icariin + 25 μM LY294002 + 200 μM H2O2; (E) 25 μM LY294002 + 200 μM H2O2. Treatment with LY294002, icariin, and H2O2 was performed for 2 h, 24 h, and 6 h, respectively.
2.4. Detection of Icariin Cytotoxicity and Cell Viability and Proliferation
NP cells at passage 3 were replated in 96-well plates at a density 1 × 105 cells per well, and the culture medium was plated after synchronization. Cells were then treated with icariin for 24 h at various concentrations (0.1, 0.5, 1, 5, 10, 20, 40, and 50 μM). Cell viability was detected according to the instructions of the CCK8 assay. Then cells were treated according to the aforementioned experimental groupings. Cell viability was detected according to the manufacturer's instructions.
2.5. Apoptosis Assay
Cells were harvested and washed with PBS twice at 4°C. Next, cells were resuspended in 200 μL of binding buffer and incubated with 10 μL of Annexin V-FITC solution (15 min, room temperature) in the dark. Then cells were incubated with 10 μL PI and 300 μL Binding Buffer and immediately analyzed in a BD FACSCalibur cytometer to separate living cells, apoptotic cells, and necrotic cells in different periods.
2.6. Detection of Intracellular ROS Levels by Flow Cytometry
Cells were treated differently according to the aforementioned experiment grouping design. Then 200 μL of culture medium from each group was gathered to detect intracellular ROS levels. Experimental steps were strictly executed according to the manufacturer's instructions.
2.7. Expression of Akt, p-Akt, p53, Bcl-2, Bax, and Caspase-3 by Western Blot Analysis
Proteins were extracted according to the instructions of the Total Extraction Sample Kit. Equal amounts of proteins (10 μg) were loaded onto 10% sodium dodecyl sulfate polyacrylamide gels, electrophoresed, and then transferred to polyvinylidene fluoride membranes. The membranes were incubated with 5% nonfat milk for 2 h followed by incubation with primary antibodies overnight at 4°C (0.5 μg/mL Akt, p53, p-Akt, Bcl-2, and caspase-3; 1 : 5000). After washing in TBST, membranes were incubated with the secondary antibody for 1.5 h at room temperature (rabbit anti-mouse or goat anti-rabbit, 1 : 5000). Bands were visualized by incubating with enhanced chemiluminescence reagent for 2 min after membranes were washed with TBST. Densitometry of p-Akt, Akt, p53, Bcl-2, and bax as well as caspase-3 levels was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.8. Statistical Analysis
Data are presented as means ± standard deviation. For group-wise comparisons, a one-way ANOVA with the LSD or Dunnett's T3 test was performed using SPSS 19.0 (IBM, Chicago, IL, USA). Values were considered significantly different for p < 0.05.
3. Results
3.1. H2O2 Induced Apoptosis in NP Cells
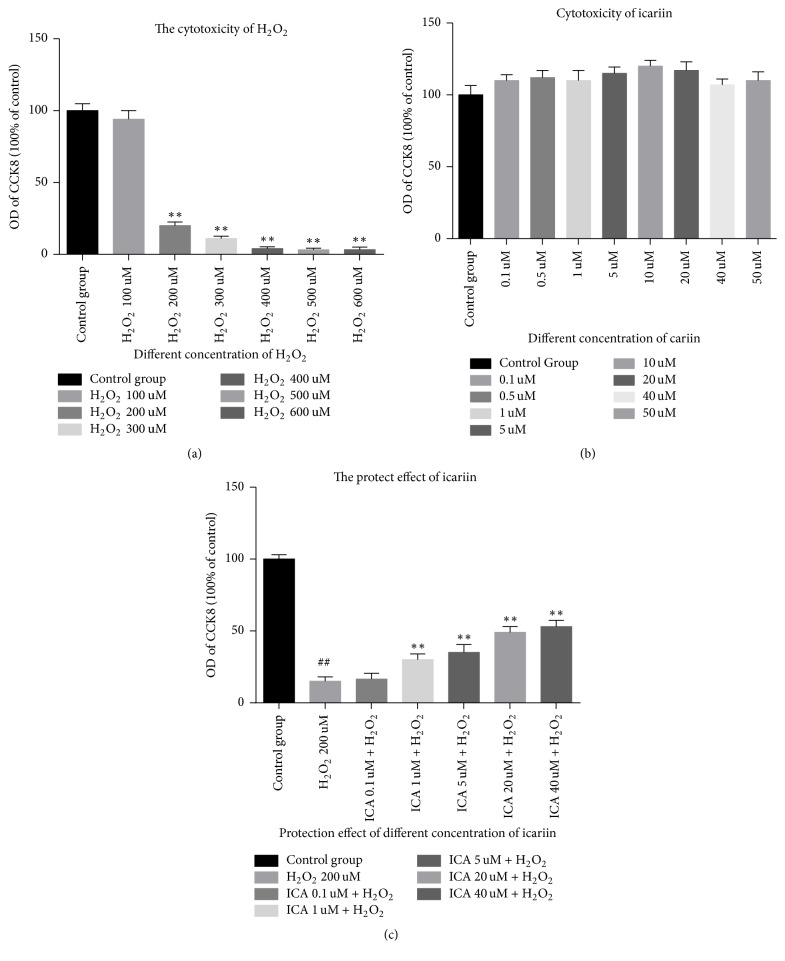
H2O2 can lead to cell dead and icariin alone has no cytotoxicity in NP cells; what is more, icariin can protect against the H2O2-induced cytotoxicity. Our date revealed the best concentration of H2O2 which can lead to a favourable cell apoptosis is 200 μM (Figure 1(a)). The viability and proliferation of cells treated with icariin for 24 h at various concentrations (0.1, 0.5, 1, 5, 10, 20, 40, and 50 μM) were not significantly different (p > 0.05) compared to the control group (Figure 1(b)). In addition, icariin provided significant protection when NP cells were exposed to 200 μM H2O2 (Figure 1(c)). The results of the above are detected by CCK8.
Figure 1.
Icariin have no cytotoxicity in NP cells and had a significant protective effect on NP cells exposed to H2O2 at the concentration of 200 uM and this protective effect was attenuated by the PI3K/AKT pathway inhibitor LY294002. (a) H2O2 led to obvious cell death at the concentration of 200 uM to 600 uM. Results are presented as mean ± SD (∗∗p < 0.01 versus control group). (b) Icariin alone has no effect on the viability and proliferation of NP cells at concentrations up to 50 μM. (c) Icariin protects NP cells against H2O2-induced toxicity (##p < 0.01 versus control group, ∗∗p < 0.01 versus H2O2 group).
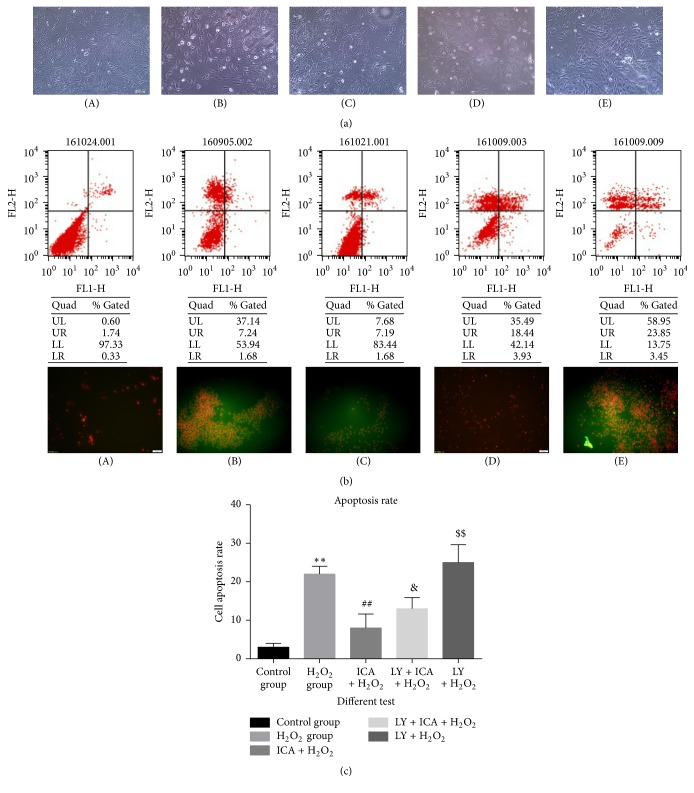
3.2. The Apoptosis Rate of Different Tests
The protective effect was weakened by a 1 h pretreatment with 20 μM of the PI3K/Akt pathway inhibitor, LY294002 (Figure 2). As shown in Figure 2, cells treated with H2O2 had a smaller size than control cells and a high percentage of dead cells in the population. This observation was confirmed by fluorescence microscopy and flow cytometry after Annexin V/PI staining of the cells (Figure 2(a)). Morphologic changes and apoptosis were also evident by fluorescence microscopy and flow cytometry with Annexin V/propidium iodide staining (Figure 2(b)). When cells were pretreated with icariin, the number of dead cells was decreased and cell morphology was similar to that of control cells. Groups (D) (200 μM H2O2 + 20 μM Ica) and (E) (200 μM H2O2 + 20 μM Ica + 25 μM LY294002) reveal that the cells became shrunk and there were many dead cells. This revealed that the protection by icariin was prevented by inhibiting the PI3K/Akt pathway (p < 0.05).
Figure 2.
The sectionalization: (A) blank control. (B) 200 μM H2O2. (C) 200 μM H2O2 + 20 μM icariin (Ica). (D) 200 μM H2O2 + 20 μM Ica. (E) 200 μM H2O2 + 20 μM Ica + 25 μM LY294002. (a) Phase-contrast light microscopy observations: the cell number of group (B) is decreased, cells are more shrunk, and there were many round dead cells. In group (C), there were fewer dead cells and the cell morphology was more normal than in group (B). However, in group (D), cells shrank and there were many dead cells. (b) Apoptosis detected by flow cytometry and fluorescence microscope: the extent of apoptosis significantly declined in group (C) compared with group (B). In group (D), the extent of apoptosis increased compared with group (C). Group (E) exhibited the most apoptotic cells of all groups. (c) Apoptosis rate: the apoptosis rate of H2O2 group increased significantly and the cytotoxicity of H2O2 is weakened by icariin (∗∗p < 0.01 versus control group; ##p < 0.01 versus H2O2 group). In addition, the lock of PI3/AKT pathway made a dent in the protection effect of icariin (&p < 0.05 versus group (C)). Group (E) (25 μM LY294002 + 200 μM H2O2): this group exhibited more apoptotic cells ($$p < 0.01 versus group (D)).
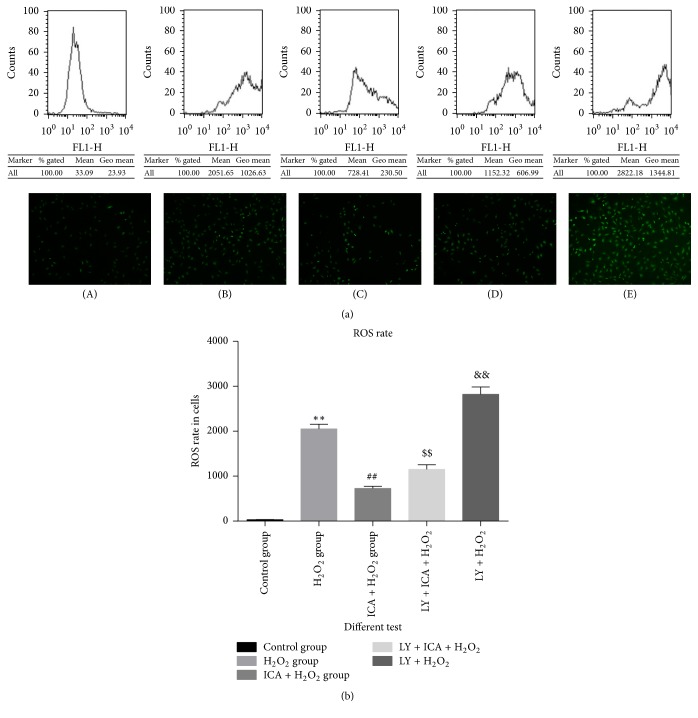
3.3. The Intracellular ROS Rate of Different Tests
With the different interventions of the (A)–(E) groups, ROS levels in NP cells varied in parallel with the extent of apoptosis (Figure 3). Similar results were observed by both fluorescence microscopy and flow cytometry. Compared to the control group, cells treated with 200 μM H2O2 showed a significant increase of intracellular ROS levels (p < 0.05). Cells pretreated with icariin showed intracellular ROS levels significantly lower compared to group (B) (p < 0.05). Intracellular ROS levels of group (D) cells (200 μM H2O2 + 20 μM Ica +25 μM LY294002) were risen compared to group (C) (p < 0.05), which demonstrates that the PI3K/AKT pathway participates in the process. Group (E) cells (25 μM LY294002 + 200 μM H2O2) showed the highest ROS levels among all groups (p < 0.05), which indicates that blocking the PI3K/Akt pathway itself can be deleterious for the cells.
Figure 3.
The sectionalization: (A) blank control. (B) 200 μM H2O2. (C) 200 μM H2O2 + 20 μM icariin (Ica). (D) 200 μM H2O2 + 20 μM Ica. (E) 200 μM H2O2 + 20 μM Ica + 25 μM LY294002. (a) Flow cytometry detection and fluorescence microscope observations: the intracellular ROS dye of group (B) rises. In group (C), there were fewer intracellular ROS than in group (B). However, in groups (D) and (E), intracellular ROS dye increased. (b) ROS rate: the ROS rate of H2O2 group increased significantly and the cytotoxicity of H2O2 is weakened by icariin (∗∗p < 0.01 versus control group; ##p < 0.01 versus H2O2 group). In addition, the lock of PI3/AKT pathway made a dent in the protection effect of icariin ($$p < 0.01 versus group (C)). Group (E) showed the highest ROS levels among all groups, which indicates LY294002 acts synergistically to H2O2 in elevating intracellular ROS (&&p < 0.01 versus group (B)).
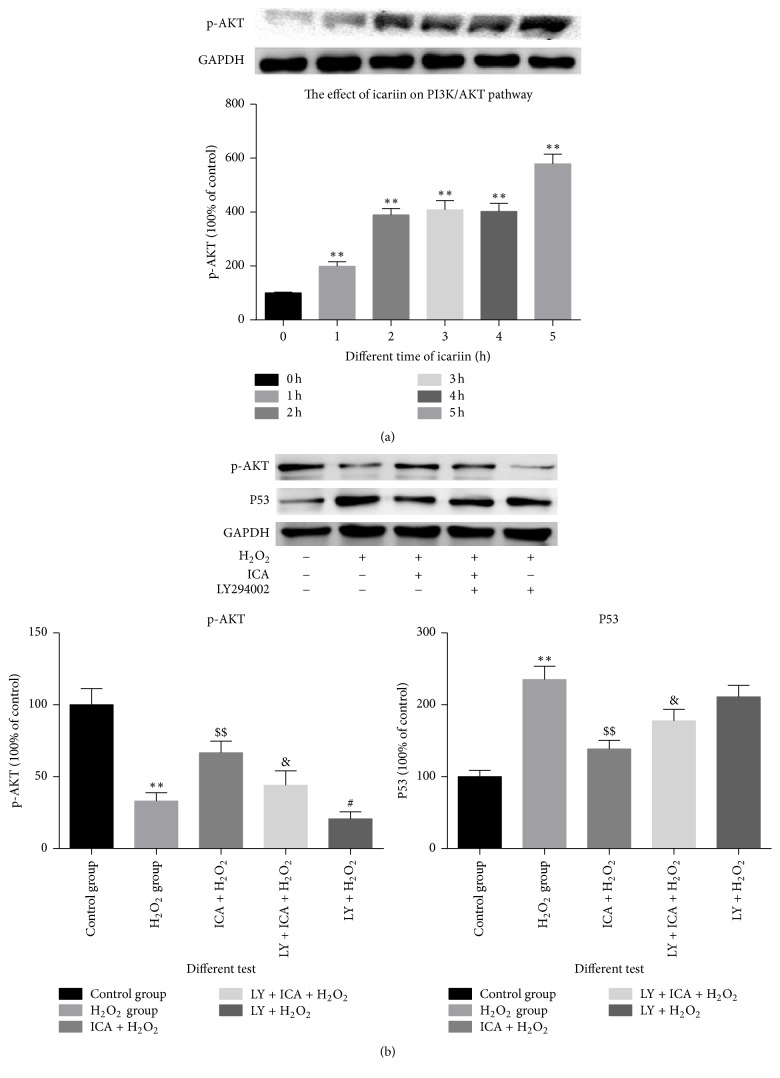
3.4. The Activation of PI3K/AKT Pathway
We observed a time-dependent activation of the PI3K/AKT pathway when NP cells were treated with icariin (p < 0.05) (Figure 4). Also, with different interventions, p-AKT and p53, the iconic molecules of PI3K/AKT, showed the inverse variation trendy with apoptosis rate, indicating that the activation of PI3K/AKT is a protection factor in the H2O2-induced apoptosis (Figure 4(b)).
Figure 4.
The effect of icariin on the PI3K/Akt pathway. (a) Prolonged culturing of NP cells with icariin increases the expression of p-Akt (∗∗p < 0.01 versus control group). (b) p-Akt and p53 levels in NP cells treated with different agents (∗∗p < 0.01 versus control group; $$p < 0.01 versus H2O2 group; &p < 0.05 versus group (C); #p < 0.05 versus group (B)). Data are expressed as means ± SD (n = 3).
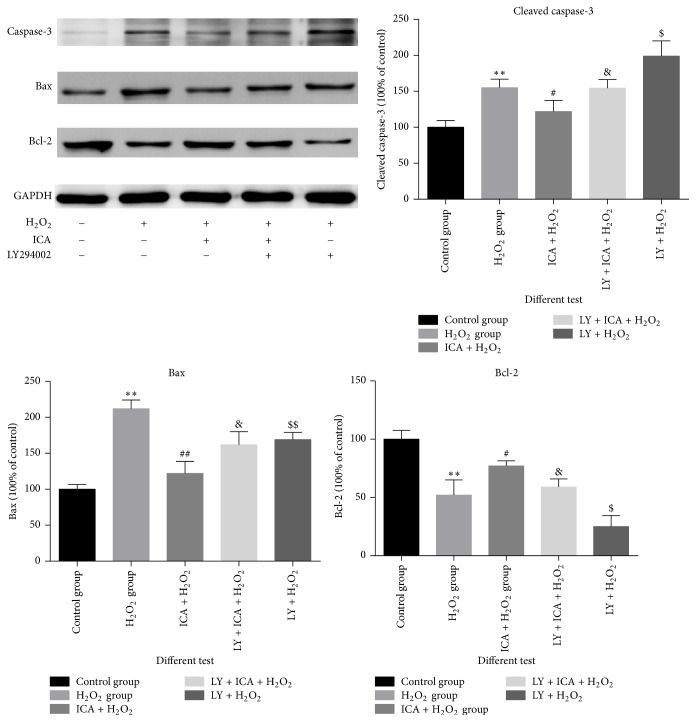
3.5. The Expression of Proteins in Apoptosis Pathway
The expression of caspase-3, Bax, and Bcl-2 proteins is shown in Figure 5. The antiapoptosis protein bcl-2 decreased when tested with H2O2 and increased when pretreated with icariin and decreased when the PI3K/AKT pathway was blocked (p < 0.05). In contrast, proapoptotic proteins caspase-3 and Bax were decreased in icariin-treated cells. Together, these results showed that icariin had a significant protective effect when NP cells were exposed to H2O2 and this protection could be impaired by LY294002.
Figure 5.
Icariin protects nucleus pulposus (NP) cells from H2O2-induced apoptosis. Bcl-2, Bax, and caspase-3 were detected by Western blotting. Bcl-2 levels are descended in NP cells treated with H2O2 (∗∗p < 0.01 versus control group) and Bcl-2 levels are increased in NP cells treated with H2O2 and icariin when compared with the H2O2 alone group (#p < 0.05 versus H2O2 group) and decreased when the PI3K/Akt pathway is blocked with LY294002 ($p < 0.05 versus group (C)). Caspase-3 and Bax are decreased in icariin-treated cells (∗∗p < 0.05 versus control group). The protective effect of icariin could be impaired by the presence of LY294002 to block the PI3K/Akt pathway (##p < 0.01 versus H2O2 group, &p < 0.05 versus group (C)). In addition, LY294002 alone exacerbates H2O2-induced damage ($$p < 0.01, $p < 0.05 versus H2O2 group).
4. Discussion
Intervertebral disc degeneration is the most important reason for LBP. There are extensive reports about the pathogenesis of spinal degeneration and the primary therapies currently in use. These include Western medicine, surgical operations, and IVD tissue engineering [11–13]. However, there are few reports on the effect of traditional Chinese medicines, such as icariin, on NP and annulus fibrosus cells, although, as an old Chinese traditional medicine, icariin has been reported to benefit osteogenesis in vivo [14], accelerate the differentiation of osteoblast and mesenchymal stem cells [15], and protect neurocytes [16]. Additional effects are described in numerous review articles [9, 15, 17–24].
The current study is the first report of the effect and the possible mechanism of icariin in NP cells exposed to H2O2. We believe the mechanism of the protective effect of icariin may involve activation of the PI3K/Akt pathway. Icariin attenuates cigarette smoke-mediated oxidative stress in human lung epithelial cells [17], inhibits neurotoxicity in PC12 cells [16], and activates rat bone marrow cells [15]. These results suggest an activation effect of icariin on the PI3K/Akt pathway.
The IVD has no blood vessels to provide nutrition. Thus, degeneration will ensue if it is exposed to acid or oxidative stress. There are reports that oxidative stress caused by H2O2 can lead to apoptosis of NP cells [25–33]. In this work, we chose H2O2 to simulate the physiopathological environment with oxidative stress in vitro, because, actually, the NP cells have to face the oxidative stress in vivo. We chose the concentration of 200 μM because this is the lowest concentration that can induce apoptosis of NP cells. We found that, even at 600 μM of H2O2, NP cells presented the state of necrosis and that, as the concentration of H2O2 increased from 200 μM to 600 μM, the NP cells showed similar apoptosis state. Related results had also been measured by the methods of CCK8.
Then we found that icariin provides substantial concentration-dependent protection to NP cells exposed to H2O2 by means of the CCK8 assay. The greatest protection was seen at 40 μM when these cells were exposed to 200 μM H2O2. Protection could also be observed by light microscopy. Importantly, icariin alone elicited no cytotoxic effect on NP cells at concentrations up to 50 μM.
We used Western blotting to explore the pathways activated in NP cells treated with icariin. We observed an increase in p-Akt, which is the active form in the PI3K/Akt signaling pathway. Because icariin activated the PI3K/AKT pathway in NP cells, we conducted a series of experiments including measurements of ROS levels, apoptosis, apoptosis-related molecules (caspase-3, Bcl-2, and Bax), and the proteins included in PI3K/AKT pathway such as p-AKT and p53. Changes in each of these parameters reflected the protective effect of icariin when NP cells were exposed to 200 μM H2O2. Importantly, all changes were diminished in cells treated with LY294002, an inhibitor of PI3K. Although the results with a single pharmacologic agent are insufficient to conclude that the PI3K/Akt pathway is the most important or only mechanism involved in the protective effect of icariin, it is clear that this pathway is a factor. The potential role of other pathways and cytokines requires more research, such as MAPK [34, 35] and HIF-1α [36] and NF-kappaB and AF-1 [37],which reportedly can be stimulated by icariin. Furthermore, the extracellular matrix of NP cells has not been examined and there may be some differences between the control and experimental groups.
In summary, this is the first report demonstrating the protective effect of icariin on NP cells exposed to H2O2. In addition, we have proposed a possible mechanism for this protection involving the PI3K/Akt pathway. These results may suggest new approaches to prevent spinal degeneration and decrease the damage that is caused by the imbalance of oxidative stress.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81472134).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Huang Y.-C., Leung V. Y. L., Lu W. W., Luk K. D. K. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine Journal. 2013;13(3):352–362. doi: 10.1016/j.spinee.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Karl M. O., Reh T. A. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends in Molecular Medicine. 2010;16(4):193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagenais S., Caro J., Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. The Spine Journal. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Hong J., Reed C., Novick D., Happich M. Costs associated with treatment of chronic low back pain: an analysis of the UK general practice research database. Spine. 2013;38(1):75–82. doi: 10.1097/brs.0b013e318276450f. [DOI] [PubMed] [Google Scholar]
- 5.Maniadakis N., Gray A. The economic burden of back pain in the UK. Pain. 2000;84(1):95–103. doi: 10.1016/S0304-3959(99)00187-6. [DOI] [PubMed] [Google Scholar]
- 6.Juniper M., Le T. K., Mladsi D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opinion on Pharmacotherapy. 2009;10(16):2581–2592. doi: 10.1517/14656560903304063. [DOI] [PubMed] [Google Scholar]
- 7.Breivik H., Eisenberg E., O'Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13(1, article 1229) doi: 10.1186/1471-2458-13-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana G., See E., Pandit A. Current trends in biologics delivery to restore intervertebral disc anabolism. Advanced Drug Delivery Reviews. 2015;84:146–158. doi: 10.1016/j.addr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Yang L., Xia Y., Guo C., Kong L. Icariin enhances cytotoxicity of doxorubicin in human multidrug-resistant osteosarcoma cells by inhibition of ABCB1 and down-regulation of the PI3K/Akt pathway. Biological and Pharmaceutical Bulletin. 2015;38:277–284. doi: 10.1248/bpb.b14-00663. [DOI] [PubMed] [Google Scholar]
- 10.Xie R. H., et al. Mechanism of chlorogenic acid on apoptosis of rat nucleus pulposus cells induced by oxidative stress. Zhong Yao Cai. 465-469: 37; 2014. [PubMed] [Google Scholar]
- 11.Colombier P., Camus A., Lescaudron L., Clouet J., Guicheux J. Intervertebral disc regeneration: a great challenge for tissue engineers. Trends in Biotechnology. 2014;32(9):433–435. doi: 10.1016/j.tibtech.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Silva-Correia J., Correia S. I., Oliveira J. M., Reis R. L. Tissue engineering strategies applied in the regeneration of the human intervertebral disk. Biotechnology Advances. 2013;31(8):1514–1531. doi: 10.1016/j.biotechadv.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Bowles R. D., Gebhard H. H., Härtl R., Bonassar L. J. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(32):13106–13111. doi: 10.1073/pnas.1107094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J., Tao Y., Ping Z., et al. Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/β-catenin signaling pathway in vivo and in vitro. Scientific Reports. 2016;6 doi: 10.1038/srep23827.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhai Y. K., Guo X. Y., Ge B. F., et al. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K–AKT–eNOS–NO–cGMP–PKG. Bone. 2014;66:189–198. doi: 10.1016/j.bone.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Zeng K.-W., Ko H., Yang H. O., Wang X.-M. Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology. 2010;59(6):542–550. doi: 10.1016/j.neuropharm.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Wu J., Xu H., Wong P. F., Xia S., Xu J., Dong J. Icaritin attenuates cigarette smoke-mediated oxidative stress in human lung epithelial cells via activation of PI3K-AKT and Nrf2 signaling. Food and Chemical Toxicology. 2014;64:307–313. doi: 10.1016/j.fct.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi H., Yu J., Hashimoto R., Ouchi Y., Okabe T. Involvement of androgen receptor in nitric oxide production induced by icariin in human umbilical vein endothelial cells. FEBS Letters. 2010;584(11):2440–2444. doi: 10.1016/j.febslet.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M.-C., Chen X.-H., Zhao X., Zhang X.-J., Chen W.-F. Involvement of IGF-1 receptor signaling pathway in the neuroprotective effects of Icaritin against MPP+-induced toxicity in MES23.5 cells. European Journal of Pharmacology. 2016;786:53–59. doi: 10.1016/j.ejphar.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Xu C.-Q., Liu B.-J., Wu J.-F., et al. Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NFκB signaling pathway. European Journal of Pharmacology. 2010;642(1–3):146–153. doi: 10.1016/j.ejphar.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D., Wang Z., Sheng C., et al. Icariin prevents amyloid beta-induced apoptosis via the pi3k/akt pathway in pc-12 cells. Evidence-Based Complementary and Alternative Medicine. 2015;2015 doi: 10.1155/2015/235265.235265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng X., Pei H., Lan C. Icariin exerts protective effect against myocardial ischemia/reperfusion injury in rats. Cell Biochemistry and Biophysics. 2015;73(1):229–235. doi: 10.1007/s12013-015-0669-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhai M., He L., Ju X., et al. Icariin acts as a potential agent for preventing cardiac ischemia/reperfusion injury. Cell Biochemistry and Biophysics. 2015;72(2):589–597. doi: 10.1007/s12013-014-0506-3. [DOI] [PubMed] [Google Scholar]
- 24.Duan X.-H., Xu C.-Q., Huang J.-H., Zhou W.-J., Sun B. Icariin delays homocysteine-induced endothelial cellular senescence involving activation of the PI3K/AKT-eNOS signaling pathway. Pharmaceutical Biology. 2013;51(4):433–440. doi: 10.3109/13880209.2012.738332. [DOI] [PubMed] [Google Scholar]
- 25.Chu H., Yu H., Ren D., Zhu K., Huang H. Plumbagin exerts protective effects in nucleus pulposus cells by attenuating hydrogen peroxide-induced oxidative stress, inflammation and apoptosis through NF-κB and Nrf-2. International Journal of Molecular Medicine. 2016;37(6):1669–1676. doi: 10.3892/ijmm.2016.2564. [DOI] [PubMed] [Google Scholar]
- 26.Dimozi A., Mavrogonatou E., Sklirou A., Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. European Cells & Materials. 2015;30:89–103. doi: 10.22203/eCM.v030a07. [DOI] [PubMed] [Google Scholar]
- 27.He B., Tao H., Liu S., Wei A. Protective effect of carboxymethylated chitosan on hydrogen peroxide-induced apoptosis in nucleus pulposus cells. Molecular Medicine Reports. 2015;11(3):1629–1638. doi: 10.3892/mmr.2014.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L., Rong Z., Zeng M., et al. Pyrroloquinoline quinone protects nucleus pulposus cells from hydrogen peroxide-induced apoptosis by inhibiting the mitochondria-mediated pathway. European Spine Journal. 2015;24(8):1702–1710. doi: 10.1007/s00586-014-3630-2. [DOI] [PubMed] [Google Scholar]
- 29.Chen J.-W., Ni B.-B., Li B., Yang Y.-H., Jiang S.-D., Jiang L.-S. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cellular Physiology and Biochemistry. 2014;34(4):1175–1189. doi: 10.1159/000366330. [DOI] [PubMed] [Google Scholar]
- 30.Xie R. H., Yin M., Yin C. C., et al. Mechanism of chlorogenic acid on apoptosis of rat nucleus pulposus cells induced by oxidative stress. Zhong Yao Cai. 2014;37(3):465–469. [PubMed] [Google Scholar]
- 31.Yang X., Jin L., Yao L., Shen F. H., Shimer A. L., Li X. Antioxidative nanofullerol prevents intervertebral disk degeneration. International Journal of Nanomedicine. 2014;9(1):2419–2430. doi: 10.2147/IJN.S60853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D., Wang D., Shimer A., Shen F. H., Li X., Yang X. Glutathione protects human nucleus pulposus cells from cell apoptosis and inhibition of matrix synthesis. Connective Tissue Research. 2014;55(2):132–139. doi: 10.3109/03008207.2013.876421. [DOI] [PubMed] [Google Scholar]
- 33.Xie J., Tong P.-J., Shan L.-T., Wu C.-L. Reseach of oxidative stress nduces aging in rabbit intervertebral disc nucleus pulposus cells injured by H2O2. China Journal of Orthopaedics and Traumatology. 2013;26(4):332–335. [PubMed] [Google Scholar]
- 34.Ding L., Liang X.-G., Zhu D.-Y., Lou Y.-J. Icariin promotes expression of PGC-1α, PPARα, and NRF-1 during cardiomyocyte differentiation of murine embryonic stem cells in vitro. Acta Pharmacologica Sinica. 2007;28(10):1541–1549. doi: 10.1111/j.1745-7254.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y., Xia L., Zhou Y., Xu Y., Jiang X. Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a MAPK-dependent manner. Cell Proliferation. 2015;48(3):375–384. doi: 10.1111/cpr.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P., Zhang F., He Q., et al. Flavonoid compound icariin activates hypoxia inducible factor-1α in chondrocytes and promotes articular cartilage repair. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0148372.e0148372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wo Y., Zhu D., Yu Y., Lou Y. Involvement of NF-κB and AP-1 activation in icariin promoted cardiac differentiation of mouse embryonic stem cells. European Journal of Pharmacology. 2008;586(1-3):59–66. doi: 10.1016/j.ejphar.2008.02.080. [DOI] [PubMed] [Google Scholar]