Abstract
Medicaid programs provide health insurance coverage for many patients with hepatitis C, a public health problem for which effective but very expensive treatments are now available. Facing constrained budgets, most states adopted prior authorization criteria for sofosbuvir, the first of these agents.
Using fee-for-service utilization data from 42 Medicaid programs in 2014, we found that strict behavioral criteria—those that limited coverage on the basis of drug or alcohol use and included specific abstinence or treatment requirements—were associated with significantly less spending on sofosbuvir.
Despite the potential cost savings, such criteria raise troubling questions in terms of public health as well as medical ethics, clinical evidence, and potentially federal law. Decision-makers should reject these requirements in Medicaid coverage policy and pursue national and state policy strategies to balance short-term budgetary realities with long-term public health benefits.
Sofosbuvir, the first polymerase inhibitor approved by the Food and Drug Administration, can achieve extremely high hepatitis C (HCV) cure rates of more than 90% with far less toxicity and shorter treatment duration than can traditional agents.1–4 As a well-tolerated, easily administered tablet used in combination with other medications, sofosbuvir is the first of a wave of new HCV medications that are significant improvements over traditional interferon-based regimens. However, the manufacturer has priced a standard treatment course in the United States at an estimated $84 000, or approximately $1000 per pill. National controversy over drug pricing has been compounded by the facts that similar treatment courses are far cheaper in other settings—for example, $50 000 to $60 000 in the United Kingdom and less than $1000 in some developing countries—and that the drug can be produced for approximately $3 per pill.5
Nearly 3 million Americans have been diagnosed with HCV, with half or more unaware of their diagnosis and approximately 1 million unaccounted for because of reasons such as homelessness or incarceration.6–8 Sofosbuvir’s approval coincided with US Preventive Services Task Force recommendations to broaden HCV screening, and therefore disease identification.9 Consequently, the impact of this agent and several other novel HCV treatments on total health care costs reflect the high drug prices multiplied by a large and increasing number of diagnosed patients across all types of insurance.10
BURDEN ON MEDICAID POPULATIONS
Although cost issues present challenges for all diagnosed patients, they are especially relevant for Medicaid beneficiaries, a low socioeconomic group with particularly high HCV disease burden compared with the general population. Prevalence of HCV in some Medicaid populations has been estimated at 7.5 times that of commercially insured populations,11 with the homeless and incarcerated representing another fraction of infected beneficiaries. In 2014, demand for new HCV medications in Medicaid populations helped drive a historic 13.1% surge in national prescription drug spending, the largest increase observed since 2001.12
At the programmatic level, state Medicaid programs also face more substantial budget constraints than do other insurers, which can particularly limit their ability to manage the use of sofosbuvir and other new HCV medications among large populations of patients clinically eligible to receive them. Initially, some programs approved sofosbuvir for use whereas others chose not to add it to their formularies, prompting some physicians to obtain direct subsidies from the drug manufacturer. By report, others pursued alternative strategies, including convincing their state to renegotiate or “carve out” sofosbuvir coverage through direct payment outside capitation.10
SOFOSBUVIR SPENDING BY MEDICAID PROGRAMS
Overall, there were rapid, widespread increases in sofosbuvir spending among state Medicaid programs in the first year after Food and Drug Administration approval.13 However, there was also substantial variation across programs, with sofosbuvir accounting for less than 0.5% of total program drug spending in some states and greater than 6.0% in others. Individual states also adjusted their coverage strategies during this period.
For example, New York—the largest Medicaid program by prescription drug spending—used more than 6% of total Medicaid medication spending on sofosbuvir in the first half of 2014 before its state health department enacted new coverage criteria.14,15 Using considerations of prescriber experience, psychosocial readiness of the patient for treatment, and disease prognosis and severity to determine drug appropriateness, the program reduced its spending on sofosbuvir in the second half of the year.
Treatment coverage restrictions have been adopted by many other Medicaid programs (as well as private and other public insurers) to address significant budget challenges. Approval criteria, however, can vary substantially.16,17 Among programs with sofosbuvir fee-for-service reimbursement criteria, for example, the majority restrict use on the basis of substance abuse history, disease severity, and prescriber type criteria.16 In some states, substance users must also demonstrate periods of abstinence before they can receive sofosbuvir, and the eligibility of patients with HIV infection can depend on their antiretroviral treatment status.
Decisions related to a potentially curative treatment that is appropriate for nearly all patients on the basis of medical evidence—decisions that may arise from a combination of medical evidence, budgetary considerations, and other unmeasured preferences17—pose significant ethical and public health considerations. Some, including the Centers for Medicare and Medicaid Services, have also raised concerns about the appropriateness and legality of applying such criteria under existing federal law requiring states to cover medications according to indications outlined on their Food and Drug Administration labels.16,18 However, the financial basis and impact of the variations in state program criteria remain unclear.
THE FINANCIAL IMPACT OF RESTRICTION
To understand the relationship between state utilization criteria and sofosbuvir spending, we evaluated drug spending by state Medicaid programs using aggregated, state-level data from the Centers for Medicare and Medicaid Services for calendar year 2014.19 The data include the number of prescriptions filled by calendar quarter, the number of medication units dispensed, and the fee-for-service and managed care Medicaid reimbursement (not accounting for rebates or cost offsets provided by manufacturers) for each medication, grouped by National Drug Code. They do not contain any identifiable or unidentifiable patient-level cost or clinical information.
These data are reported by state programs to the Centers for Medicare and Medicaid Services, which aggregates and provides files as publically available data on the Medicaid Web site (www.Medicaid.gov). We excluded one quarter with implausible values as an erroneous entry (Virginia, second quarter) before merging data with the National Drug Data File to include medication-specific information.20 We also collected information about decisions to expand Medicaid as of 2014 and publically available state-level HCV incidence data from the Centers for Disease Control and Prevention.
Our outcome was the proportion of Medicaid fee-for-service prescription drug spending accounted for by sofosbuvir. We used data from previous analyses16,17 to identify criteria used in state Medicaid fee-for-service prior authorization policies through the end of 2014, categorizing each criterion as clinical, administrative, or behavioral (Table 1). We also divided behavioral criteria into 2 subdomains: treatment criteria (those requiring patients to be in treatment for substance or alcohol abuse) and abstinence criteria (those requiring patients to be abstinent from alcohol or illicit substances). Although there can be multiple features of abstinence, including achievement of abstinence and the presence or length of an abstinence waiting period, these were considered together for the purposes of defining criteria.
TABLE 1—
Definitions for Sofosbuvir Utilization Criteria: United States, 2014
| Utilization Criteria Category | Components | Strict | Lenient | |
| Clinical | Minimum fibrosis stagea | Minimum fibrosis stage of ≥ F3 and either documentation HIV treatment or exclusion of cirrhotic patients | All other clinical policiesc | |
| Exclusion of cirrhotic patientsb | ||||
| Documentation of HIV treatment | ||||
| Administrative | Restriction of prescriber type | Prescription either by or in consultation with infectious disease physicians, gastroenterologists, or liver transplant physicians | No prescriber limitation | |
| Behavioral | Presence of any criterion related to alcohol or drug use | Presence of any criteria with specific requirements related to drug or alcohol use and either abstinence requirement for all patients or treatment requirementd | All other behavioral policiese | |
| Abstinence from drugs or alcohol | ||||
| Treatment of drug or alcohol abuse | ||||
| Treatment subdomain | Presence of any criteria with specific requirements related to drug or alcohol use and treatment requirement | All less stringent treatment policies | ||
| Abstinence subdomain | Presence of any criteria with specific requirements related to drug or alcohol use and abstinence requirement for all patients | All less stringent abstinence policies |
METAVIR fibrosis score, which is graded on a 5-point scale, ranging from 0 to 4; F0 = no fibrosis; F1 = portal fibrosis without septa; F2 = portal fibrosis with few septa; F3 = numerous septa without cirrhosis; F4 = cirrhosis.
Six states (AK, AR, IA, LA, NE, TN) mandated liver biopsy to confirm cirrhosis.
Includes clinical policies that have no minimum fibrosis stage requirement, those with requirements of ≤ F2, and those with minimum fibrosis stage requirements of ≥ F3 but without concurrent limitations on the basis of HIV or cirrhosis status.
Includes treatment policies that allow bypass and do not require treatment completion.
Includes behavioral policies that have no criteria related to alcohol or drug use, those with criteria without specific requirements, and those with specific requirements but not related to required abstinence or treatment.
For each state, we defined clinical, administrative, and behavioral utilization criteria as strict or lenient (Table 1). We designated states as having strict clinical criteria if they limited sofosbuvir to patients with severe fibrosis (minimum fibrosis stage of ≥ F3, which includes patients either with numerous septa without cirrhosis or with cirrhosis) while also either excluding cirrhotic patients (with or without biopsy confirmation) or requiring treatment documentation for HIV-positive individuals. We deemed states that required sofosbuvir prescription either by, or in consultation with, subspecialists in gastroenterology, infectious diseases, or liver transplantation to have strict administrative criteria. Finally, we considered states to have strict behavioral criteria if their policies included any stipulations related to drug or alcohol use as well as specific requirements related to either abstinence or treatment.
We excluded 9 Medicaid programs (in GA, HI, KS, MI, ND, NJ, NM, SC, TX) for which there was missing or incomplete reporting of sofosbuvir utilization data or no available data from published analyses of 2014 fee-for-service sofosbuvir utilization criteria. To evaluate the associations between these criteria and state program sofosbuvir spending among the remaining 41 states and Washington, DC, we used multivariable linear regression analyses. We included as independent variables the clinical, administrative, and behavioral criteria as well as state decisions regarding Medicaid expansion (defined as expansion in place for all 4 quarters of 2014). We also specified regression models that subdivided behavioral criteria into 2 distinct variables for treatment and abstinence criteria. State-level HCV prevalence information is not available and could not be incorporated into analytic models. However, as sensitivity analyses, we developed additional models including 5-year state-level HCV incidence rates (2009–2013) as covariates. We performed analyses using SAS version 9.4 (SAS Institute, Cary, NC), and all tests of significance were 2-tailed at α = 0.05.
ANALYTIC FINDINGS
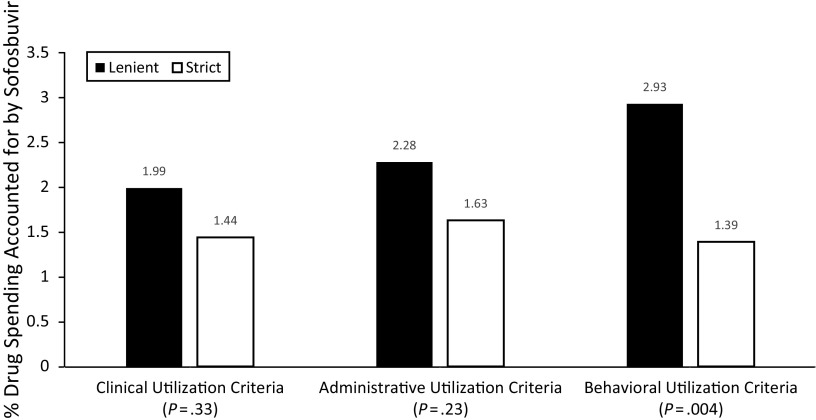
We found that the proportion of total Medicaid fee-for-service drug spending accounted for by sofosbuvir varied substantially across states, ranging from 0.08% to 6.70% (Table 2). Most state programs adopted some type of strict criteria, with 29%, 71%, and 69% using strict clinical, behavioral, and administrative criteria, respectively. Compared with lenient criteria, the presence of strict clinical (1.44% vs 1.99%; P = .33) or administrative (1.63% vs 2.28%; P = .23) criteria was not significantly associated with sofosbuvir spending (Figure 1). By contrast, the presence of strict behavioral utilization criteria was associated with a statistically significantly smaller proportion of state Medicaid drug spending accounted for by sofosbuvir (1.39% vs 2.93%; P = .004).
TABLE 2—
Fee-for-Service Drug Spending on Sofosbuvir, Utilization Criteria, and Medicaid Expansion Decisions, by State: United States, 2014
| State | FFS Drug Spending Accounted for by Sofosbuvir, % | Medicaid Expansiona | Strict Clinical Criteria | Strict Behavioral Criteria | Strict Administrative Criteria |
| Maryland | 0.08 | Y | N | Y | Y |
| Wisconsin | 0.18 | N | N | Y | Y |
| New Hampshire | 0.25 | N | N | N | Y |
| Alaska | 0.27 | N | Y | Y | N |
| West Virginia | 0.28 | Y | Y | Y | Y |
| Iowa | 0.29 | Y | N | Y | Y |
| Oregon | 0.30 | Y | N | Y | Y |
| Pennsylvania | 0.35 | N | N | Y | Y |
| Virginiab | 0.47 | N | N | Y | Y |
| Kentucky | 0.66 | Y | Y | Y | Y |
| Rhode Island | 0.67 | Y | N | Y | Y |
| Delaware | 0.71 | Y | Y | Y | N |
| Mississippi | 0.73 | N | N | Y | Y |
| District of Columbia | 0.76 | Y | Y | Y | Y |
| Arkansas | 0.79 | N | N | N | N |
| Idaho | 0.88 | N | Y | N | Y |
| Colorado | 1.11 | Y | N | Y | Y |
| Washington | 1.19 | Y | Y | Y | Y |
| California | 1.20 | Y | Y | Y | Y |
| Arizona | 1.25 | Y | Y | N | Y |
| Nebraska | 1.25 | N | N | Y | N |
| Louisiana | 1.34 | N | N | Y | Y |
| Utah | 1.41 | N | N | N | N |
| Ohio | 1.64 | Y | N | Y | Y |
| Alabama | 1.77 | N | N | Y | N |
| South Dakota | 1.87 | N | N | Y | Y |
| Illinois | 1.92 | Y | N | Y | Y |
| Vermont | 1.94 | Y | N | Y | N |
| Florida | 2.05 | N | Y | Y | Y |
| North Carolina | 2.10 | N | N | Y | N |
| Wyoming | 2.52 | N | N | Y | N |
| Missouri | 2.77 | N | N | Y | N |
| Maine | 2.84 | N | N | N | Y |
| Minnesota | 3.06 | Y | N | N | N |
| Montana | 3.21 | Y | N | Y | Y |
| Indiana | 3.29 | N | N | N | Y |
| New York | 3.63 | Y | Y | N | Y |
| Oklahoma | 3.73 | N | N | Y | Y |
| Tennessee | 4.44 | N | Y | Y | Y |
| Nevadac | 5.26 | Y | N | N | N |
| Massachusetts | 5.76 | Y | N | N | N |
| Connecticut | 6.68 | Y | N | N | Y |
Note. FFS = fee for service.
Y indicates expansion over all 4 quarters of 2014 (excludes states with expansion beginning in 2014 or after).
Calculated from 3 quarters of data (data from 2nd quarter excluded as erroneous entry).
Did not use prior authorization criteria.
FIGURE 1—
Associations Between State Medicaid Program Utilization Criteria and Fee-for-Service Drug Spending Accounted for by Sofosbuvir: United States, 2014
The relationship between spending and behavioral criteria was relatively unaffected by adjustment for all 3 types of criteria and state decisions regarding Medicaid expansion (Table 3). In multivariable analyses, the presence of strict behavioral criteria was associated with a 1.44% decrease in the portion of total Medicaid drug spending accounted for by sofosbuvir (P = .007). In analyses that subdivided behavioral criteria into treatment and abstinence components, we found that the presence of strict abstinence criteria—that is, periods of abstinence required for all patients before treatment with sofosbuvir—was associated with a 1.03% decrease in the portion of total Medicaid drug spending accounted for by sofosbuvir (P = .045), whereas there was not a significant relationship between strict treatment criteria and spending (P = .16). In multivariable analyses, we found no significant associations between sofosbuvir spending and the presence of strict clinical or administrative criteria. Sensitivity analyses corroborated these findings: the presence of strict behavioral criteria, but not strict clinical or administrative criteria, was significantly associated with spending.
TABLE 3—
Association Between Sofosbuvir Utilization Criteria and Medicaid Fee-for-Service Drug Spending Accounted for by Sofosbuvir (%): United States, 2014
| Criteria | b | P | B | P |
| Strict clinical criteria | −0.54 | .33 | −0.49 | .35 |
| Strict administrative criteria | −0.65 | .23 | −0.42 | .41 |
| Strict behavioral criteriaa | −1.53 | .004 | −1.44 | .007 |
| Medicaid expansion | . . . | . . . | 0.45 | .34 |
In analyses that subdivided behavioral criteria into abstinence and treatment components, strict abstinence criteria were associated with lower spending on Sofosbuvir, whereas strict treatment criteria were not.
These findings highlight the budgetary challenges that must be confronted when developing Medicaid policy for sofosbuvir coverage. A potential decrease in spending on sofosbuvir of 1.44% of overall drug spending is a substantial portion of use for programs that, to date, have committed as much as 5% to 7% of their overall drug budgets to the medication.13 The absolute amount of such savings could also be significant. For instance, in states with a large proportion of fee-for-service drug spending accounted for by sofosbuvir, such as Connecticut and Massachusetts, such changes would correspond to an estimated $40 million to $50 million decrease in drug spending. Savings could be even more significant in larger state programs such as New York Medicaid, which spent nearly $5.3 billion on prescription drugs in 2014.
Although the exact amount of potential savings would vary across states depending on the overall prescription drug budget, the magnitude of funds would be considerable and potentially useful for meeting either the HCV or non-HCV medical needs of other Medicaid recipients. This tension is particularly notable because state programs face short-term budgetary realities that are at odds with these long-term benefits. For example, although substantial, the collective $1.3 billion spent on sofosbuvir by Medicaid programs in 201413 represents medication access for only 2.4% of Medicaid beneficiaries clinically eligible for treatment.21
COVERAGE AS A PUBLIC HEALTH PRIORITY
Although some trade-offs are inevitable, policies restricting sofosbuvir access on the basis of strict alcohol and drug utilization criteria, which were adopted by 30 states in 2014 and were associated with lower spending on sofosbuvir, run contrary to evidence supporting HCV treatment among drug users.
In particular, individuals who inject drugs have displayed adherence and treatment response to traditional interferon-based regimens that are comparable to those of individuals who do not.22 Because injection drug use is also the most common risk factor for HCV infection,23 efforts to eradicate existing HCV infections and prevent future spread should include specific treatment of those who inject drugs.24,25 Doing so with a newer, less toxic agent such as sofosbuvir or another direct-acting antiviral aligns with public health priorities and is also likely be both clinically and cost-effective.26
Moreover, data do not support pretreatment substance use screening, and recent consensus guidelines from clinical experts in liver disease strongly recommend that policymakers abandon these requirements.27 In this context, abstinence criteria for sofosbuvir approval can burden patients with undue hurdles and stigma. In turn, they conflict with imperatives related to public health, medical evidence, and ethics; they also depart from the spirit and letter of Medicaid law, which does not restrict coverage for other medications on the basis of substance use.
Finally, although we did not observe associations between strict clinical criteria and sofosbuvir spending overall, policymakers should regularly evaluate all utilization criteria for appropriateness. Anecdotally, for example, the requirement in some states that patients undergo liver biopsy to prove cirrhosis could deter patients otherwise appropriate for treatment. Ultimately, such efforts are important because the overall health and financial costs of chronic HCV infections are expected to increase in the coming years.28 Providing HCV treatment to clinically eligible Medicaid beneficiaries is an important public health intervention that could prevent new infections and significant virus-related complications29 while being cost-effective, depending on the time horizon.30
POTENTIAL SOLUTIONS
Considering these realities, policymakers would ideally remove abstinence requirements from Medicaid utilization criteria. However, the basis of the criteria—a pressing need to control costs in a large population—reflects the underlying problem of medication affordability.
At the price of $1000 per pill, sofosbuvir is fundamentally unaffordable for state programs. Cost control strategies used in private markets, such as cost sharing via coinsurance, are untenable in low-income Medicaid populations. The status quo of price negotiations and rebate arrangements between individual states and the drug manufacturers may modestly attenuate the budget impact of sofosbuvir, but it is insufficient to create widespread parity in access for beneficiaries clinically eligible to receive it. Likewise, patient assistance programs provided by the manufacturers of HCV medications may increase access for some patients but cannot address the scale of treatment needed nationally. Without strategies to increase overall medication affordability, removal of problematic substance use criteria is likely to have limited benefits.
National Policy
One broad solution for addressing high drug prices would be federal reform. Some have suggested that the federal government acquire the drug patent, the threat of which has been used previously.31 In contrast to those other special circumstances, however, the sofosbuvir price controversy is not an isolated issue. Instead, the drug is the first of a wave of costly new “specialty medications” for the treatment of HCV as well as cancer, multiple sclerosis, autoimmune conditions, and hyperlipidemia—all of which will have significant effects on health care costs.32 Forcibly seizing the patent rights to sofosbuvir would set a problematic precedent as other HCV and non-HCV drugs enter the market.
Alternatively, advocacy groups have proposed an HCV-specific analog to the AIDS Drug Assistance Program, which was created in the late 1980s to address the HIV/AIDS epidemic and pay for costly antiretroviral medications.31,33 Because HCV and HIV are both communicable diseases that present significant individual and public health harms, AIDS Drug Assistance Program–like appropriations have merit and the potential to significantly expand drug coverage. Implementation, however, would be complex. Such a program would encounter the same issues facing the AIDS Drug Assistance Program itself, including waiting lists and unmet needs. More specifically, the AIDS Drug Assistance Program budget has been highly variable from year to year, because it is influenced by a variety of factors and is contingent on buy-in from multiple parties and transfers from other funds.33
State Policy
Considering the challenges to enacting any broad national legislation, policymakers could pursue several state-level approaches to control costs related to sofosbuvir use. First, state Medicaid programs could advance policies that increase the transparency of sofosbuvir pricing. Individual states have negotiated rebates, either individually or through pharmacy benefit managers, resulting in net prices that are lower than widely publicized wholesale acquisition costs. This information, however, is unavailable even between Medicaid programs and contributes to persistent disparities in access and affordability between—and in some cases within—states.
Although individual states are unable to exert market share leverage as the Departments of Veterans Affairs and Defense have done nationally to negotiate drug prices,34 greater price transparency could still help programs compare arrangements, benchmark them against the relatively minimal manufacturing costs (which have been estimated to be as low as $68–$136 for a 12-week treatment course in some settings35), and cover more patients with fixed budgets. Such efforts are aided by the fact that other medications have emerged as treatment alternatives to sofosbuvir.
Second, because a major element of sofosbuvir’s appeal is its ability to reduce downstream disease burden and costs, Medicaid policymakers could pursue “shared savings” arrangements that are consistent with national emphases on value-based care and emerging collaboration between payers and drug manufacturers.36 As some have suggested for Medicare,37 this could involve setting discounted upfront drug prices, prospectively withholding portions of drug payments, and paying them back as shared savings if downstream gains materialize. Although potentially complex to design, this kind of shared accountability has been demonstrated in other countries.38 It might even take on nonfinancial forms in cases of unrealized benefit, with manufacturers providing additional treatment courses at no additional cost to beneficiaries or payers.39
LIMITATIONS AND NEXT STEPS
Our analysis possesses several limitations. Although Medicaid criteria under managed care and fee-for-service may align, information on authorization criteria for Medicaid managed care are not publicly available. Accordingly, our analysis does not include Medicaid managed care organizations and therefore reflects only a portion of overall Medicaid utilization criteria for sofosbuvir. Our analysis excluded 9 states, and patient-level data were not available for analysis. Because neither accurate HCV prevalence data nor information about price negotiations and rebate agreements between states and drug manufacturers are publicly or privately available, these influences should be explored in future analyses.
Additionally, although our study provides unique insight during the first year after sofosbuvir’s Food and Drug Administration approval, future work could incorporate data across multiple years to describe how Medicaid program drug spending strategies and utilization criteria have evolved in view of alternative agents and regimens (e.g., protease inhibitors, protease and polymerase combinations) and advocacy efforts. Finally, the observational nature of our study precludes conclusions about causality.
Nonetheless, this work highlights problematic elements in Medicaid utilization criteria that underscore the need for further study and evaluation. With respect to HCV treatment, utilization criteria should be studied in Medicaid managed care organizations, and direct cost- and comparative-effectiveness studies for sofosbuvir and other novel HCV medications are also sorely needed. As clinicians grow more comfortable with new agents, data about long-term provider behavior, clinical outcomes, and metrics such as discontinuation rates, reported to be as high as 8% in private insurance markets,40 will also inform future Medicaid drug policy. Considering the rapidly changing landscape of HCV treatment regimens, evaluation of other direct antiviral agents is also warranted.
Such information is also urgently needed to inform policy and public health efforts going forward. Although only one medication, sofosbuvir is a salient example of the economic and public health challenges facing Medicaid policymakers and public health leaders amid the surge in costly new medications entering the market. Experience with sofosbuvir, and evaluation of new HCV treatments more broadly, may help leaders more equitably advocate and allocate budgets and resources for beneficiaries, a task that is particularly urgent because national cost containment strategies (e.g., value-based reimbursement) also compel Medicaid programs to weigh both the relative value of novel and traditional drugs and nonmedication health care services (e.g., inpatient and outpatient care).
CONCLUSIONS
Although restricting access to sofosbuvir among Medicaid beneficiaries with ongoing substance use was associated with reduced spending, such criteria raise important concerns related to medical ethics, clinical evidence, and potentially federal law. Providing HCV treatment to clinically eligible Medicaid beneficiaries is an important public health intervention, and decision-makers should reject these requirements in Medicaid coverage policy and pursue strategies to balance short-term budgetary realities with long-term treatment benefits.
ACKNOWLEDGMENTS
J. M. Liao is a member of the Pennsylvania State Medicaid Pharmacy and Therapeutics Committee. M. A. Fischer is a clinical consultant to Alosa Health, a medical education nonprofit, and has received institutional research support from CVS-Caremark and Otsuka America.
HUMAN PARTICIPANT PROTECTION
No protocol approval was necessary because all data were publically available and published as aggregate data at the state level; there were no patient-level data.
REFERENCES
- 1.Ghany MG, Liang TJ. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;369(7):679–680. doi: 10.1056/NEJMc1307589. Comment in: Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowdley KV, Gordon SC, Reddy KR et al. Ledipasvir and Sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 3.Afdhal N, Zeuzem S, Kwo P. Ledipasvir and Sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 4.Zeuzem S, Dusheiko GM, Salupere R et al. Sofosbuvir and Ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 5.Flick M, Hirschler B. Gilead offers Egypt new hepatitis C drug at 99 percent discount. 2014. Available at: http://www.reuters.com/article/2014/03/21/us-hepatitis-egypt-gilead-sciences-idUSBREA2K1VF20140321. Accessed December 1, 2016.
- 6.Denniston MM, Miles RB, Drobeniuc J et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2009. Hepatology. 2012;55(6):1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlin BR, Eckhardt BJ, Shua MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353–1363. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Preventive Services Task Force. Hepatitis C: screening. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm. Accessed November 30, 2016.
- 10.Brennan T, Shrank W. New expensive treatments for hepatitis C infection. JAMA. 2014;312(6):593–594. doi: 10.1001/jama.2014.8897. Comment in: Costs of new treatments for hepatitis C infection—reply. JAMA. 2014; Costs of new treatments for hepatitis C infection. JAMA. 2014. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RL, Blumen HE, Ferro C. The burden of hepatitis C virus disease in commercial and managed Medicaid populations. 2015. Available at: http://us.milliman.com/uploadedFiles/insight/2015/milliman-hcv-burden.pdf. Accessed December 1, 2016.
- 12.IMS Institute. Medicines use and spending shifts: a review of the use of medicines in the US in 2014. 2015. Available at: https://www.imshealth.com/files/web/IMSH20Institute/Reports/Medicines_Use_and_Spending_Shifts/Medicine-Spending-and-Growth_1995-2014.pdf. Accessed December 1, 2016.
- 13.Liao JM, Fischer MA. Early patterns of Sofosbuvir utilization by state Medicaid programs. N Engl J Med. 2015;373(13):1279–1281. doi: 10.1056/NEJMc1506108. [DOI] [PubMed] [Google Scholar]
- 14.New York State Department of Health. New York State Medicaid drug utilization review (DUR): board meeting summary for September 18, 2014. 2014. Available at: http://www.health.ny.gov/health_care/medicaid/program/dur/meetings/2014/09/sum_091814_durb.pdf. Accessed November 30, 2016.
- 15.Magellan Health, Inc. NYS Medicaid pharmacy prior authorization programs. 2014. Available at: https://newyork.fhsc.com/providers/pdp_hepatitisc.asp. Accessed November 30, 2016.
- 16.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of Sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215–223. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- 17.Canary LA, Klevens M, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med. 2015;163(3):226–228. doi: 10.7326/M15-0320. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicaid and CHIP Services. Medicaid drug rebate program notice. Available at: https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Prescription-Drugs/Downloads/Rx-Releases/State-Releases/state-rel-172.pdf. Accessed March 8, 2017.
- 19.Centers for Medicaid and CHIP Services. Medicaid. Available at: https://www.medicaid.gov/medicaid/prescription-drugs/medicaid-drug-rebate-program/data/index.html. Accessed March 8, 2017.
- 20.First Databank, National Drug Data File. San Francisco, CA: Hearst Corporation; 2011. [Google Scholar]
- 21.Wyden R, Grassley C, Hatch OG The price of Sovaldi and its impact on the US health care system. Available at: http://www.finance.senate.gov/imo/media/doc/1%20The%20Price%20of%20Sovaldi%20and%20Its%20Impact%20on%20the%20U.S.%20Health%20Care%20System%20(Full%20Report).pdf. Accessed December 1, 2016.
- 22.Aspinall EJ, Corson S, Doyle JS et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review of meta-analysis. Clin Infect Dis. 2013;57(suppl 2):S80–S89. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- 23.Amon JJ, Garfein RS, Ahdieh-Grant L et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis. 2008;46(12):1852–1858. doi: 10.1086/588297. [DOI] [PubMed] [Google Scholar]
- 24.Hellard M, Doyle JS, Sacks-Davis R, Thompson AJ, McBryde E. Eradication of hepatitis C infection: the importance of targeting people who inject drugs. Hepatology. 2014;59(2):366–369. doi: 10.1002/hep.26623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edlin BR, Winkelstein ER. Can hepatitis C be eradicated in the United States? Antiviral Res. 2014;110:79–93. doi: 10.1016/j.antiviral.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Martin NK, Vickerman P, Miners A et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55(1):49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 27.American Association for the Study of Liver Diseases; Infectious Diseases Society of America. When and in whom to initiate HCV therapy. Available at: http://www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Accessed December 1, 2016.
- 28.Razavi H, Elkhoury AC, Elbasha E et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 suppl 1):S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 30.Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology. 2015;61(6):1860–1869. doi: 10.1002/hep.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trooskin SB, Reynolds H, Kostman JR. Access to costly new hepatitis C drugs: medicine, money and advocacy. Clin Infect Dis. 2015;61(12):1825–1830. doi: 10.1093/cid/civ677. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch BR, Balu S, Schulman KA. The impact of specialty pharmaceuticals as drivers of health care costs. Health Aff (Millwood) 2014;33(10):1714–1720. doi: 10.1377/hlthaff.2014.0558. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser Family Foundation. AIDS drug assistance programs (ADAPs) 2014. Available at: http://kff.org/hivaids/fact-sheet/aids-drug-assistance-programs. Accessed December 1, 2016.
- 34.Blumenthal D, Squires D. Drug price control: how some government programs do it. Available at: http://www.commonwealthfund.org/publications/blog/2016/may/drug-price-control-how-some-government-programs-do-it. Accessed January 25, 2016.
- 35.Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-activing antivirals for use in large-scaled treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928–936. doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PricewaterhouseCoopers Health Research Institute. 21st century pharmaceutical collaboration: the value convergence. Available at: http://www.pwc.com/us/en/health-industries/health-research-institute/hri-pharma-life-sciences-fda.html. Accessed December 1, 2016.
- 37.Neuman T, Hoadley J, Cubanski J. The cost of a cure: Medicare’s role in treating hepatitis C. 2014. Available at: http://healthaffairs.org/blog/2014/06/05/the-cost-of-a-cure-medicares-role-in-treating-hepatitis-c. Accessed November 30, 2016.
- 38.ConvergeHEALTH. Value-based pricing for pharmaceuticals: implications of the shift from volume to value. Available at: https://www2.deloitte.com/us/en/pages/consulting/articles/value-based-pricing-for-pharmaceuticals.html. Accessed November 29, 2016.
- 39.Islam I. Rising costs of drugs: where do we go from here? Available at: http://healthaffairs.org/blog/2015/08/31/rising-cost-of-drugs-where-do-we-go-from-here. Accessed December 1, 2016.
- 40.Milllman J. The new $84,000 hepatitis C treatment is losing momentum, for now. 2015. Available at: http://www.washingtonpost.com/blogs/wonkblog/wp/2014/09/18/the-new-84000-hepatitis-c-treatment-is-losing-momentum-for-now. Accessed November 28, 2016.