Abstract
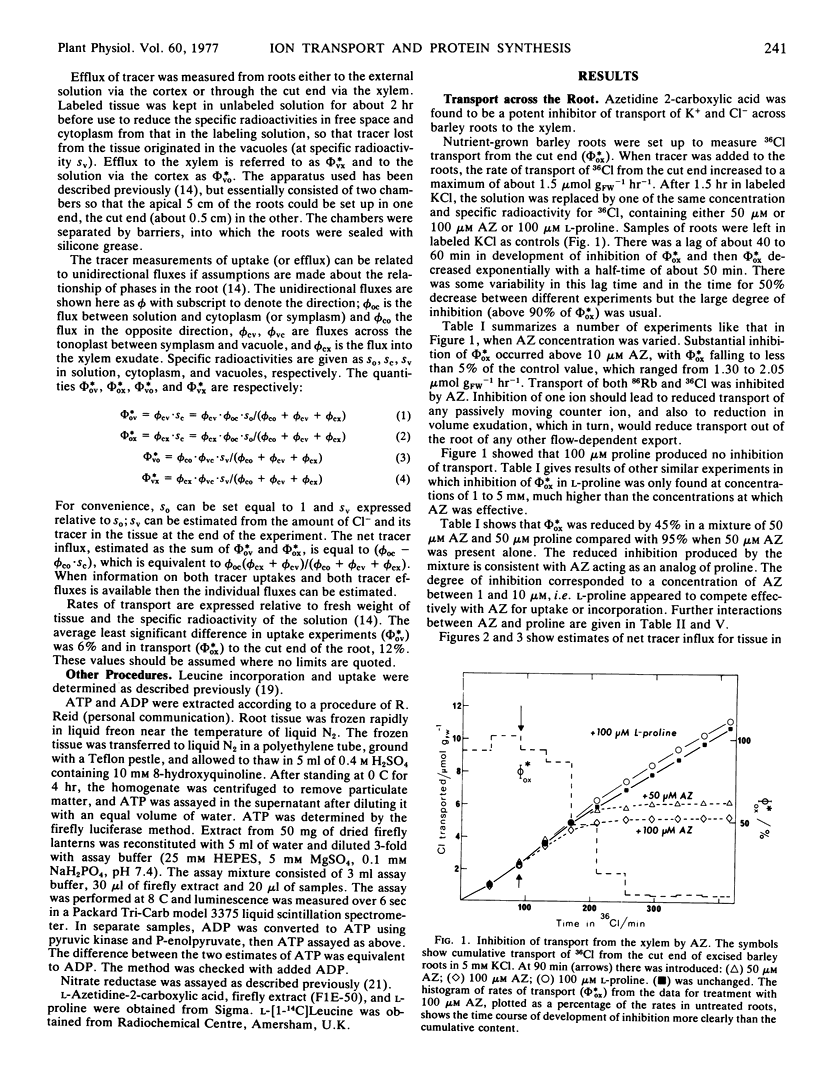
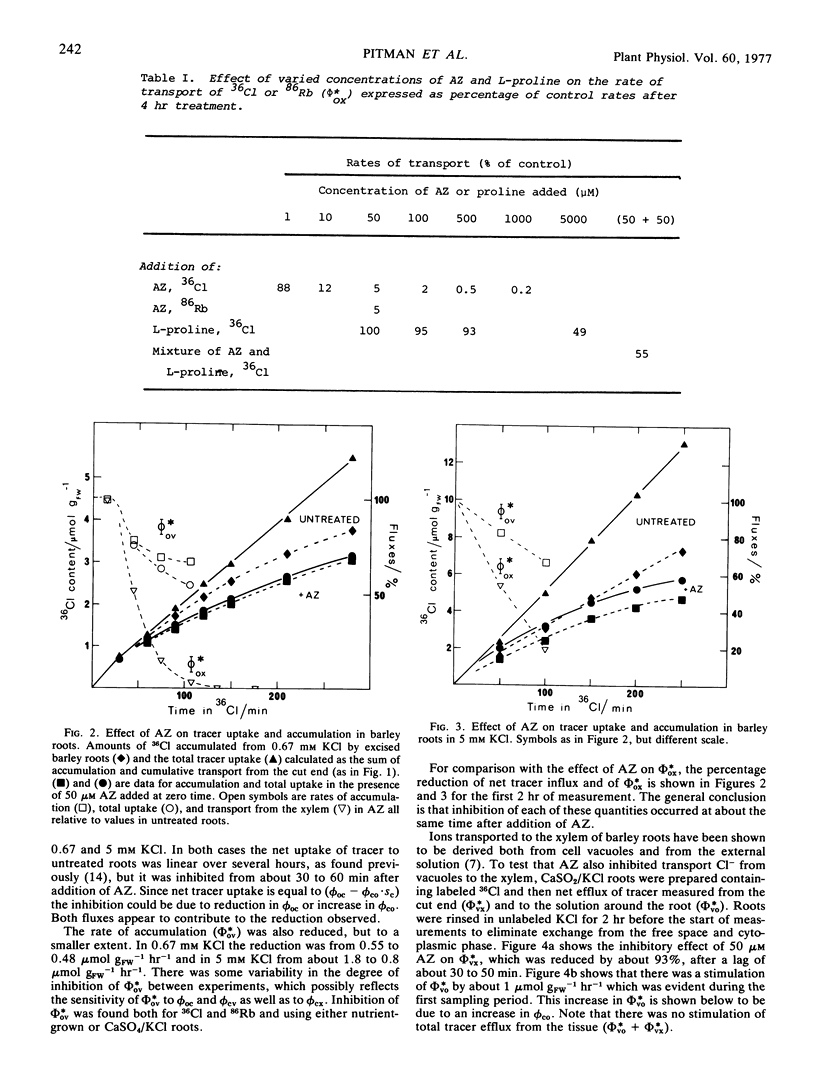
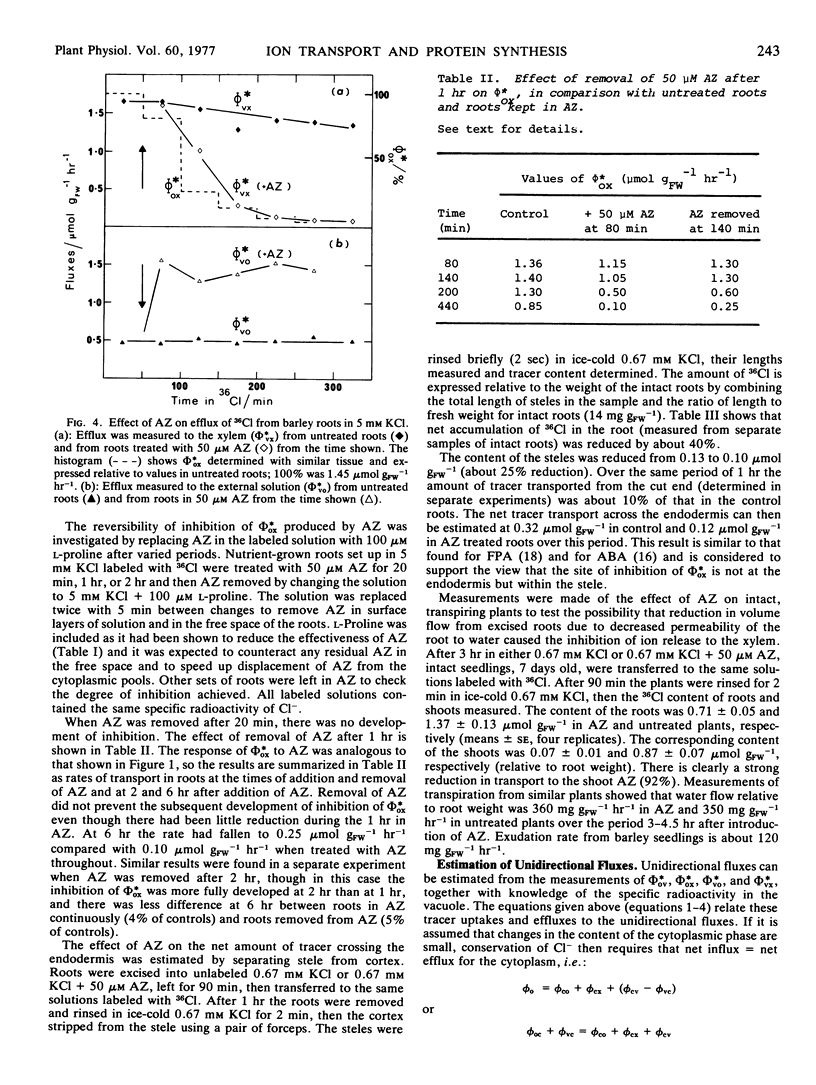
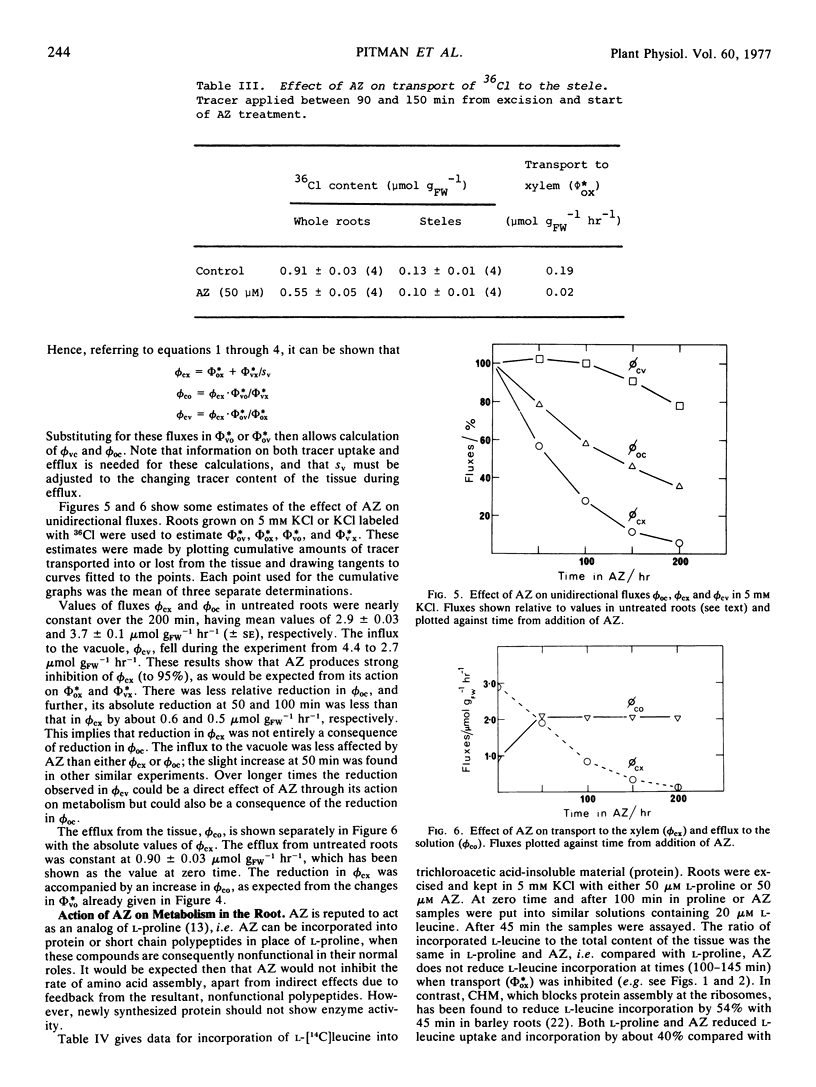
Azetidine 2-carboxylic acid (AZ) was used as an analog of proline to investigate further the relationship between protein synthesis and ion transport. AZ does not inhibit protein assembly, but the proteins formed are ineffective as enzymes. At relatively low concentrations (50 μM) AZ was a potent inhibitor of release of ions to the xylem of excised roots of barley (Hordeum vulgare L.) and intact plants. Uptake to the root was also inhibited but to a lesser degree. A procedure was introduced for estimating unidirectional fluxes from measurements of net tracer uptake, net transport to the xylem, and net efflux from the roots. It was shown that inhibition of release to the xylem was not caused by reduction in influx at the plasmalemma or to stimulation of influx to the vacuoles. It was suggested that AZ was acting on the process of release from symplast to the xylem. The action of AZ is compared with similar effects on ion transport produced by p-fluorophenylalanine, cycloheximide, and abscisic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis R. F., Higinbotham N. Electrochemical gradients and k and cl fluxes in excised corn roots. Plant Physiol. 1976 Feb;57(2):129–136. doi: 10.1104/pp.57.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z. Abscisic Acid effect on root exudation related to increased permeability to water. Plant Physiol. 1973 Jan;51(1):217–219. doi: 10.1104/pp.51.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Vaadia Y. Uptake and Transport of Radiochloride and Tritiated Water by Various Zones of Onion Roots of Different Chloride Status. Plant Physiol. 1964 Jan;39(1):104–108. doi: 10.1104/pp.39.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. G., Sucoff E. Effects of kinetin and root tip removal on exudation and potassium (rubidium) transport in roots of honey locust. Plant Physiol. 1976 Feb;57(2):230–236. doi: 10.1104/pp.57.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A., Spurr A. R., Epstein E. Lateral Transport of Ions into the Xylem of Corn Roots: II. Evaluation of a Stelar Pump. Plant Physiol. 1971 Aug;48(2):118–124. doi: 10.1104/pp.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U., Läuchli A., Ball E., Pitman M. G. Cycloheximide: a specific inhibitor of protein synthesis and intercellular ion transport in plant roots. Experientia. 1974 May 15;30(5):470–471. doi: 10.1007/BF01926298. [DOI] [PubMed] [Google Scholar]
- McMahon D. Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 1975 May;55(5):815–821. doi: 10.1104/pp.55.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Imber D. Abnormal Stomatal Behavior and Hormonal Imbalance in Flacca, a Wilty Mutant of Tomato: III. Hormonal Effects on the Water Status in the Plant. Plant Physiol. 1971 Jun;47(6):849–850. doi: 10.1104/pp.47.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]


