Abstract
Objective(s):
To investigate the effects of Nonylphenol (NP) in pups from dams exposed during gestational and lactational periods on immediate early genes (c-jun, c-fos) in hippocampus and the learning and memory of F1 rats.
Materials and Methods:
Twenty eight pregnant dams, stratified by pregnancy date, were randomly assigned into 4 groups, which were gavaged with NP at the doses of 50 mg/kg/day, 100 mg/kg/day, 200 mg/kg/day and groundnut oil, respectively. Step-down avoidance test, and learning and memory effects of NP were evaluated on 8-weeks-old pups. The expressions of c-jun and c-fos and the activities of choline acetyltransferase (ChAT) and acetylcholinesterase (AchE) were evaluated in hippocampus of pups.
Results:
Compared to the control, reaction time (RT) that pups spent to jump to the platform was longer (P=0.02), the number of errors were higher (P=0.01), and the step-down latency was shorter in the 200 mg/kg/day NP-treated group (P=0.04). Exposure to NP induced a significant reduction in ChAT activity in hippocampus in the 100 (P=0.005) and 200 mg/kg/day NP-treated groups (P=0.002), whereas exposure to 200 mg/kg/day caused a significant increase in AchE activity (P=0.004); a dose–response relationship was revealed between ChAT and AchE activities and NP exposure in the hippocampus of pups (r=-0.821, P=0.01; r=0.757, P=0.04). Exposure to NP in the 100 and 200 mg/kg/day NP-treated groups exhibited an increase in number of c-fos and c-jun positive cells.
Conclusion:
Exposure to NP might negatively affect learning and memory ability in F1 rats, possibly due to the alterations in the expression of c-jun and c-fos, and ChAT, AchE activities in hippocampus of pups.
Keywords: Learning and memory, Neurotransmitters, Nonylphenol, Pups
Introduction
The potential effects of gestational exposure to endocrine disrupting chemicals (EDCs) on central nervous system (CNS) development and function in offspring rats have raised concerns in recent years (1, 2). Nonylphenol (NP), an environmental EDC, possesses weak estrogenic activity, which is used in the production of many consumer compounds, including agricultural chemicals and spermicides (3). Accumulating data suggested that NP has toxic impact on the immune, reproductive, and endocrine system in offspring rats; the adverse effects of NP exposure via placenta on nervous system development in F1 rats were reported sparsely.
Immediate early genes (IEGs) are a class of genes, which can be activated and transcribed dynamically in response to neuronal activity. As activity-dependent transcription factors, it has been proposed that c-fos and c-jun have important roles in signal transduction, cell proliferation and differentiation and neuronal plasticity that is implicated with long-term memory consolidation in the brain (4, 5).
Acetylcholine (Ach), as a neurotransmitter and a neuromodulator in the brain, is necessary for the process of neuronal excitability and synaptic transmission as well as synaptic plasticity. Acetylcholinesterase (AchE) is implicated with the termination of cholinergic transmission by Ach hydrolysis, and degradation of Ach to inactive precursors (acetate and choline) in the synaptic cleft. Choline acetyltransferase (ChAT) and AchE are important for maintaining a stable level of Ach in living brain systems. Thus, modulation of the cholinergic signaling pathway, including the inhibition of AchE, the activation of ChAT, and the promotion of Ach synthesis, may serve as strategies for the treatment of memory dysfunction induced by NP neurotoxicity.
In this report, we performed behavioral toxicity test method together with the histology and morphology observation of brain to explore the impact of NP on neurobehavioral development and ChAT and AchE activities as well as c-jun and c-fos expressions in hippocampus of F1 rats.
Materials and Methods
Animals and treatments
Sixty Sprague-Dawley rats were purchased from Animal Center of the Third Military Medical University (Chongqing, China). After acclimatization for 7 days, females were caged with males (ratio 3:1). Vaginal smears were collected daily, and gestational day (GD) 0 was confirmed by sperm-positive vaginal smear. Subsequently, the 28 dams were randomly assigned to 4 groups (n=7 per group), which were given NP (dissolved in groundnut oil) by gavage at the doses of 50 mg/kg/day, 100 mg/kg/day, 200 mg/kg/day and groundnut oil (vehicle control; 10 ml/kg/day), respectively, from GD 6 until GD 21. After postnatal day (PND) 60, filial generation (F1) rats were reared in the laboratory with free access to standard rat fodder (8 gram fodder/100 gram body weight). The animals were group housed under controlled temperature maintained at 20±2 °C and humidity at 60±5 % on a 12:12-hr light-dark cycle (lights on 09:00–21:00 hr). Food and water were available ad libitum. All rat procedures and handling were in accordance with Animal Care and Use Guidelines in China.
After parturition, pups remained with their biological mother. Male pups in the group (n = 10 per group) were subjected to the measurement of learning and memory at the age of 8 weeks, expression of immediate early genges (c-jun and c-fos), and ChAT and AchE activities in hippocampus on PND 60.
Reagents
Nonylphenol for gavage (purity of 99%) was purchased from the Tokyo Chemical Co, Ltd (Tokyo, Japan). Rat ChAT and AchE kits were purchased from the Nanjing Jiancheng biological technology Co, LTD (NanJing, China). Anti-mouse-UL trapture antibody produced in 5 MG rabbit were obtained from the Beijing Zhongshan Biotechnology Reagents Co, Ltd. (Beijing, China). Goat anti-rabbit antibody was purchased from the Beijing Zhongshan Golden Bridge Biotechnology Co, Ltd. (Beijing, China). diaminobenzedine (DAB) chromogenic kit was purchased from the Dako Co (Glostrup, Denmark). All other chemicals were commercially available.
Step-down avoidance test
Learning and memory capacity was assessed by step-down avoidance test as described in our previous study (6). Briefly, the apparatus was a plastic box (27x15x12 cm3) whose floor was made of parallel bronze bars. The left end of the grid was occupied by a 4 cm diameter, 5 cm high wooden platform. The behavior of rats was recorded in a personal computer using a step-down test video monitoring system (Shanghai Jiliang Software Technology Co, Ltd, China) coupled to an infrared sensor located in the apparatus. The experiments were carried out from 10:00 to 14:00 hr.
In the period of learning, before the beginning of the training session, rats were placed on the apparatus to adapt for 5 min. In the training session, rats were put on the grid floor and then a continuous electric shock (0.4 mA) was delivered to the grid floor by an isolated stimulator. When the electric shock was delivered, the rat escapes from the grid floor back up onto the platform. The time for offspring rats to escape from the grid floor back up onto the platform was defined as reaction time (RT). The duration of training test was for 5 min and the shock was maintained for this period. The time of electronic shock suffered by rats when escaping from platform to the grid floor in 5 min was defined as the number of errors. Latency to first step-down (step-down latency, SDL), RT and the number of errors were recorded as learning credit from memory. The number of errors at 24 and 96 hr in pups were recorded after the first day of step-down avoidance test training. In the period of memory consolidation, the tests were administered again 2 days after the learning period. Latency to first step-down and the number of errors were recorded as memory consolidation test. In the period of memory fade away, the tests were administered again 3 days after the memory consolidation period, and the latency to first step-down, RT and the number of errors were recorded.
ChAT and AchE activities detection in hippocampus
Following step-down avoidance test, the rats in each group were killed by decapitation under anesthesia at the age of 8 weeks. The brains were quickly removed. The hippocampi were dissected on ice, weighed and homogenized to 10% homogenate (100 mg sample in 1 ml ice-cold saline) for AchE. Aliquots of the homogenate were further diluted with saline to 5% for ChAT assay. The supernatant was collected for the measurement of AchE and ChAT activities. The activities of ChAT and AchE were measured by spectrophotometry using the corresponding commercial kits (Nanjing Jiancheng biological technology Co, LTD, Nanjing, China). ChAT activity was indicated by unite per g protein (U/g protein). AchE activity was indicated by unite per mg protein (U/mg protein) (7).
Immunohistochemistry
Immunohistochemical test followed previously described study (8) with some modifications. The brains were fixed in 4% paraformaldehyde for 24 hr, and then embedded in paraffin. Coronal sections (5 mm) were collected on Super Frost glass slides. The sections were deparaffinized, rehydrated, and quenched for endogenous peroxidase with 3% H2O2 for 10 min. All sections were then washed with phosphate-buffered saline (PBS) and incubated with a rabbit anti-c-jun or anti-c-fos antibody at 37.8 °C for 30 min. Sections were then washed three times with PBS and additionally incubated with biotinylated secondary antibodies at 37.8 °C for 30 min. Then, the sections washed three times in PBS followed by incubation for 10 min in avidin–biotin–peroxidase (Maixin). Sections were then reacted with 0.05% nickel-enhanced 3,3-diaminobenzidine. Finally, the sections were counterstained by Mayer’s hematoxylin solution, rinsed, and mounted in neutral gum (8). The Image-Pro Plus 6.0 (IPP6.0) image analysis software was used to analyze the pictures, which were taken under 400× magnification fields of view. IPP6.0 was used to analyze a cluster of pictures with positive staining areas. Based on the features of positive immunohistochemical staining of each protein, the definite areas of these pictures were selected and the integrated optical density (IOD) mean value in these areas was then calculated (9).
Image analysis
The c-jun and c-fos immunoreaction products in the hippocampal region were analyzed by the Image Analysis System attached to a microscope at 400× magnification.
Statistical analysis
All data are expressed as mean ± standard deviation. The statistical analyses were performed with SPSS software, version 22.0 for Windows (SPSS Inc., Chicago, IL). Statistical significance between groups was analyzed with one-way analysis of variance (ANOVA) with LSD-t test to determine the effects of different treatments. Statistical significance was reached when the P value was less than 0.05.
Results
Step-down avoidance test
At the age of 8 weeks, the pups from dams treated with 200 mg/kg/day NP and when compared to control group, reaction time significantly increased in pups (P=0.02). The NP-treated group had reduced step-down latency in contrast to the control offspring in step-down avoidance test (P=0.01, Table 1). In the periods of memory consolidation and fade away, the group with 200 mg/kg/day NP significantly increased in the number of errors (P=0.01), and significantly reduced step-down latency compared to the control group (P=0.04). No differences were observed in the 50 and 100 mg/kg NP-treated groups as compared to the control (P=0.16) (Figure 1).
Table 1.
The reaction time and latency to first step-down in pups at the age of 8 weeks in each treatment group
| Treatment dose (mg/kg/day) | Reaction time Latency to first step-down | |
|---|---|---|
| (second) | (second) | |
| Control | 27.1±3.8 | 39.1±3.5 |
| NP (50) | 26.4±3.9 | 40.2±3.9 |
| NP (100) | 28.0±5.2 | 39.8±5.3 |
| NP (200) | 37.5±6.3* | 25.5±3.8* |
ANOVA: vs control.
P<0.05
Figure 1.
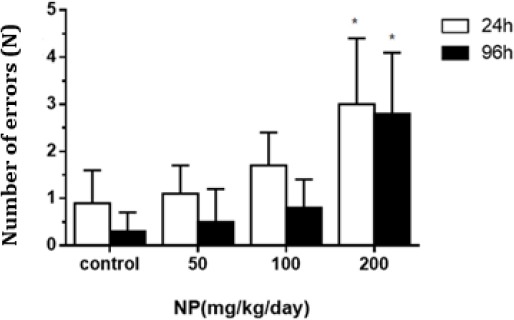
The number of errors at 24 and 96 hr in pups at the age of 8 weeks in each treatment group (x̄±S). Experimental groups at the age of 8 weeks included 50 mg/kg/day, 100 mg/kg/day and 200 mg/kg/day nonylphenol (NP)-treated groups, and groundnut oil alone (vehicle control). (ANOVA : vs control, *P<0.05, n=10)
The impact of NP on ChAT and AchE
ChAT activities in the hippocampus of 100 (P=0.005) and 200 mg/kg/day NP-treated groups (P=0.002) were decreased in contrast to the control. AchE activity in the hippocampus of 200 mg/kg/day NP-treated group was increased when compared to theTable 1. The reaction time and latency to first step-down in pups at the age of 8 weeks in each treatment group ANOVA: vs control. *P<0.05 control (P=0.004), and a dose–response relationship was revealed between ChAT and AchE activities and NP exposure in the hippocampus of pups (r=-0.821, P=0.01; r= 0.757, P=0.04) (Figure 2-3).
Figure 2.
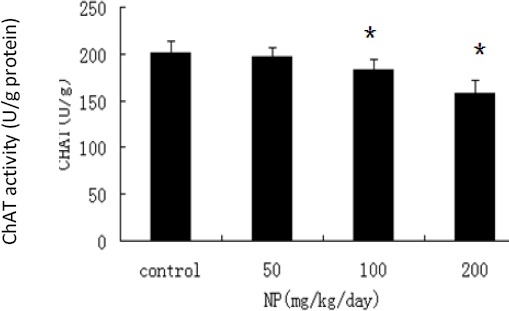
The impact of nonylphenol (NP) on the choline acetyltransferase (ChAT) activity in each treatment group (x̄±S). Experimental groups on postnatal day (PND) 60 were on: 50 mg/kg/day, 100 mg/kg/day and 200 mg/kg/day NP-treated groups, and groundnut oil alone (vehicle control). (ANOVA : vs control, *P<0.05, n=10)
Figure 3.
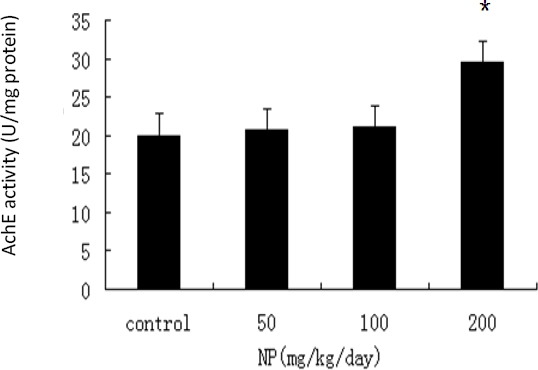
The impact of nonylphenol (NP) on the acetylcholinesterase (AchE) activity in each treatment group (x̄±S). Experimental groups on postnatal day (PND) 60 were on: NP at dose levels of 50 mg/kg/day, 100 mg/kg/day, and 200 mg/kg/day and groundnut oil alone (vehicle control). (ANOVA : vs control, *P<0.05, n=10)
The impact of NP on the expression of c-fos and c-jun protein
Gestational and lactational exposure to NP leads to a significant increase in positive cell number per visual fields under microscope and integrated optical density at high dose (200 mg/kg/day) and middle dose (100 mg/kg/day) when compared to the control (P=0.03) and 50 mg/kg NP group (P=0.01) (Table 2).
Table 2.
The impact of nonylphenol (NP) on the expression of c-fos and c-jun proteins in the hippocampus of rats offspring by immunohistochemistry (X̄±S, n=10)
| Treatment dose (mg/kg/day) | Positive cell number per visual fields under microscope c-fos protein c-jun protein | Integrated optical density c-fos protein c-jun protein | ||
|---|---|---|---|---|
| Control | 11.83±.75 | 5.83±.75 | 23.54±1.69 | 16.34±1.58 |
| NP (50) | 19.16±2.85 | 23.66±2.06 | 125.63±16.46 | 105.59±5.16 |
| NP (100) | 44.33±3.38 | 30.83±2.13 | 525.23±32.65 | 240.02±10.83 |
| NP (200) | 70.33±3.98* | 125.33±7.60* | 1404.60±40.86* | 1986.35±191.42* |
ANOVA: vs control.
P<0.05
c-fos and c-jun -positive cells with brown color were present in cytoplasm and nucleus in immunohistochemistry test. c-fos and c-jun protein expressions in normal hippocampus is weak. Decreases in the number of c-los and c-jun -positive cells were observed at low dose (50 mg/kg/day) NP group, and lightly stained cells were observed. While exposure to NP at both dose levels of 100 and 200 mg/kg/day exhibited an increase in the number of c-fos and c-jun -positive cells, especially at high doses, the color of these cells was dark, and cells were concentrated (Figure 4, 5).
Figure 4.
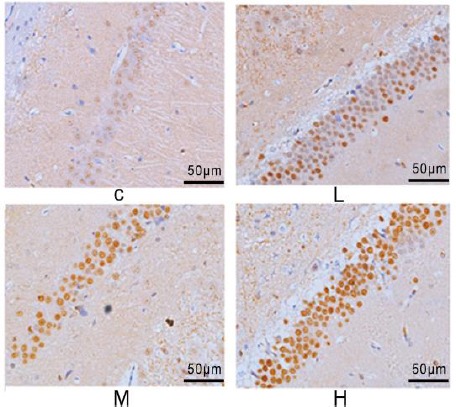
The impact of nonylphenol (NP) on the protein expression of c-fos in the hippocampus of rats’ offspring on postnatal day (PND) 60 in each treatment group (200×)
Figure 5.
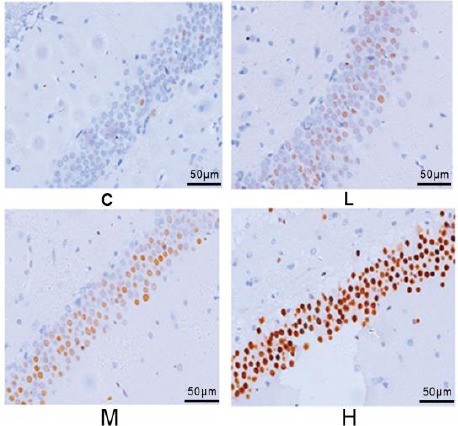
The impact of nonylphenol (NP) on the protein expression of c-jun in the hippocampus of rats’ offspring on postnatal day (PND) 60 in each treatment group (200×)
Discussion
The adverse health effects of NP on central nervous system have attracted attention in the last decade (9). Results of experiments in recent years demonstrated that gestational exposure to environ-mental contaminants can profoundly alter learning and memory in F1 rats (9,10); however, the impact of NP exposure through placenta on learning and memory function of male pups in rats were reported sparsely. In this study, exposure to NP caused a decrease in the step-down latency and increase in the number of errors in pups compared to the control, which showed gestational exposure of F1 rats to NP results in toxic impact on learning and memory.
There is increasing convergence of research on the essential role of Ach in learning and memory function. Results of the current study showed decreased ChAT activity and enhanced AchE activity in hippocampus tissues in rats. Since Ach is closely related to many physiological functions such as body movement, and learning and memory, the damage of the cholinergic nerve function can lead to developmental disorder with behavioral retardation and other symptoms in rats (11), which is highly consistent with the delayed physiological and neurological development indicators observed in our previous studies (6). Therefore, within the dose range of the experiment design, decreased ChAT activity and enhanced AchE activity may change the Ach levels in the brain. Once Ach changes as the first messenger in the nerve signal transmission system, the second and third cellular messengers will be further affected (12). Hence, we speculated that NP could have an adverse effect on the release and transmission of neurotransmitter via regulating the dynamic balance of Ach, resulting in neurological behavior changes in the offspring rats.
Exposure to NP during embryonic period and lactation might cause an increase in the level of estrogen and a decrease in the level of androgen in pups. The potential mechanism of NP-induced neurotoxicity may stem from NP’s weak estrogen-like activity: NP vies for estrogen receptor to cause the imbalance of endocrine system, alteration of the endocrine milieu of brain development, which affects further cholinergic neurotransmitter to deliver Ach. NP affects c-fos and c-jun protein expressions in hippocampus due to the facts that NP may induce abnormal changes in the morphology, structures and functions of astrocytes, and glial fibrillary acidic protein (GFAP) expression. These alterations subsequently promote apoptosis, and lead to the retardation of neural reflex time in nerve developmental stages of rats.
The aatural Science Foundation of Chictivations and expressions of IEGs are often regarded as the indicators for neural activity and gene activity functions (13). The current experimental results showed that the number of brown positive cell with c-jun and c-fos positive reaction in the hippocampus of offspring in the high dose group was higher when compared to the control group. Previous studies have found that c-jun can be specifically expressed in nervous system, and as one of the transcription factors, its regulatory range is quite wide, which can be activated and affected by a variety of chemical substances in various tissues (14). Cerebral ischemia and hypoxia can induce c-jun expression (15) in nerve cells of the corresponding hypoxia ischemia parts. When the cells are stimulated, the concentration of the heterologous Fos-jun complexes composed of c-jun and c-fos nuclear proteins could be rapidly increased in a very short period (16).
This study found that NP exposure during pregnancy would induce NP accumulation in the brain tissue of offspring rats, and exposure to NP has an adverse effect on the neural transmitter and IEGs in the signaling pathway of hippocampal neural cells. The proposed response process could be: after exogenous signal stimulation of NP to nervous system, excitement of the first level neurons that stimulates secretion of neurotransmitter, and interfering with the dynamic balance of acetylcholine. Ach, a key neurotransmitter in the central nervous system, functions on the cell membrane of target cells as the first messenger (17), followed by trans-membrane transduction mechanism of transferring external stimulation signals into the cells, and activating the intracellular second messenger. When causing in transient stress response in cells, the second messenger could activate the transcription of c-jun and c-fos, which are regarded as the third messenger, with the generated mRNA moved from the nucleus to the cytoplasm and being translated into c-fos and c-jun protein.
Conclusion
In the current study, we conducted toxicological behavioral tests and testified the toxic effect of gestational exposure to NP on c-jun, and c-fos proteins expression and learning and memory function in hippocampus of offspring rats, together with the pathological abnormalities in hippocampus ultrastructure of F1 rats exposed at dose level of 200 mg/kg/day. We made a conclusion that NP exposure might induce a reduction in learning and memory ability of F1 rats, the reasons may be due to the alterations in the expressions of c-jun and c-fos, as well as ChAT and AchE activities in hippocampus of pups.
Acknowledgment
This work was supported by the foundation of the National Natural Science Foundation of China (81360439, 81560527); Fund of Department of Science and Technology of Guizhou Province, China (LH[2014]7543, LH[2015]7521, J[2014]2177, J[2014]2185); Youth Foundation of Department of Education of Guizhou Province (KY[2013]198); Bidding project of Zunyi Medical University of China (2013F-68); 2015 Fund for key discipline construction in Zunyi Medical University (No.0996034); Scientific and Technological Fund of Department of Health of Guizhou Province, China (Fund No. gzwkj20131127, No. gzwjkj2016-1-045). 2016 College Student Innovation Program of Guizhou Province (Fund No. 201610661011), China; College Student Innovation Program of Zunyi Medical College of China (Fund No. 20155011, No.2016018).
References
- 1.Jie X, Yang W, Jie Y, Fan QY, Liu XY, Yan L, et al. Immune effects of nonylphenol on offspring of rats Exposed During Pregnancy. Hum Ecol Risk Assess. 2010;16:444–452. [Google Scholar]
- 2.Yu Jie Fan QY, Binli H, Biao Z, Zheng F, Jianmei L, et al. Joint neurodevelopmental and behavioral effects of nonylphenol and estradiol on F(1) male rats. Int J Environ Heal R. 2013;23:321–330. doi: 10.1080/09603123.2012.733936. [DOI] [PubMed] [Google Scholar]
- 3.White R, Jobling S, Hoare S, Sumpter J, Parker M. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 2014;135:175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Cadahia B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol. 2011;89:61–73. doi: 10.1139/O10-138. [DOI] [PubMed] [Google Scholar]
- 5.Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- 6.Jie X, Yang W, Jie Y, Hashim JH, Liu XY, Fan QY, et al. Toxic effect of gestational exposure to nonylphenol on F1 male rats. Birth Defects Res B Dev Reprod Toxicol. 2010;89:418–428. doi: 10.1002/bdrb.20268. [DOI] [PubMed] [Google Scholar]
- 7.Hollnagel JO, Ul Haq R, Behrens CJ, Maslarova A, Mody I, Heinemann U. No evidence for role of extracellular choline-acetyltransferase in generation of gamma oscillations in rat hippocampal slices in vitro. Neuroscience. 2015;284:459–469. doi: 10.1016/j.neuroscience.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Uyanıkgil Y, Turgut M, Baka M. Effects of melatonin on the cerebellum of infant rat following kaolin-induced hydrocephalus: a histochemical and immunohistochemical study. Cerebellum. 2017;16:142–150. doi: 10.1007/s12311-016-0778-9. [DOI] [PubMed] [Google Scholar]
- 9.Jie Y, Xuefeng Y, Mengxue Y, Xuesong Y, Jing Y, Yin T, et al. Mechanism of nonylphenol-induced neurotoxicity in F1 rats during sexual maturity. Wien Klin Wochenschr. 2016;128:426–434. doi: 10.1007/s00508-016-0960-6. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 11.Vasilopoulou CG, Constantinou C, Giannakopoulou D, Giompres P, Margarity M. Effect of a1dult onset hypothyroidism on behavioral parameters and acetylcholinesterase isoforms activity in specific brain regions of male mice. Physiol Behav. 2016;164:284–291. doi: 10.1016/j.physbeh.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Hirshman CA, Bergman NA. Factors influencing intrapulmonary airway calibre during anaesthesia. Br J Anaesth. 1990;65:30–42. doi: 10.1093/bja/65.1.30. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig M, Tobin VA, Callahan MF, Papadaki E, Becker A, Engelmann M, et al. Intranasal application of vasopressin fails to elicit changes in brain immediate early gene expression, neural activity and behavioural performance of rats. J Neuroendocrinol. 2013;25:655–667. doi: 10.1111/jne.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herdegen T, Skene P, Bähr M. The c-Jun transcription factor–bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 15.Mirante O, Price M, Puentes W, Castillo X, Benakis C, Thevenet J, et al. Endogenous protease nexin-1 protects against cerebral ischemia. Int J Mol Sci. 2013;14:16719–16731. doi: 10.3390/ijms140816719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.rthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, et al. c-Jun reprograms schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wachtel H. Dysbalance of neuronal second messenger function in the aetiology of affective disorders: A pathophysiological concept hypothesising defects beyond first messenger receptors. J Neural Transm. 1989;75:21–29. doi: 10.1007/BF01250641. [DOI] [PubMed] [Google Scholar]


