Abstract
Objective(s):
The present study aims to evaluate the protective effect of the compounds isolated from Echinophora cinerea (E. cinerea) against oxidative stress and apoptosis induced by cisplatin (CIS) in PC12 cells.
Materials and Methods:
Six compounds were isolated as quercetrin-3-O-β-D-glucopyranoside (QUE), osthol (OST), verbenone-5-O-β-D-glycopyranoside (VER), Isoimperatorin (ISO), kaempferol-3-O-β-D-glucopyranoside (KAM), and echinophorin B (ECH). For this study, we used MTT reduction assay for detection of protective effects of isolated compounds on CIS-induced cytotoxicity in PC12 cells. The effects of isolated compounds against apoptosis induced by CIS were investigated through the measurement of mitochondrial membrane potential (MMP), Bax and Bcl2 mRNA expression, and caspase-3 activation. We also assessed oxidative stress by measuring reactive oxygen species (ROS) generation with 2’, 7’-dichlorofluorescein diacetate (DCFH-DA).
Results:
Treatment of cells with QUE and OST before exposure to the CIS increased cell viability, i.e., these compounds protected the cells against CIS -induced cytotoxicity. In addition, pre-treatment with QUE and OST decreased CIS-induced apoptosis through up-regulation of Bcl-2, inhibition of caspase-3 activity, and mitochondrial membrane potential (MMP) increase. OST decreased ROS generation induced by CIS, as well.
Conclusion:
Our in vitro experiment showed that QUE and OST are apoptotic inhibitors that effectively block CIS-induced neurotoxicity predicting their therapeutic potential in the prevention of chemotherapy-induced neurotoxicity.
Keywords: Apoptosis, Cisplatin, Echinophora cinerea, Neuroprotection, Osthole, Quercetin
Introduction
Cisplatin (CIS), a highly effective chemotherapy medication, is used to treat a wide variety of both childhood and adult tumors (1). However, the chemotherapeutic use of CIS is limited by its serious side-effects such as nephrotoxicity, ototoxicity, and neurotoxicity that causes neurodegenerative diseases (2). Despite the well-known side effects of CIS treatment related to the kidney, little is known about its effects on the brain. The mechanisms by which CIS induces neuronal cell death are unclear; however, some evidence suggests that the cells may undergo changes in p53 protein expression and DNA damage (3). In addition, it has been shown that CIS induces oxidative stress by increasing levels of superoxide anion, H2O2, and hydroxyl radical. CIS-induced oxidative stress in the neurons was partially prevented by antioxidant treatments using superoxide dismutase, glutathione, selenium, and flavonoids (4-6). In the recent years, several natural antioxidants have been studied as potential neuro-protective agents and have produced promising results in in vitro model of neurodegenerative diseases and neuronal toxicity (7-9). Many research studies have been carried out to identify plants with significant antioxidant and anticancer potential by analyzing their cytotoxic, antiproliferative, apoptotic, and radical scavenging activities (10). Previous studies reported the antioxidant effect of the Echinophora species (11-13). Among them Echinophora cinerea, a plant which is used in yogurt and food as a preservative and flavoring agent (14, 15). In our previous study we showed that a flavonoid glucoside from hydro-ethanolic extract of E. cinerea showed cytoprotective effect against oxidative stress induced by hydrogen peroxide in PC12 cells (16) In the current study, we isolated the major compounds of E. cinerea acetone extract and also assessed whether these compounds are able to protect the PC12 cells against CIS-induced toxicity. Also, we investigated the mechanism underlying the protective effect of the most effective compounds. PC12 cells, derived from an adrenal tumor, possess neuronal cell features and respond positively to the nerve growth factor (NGF), differentiating into neuron-like cells (17). These cells are used as a model to investigate the neurotoxic effects of chemical agents.
Materials and Methods
General instruments and chemicals
NMR spectra were measured on a Bruker® (500 MHz) spectrometer. Chemical shifts were referenced to the residual solvent signal (CDCl3: δH 7.26,). ESI-MS, EI mass, and GC-mass spectra were performed on an Agilent 6410 qq, Agilent and Triple Quad 7890 Network mass selective spectrometer. Open column chromatographies were performed using silica gel (70–230 mesh); separations were monitored by TLC on Merck 60 F254 (0.25 mm) plates and were visualized by UV inspection and/or staining with 0.2% cerium sulfate/ 4.2% sodium molybdate and heating; HPLC were achieved on a Young Lin apparatus equipped with a binary pump (YL 9111S) and PDA detector (YL 9160). HPLC apparatus was used to purify all final products. Vertica® (Reversed phase, RP18 250×30 mm) columns were used, with 10 ml/min as flow rate. CIS, 3-(4, 5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) and 2,5 dichlorofluorescin diacetate (DCF-DA) were bought from Sigma-Aldrich (St. Louis, MO, USA). Cell culture medium, penicillin-streptomycin, and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). All the solvents used for extraction and purification were purchased from Merck (Germany) and Dr Mojallali (Iran).
Plant materials
Aerial parts of E. cinerea (Boiss.) Hedge et Lamond were collected from Pazanan Mountain, Kohkiloye and Boyer Ahmad, Iran, at an altitude of 1800 m above sea level. Plant materials were identified by Dr Azizollah Jafari, University of Yasouj, Yasouj, Iran. A specimen was kept at the Herbarium of Isfahan School of Pharmacy (No. 1122).
Extraction and isolation of flavonoids & coumarins
Air-dried plant material was ground to powder. 1000 g of the powdered plant (aerial parts) was macerated with (8 l × 3) acetone for two days at room temperature with mixing. After discarding the extract, the plant residue was extracted with EtOH: H2O (8:2) for two days (8 l × 3) which resulted in 100 g hydroalcoholic extract. A 30 g aliquot of the latter was fractionated by vacuum liquid chromatography (sorbent, RP18) using a gradient of MeOH and H2O from 5% of the premier to 100%, to afford 6 fractions (F1-F6).
F2 was purified by reversed phase HPLC using a gradient of MeOH and H2O from 20% of premier to 100% to obtain 6 fractions (F2a-F2f). F2c (5.9 mg) and F2f (2 mg) showed to be impure compounds 1 and 2 which were purified using further HPLC analyses.
F4 was purified by reversed phase HPLC using a gradient of MeOH and H2O from 50% of premier to 100% to obtain 8 fractions (F4a-F4h). (F4g) (49 mg), and (F4h) (22 mg) showed to be compounds 3 and 4, respectively.
F5 was purified by reversed phase HPLC using a gradient of MeOH and H2O from 80% of premier to 100% to obtain 5 fractions (F5a-F5e). F5c (12 mg) and F5e (21 mg) showed to be compounds 5 and 6, respectively.
All structures were elucidated using extensive NMR and mass experiments and comparison to literature (Figure 1)(16, 18).
Figure 1.
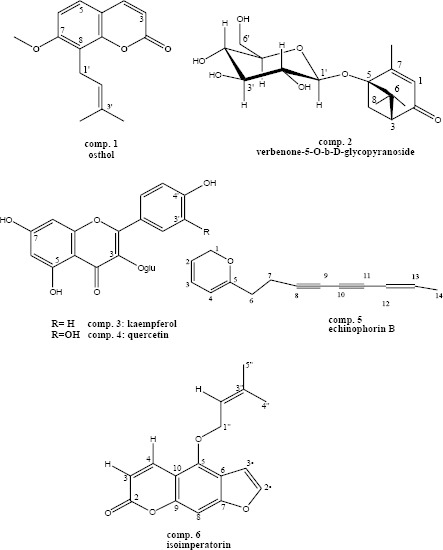
Structures of isolated compounds from Echinophora cinerea
Compound 1; osthol; osthole; 1HNMR(500 MHz, CD3OD, J in Hz) δ:7.60 (1H, d, J=9.5, H-4), 7.15(1H, d, J=8.84,H-5), 6.79(1H, d, J=8, H-6), 6.34(1H, d, J=9.5, H-3), 5.38(1H,t, J=7.3, H-2’), 4.02(3H, S, 7-OCH3), 3.6(2H,d,J=7.0, H-1’), 1.91(3H,S,H-4’), 1.66(3H, S, H-5’).
Compound 2; verbenone-5-O-β-D-glycopyranoside; 1HNMR(500 MHz, CD3OD, J in Hz) δ: 5.69 (1H, q, J=1.4 H-3), 4.58(1H, d, J=8.2, H-1’), 3.84(1H, dd, J=2.12,12, H-6’a), 3.62 (1H, dd, J=5.2,12.0, H-6’b), 3.57(1H, t, J=8.6, H-2’), 3.40(1H, m, H-3’), 3.33(1H, m,H-5’), 3.31(1H, m, H-4’), 3.19(1H, dd, J=7.2, 16.4, H-7b), 2.56(1H, dd, J=2.55-7.10, H-1), 2.49(1H,d, J=9.6, H7a), 2.1(3H, d, J=1.20, H-10), 1.48(3H,s, H-8), 1.06(3H, s, H-9). EI-mass (aglycone), m/z [ion]+: 166[M]+, 151[M-Me]+, 138 [M-2Me]+, 124 [M-3Me]+, 111[M-C8H15]+. ESI-mass, m/z[ion]+: 840[2M+166+ 23]+, 679[2M+23]+, 351[M+23]+.
Compound 3; Quercetin-3-O-β-D-glucopyranoside; 1H NMR (500 MHz, CD3OD, J in Hz) δ: 7.70 (1H, d,J=2, H-2’), 7.61(1H, dd, J=2, 8.4, H-6’), 6.89(1H, d, J=8.47, H-5’), 6.42(1H, d, J=1.5, H-8), 6.23(1H, d, J=1.5, H-6), 5.28(1H, d, J=7.62, H=1”), 3.19-3.73(6H, m, H-2” to H-6”). EI-mass (aglycone), m/z [ion]+:302[M]+, 274 [M-CO]+, 153 [M-C7O4H4]+, 137[M-C7O3H5]+
Compound 4; Kaempferol-3-O-β-D-glucopyranoside; 1HNMR(500 MHz, CD3OD, J in Hz) δ : 8.08(2H, d, J=8.86, H-2’ and H-6’), 6.9(2H, d, J=8.6, H-3’ and H-5’), 6.43(1H, s, H-8), 6.23(1H, s, H-6), 5.28(1H, d, J=7.24, H=1”), 3.19-3.71 (6H, m, H-2” to H-6”). EI-mass (aglycone), m/z [ion]+:286[M]+, 258 [M-CO]+, 153 [M-C7O4H4]+, 121[M-C7O2H5]+.
Compound 5; echinophorin B; 1HNMR(500 MHz, CD3OD, J in Hz) δ: 7.51 (1H, m, H-3), 6.30(2H, m, J=3.7, H-4), 6.26(1H, m, H-13), 6.17(1H,d, J=5.9, H-2), 5.53(1H, d, J=11.6, H-12), 2.73-2.76(2H, m, H-6), 2.76-2.81(2H,m, H-7), 1.89(3H, dd, J=1.5, 6.9, H-14). EI-mass, m/z [ion]+:212[M]+, 211 [M-H]+, 197 [M-CH3]+, 183 [M-C2H6]+, 169[M-C3H7]+,115[M-C9H7], 95[M-ester], 77[M-C6H5]+.
Compound 6; isoimperatorin; 1H NMR(500 MHz, CD3OD, J in Hz) δ: 8.28 (1H, d, J=9.80, H-4), 7.82(1H, d, J=2.30, H-2’), 7.23(1H, s, H-8), 6.99(1H, d, J=2.3, H-3’), 5.59(1H, tJ=6.80,.2’’-H),5.03(2H, d, J=6.80, 1’’-OCH2), 1.85(2H,s, 5’’- CH3), 1.79(2H, s, 4’’-CH3)
Cell culture conditions
Rat pheochromocytoma-derived cell line (PC12) was obtained from Pasteur Institute (Tehran, Iran) and maintained at 37 °C in a humidified atmosphere (90%) containing 5% CO2. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% (v/v) heat-inactivated FBS, 100 Uml−1 penicillin, and 100 mg/ml−1 streptomycin.
Cell viability assay
Cellular toxicities of CIS and compounds were analyzed in PC12 cells using the methylthiazol tetrazolium bromide method (MTT) (19). Cells were cultured in a 96-well plate at a density of 7,000 cells/well and in a volume of 200 µl. Stock solutions of the compounds were prepared in dimethyl sulfoxide (DMSO). The final concentration of the solvent in the medium was always 0.5%. At appropriate time intervals, the medium was removed and replaced with 200 μl of 0.5 mg/ml of MTT in the growth medium and then the plates were transferred to a 37°C incubator for 3 hr. Then, the medium was removed, and the purple formazan crystals were dissolved in DMSO (200 μ/well). Absorbance was determined on an ELISA plate reader (Biotek, H1M) with a test wavelength of 570 nm and a reference wavelength of 630 nm to obtain the sample signal (OD570–OD630).
Determination of intracellular ROS
Intracellular ROS levels were examined using DCF-DA. DCF-DA is a non-fluorescent lipophilic ester that easily crosses the plasma membrane and passes into the cytosol, where the acetate group is rapidly removed by unspecific esterases (20). The oxidation of this molecule to the fluorochrome DCF results in green fluorescence. The intensity of this fluorescence is generally considered to reflect the level to which ROS are present (21). After seeding for 24 hr, PC12 cells were washed with PBS buffer (pH 7.4). The cells pretreated with selective compounds for 6 hr, then were treated with CIS for an additional 24 hr. After washing with PBS, the cells were incubated with 20 µl DCF-DA at 37 °C for 30 min. The percentage of DMSO in solution did not exceed 0.5%. After incubation, cells were lysed with Triton X-100. The fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 528 nm using a fluorescence microplate reader (BioTek, H1M, USA).
Measurement of mitochondrial membrane potential
Mitochondrial dysfunction has been shown to participate in the induction of apoptosis. In this study, mitochondrial membrane potential was measured by using rhodamine 123 fluorescent dye. R123 influx mainly depends on the protonic membrane potential of mitochondria with negative charge inside in mitochondrial matrix. Depolarization of mitochondria during cell apoptosis results in the loss of rhodamine 123 from the mitochondria and a decrease in intracellular fluorescence intensity (22). At the end of treatment, cells were washed with PBS, incubated with rhodamine 123 for 30 min at 37 °C, and then washed twice with PBS. The fluorescence intensity was measured at an excitation wavelength of 488 nm and an emission wavelength of 520 nm using a fluorescence microplate reader (BioTek, H1M, USA).
Determination of Caspase-3 activity
Caspases are a family of proteases that mediate cell death and are important in the apoptosis. Caspase 3, an effector caspase, is the most studied of mammalian caspases (23). The caspase-3 activity was measured using commercial caspase-3 assay kit Sigma (USA), according to the manufacturer’s protocol. The kit is based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-vial-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase 3, resulting in the release of the p-nitroaniline (pNA) moiety. Briefly, 1×106 cells were collected and lysed with 50 μl of chilled lysis buffer and incubated on ice for 10 min. Cell lysates were centrifuged at maximum speed for 5 min at 4 °C, 10 μl of cell lysate was combined with an equal amount of substrate reaction buffer containing a caspase-3colorimetric substrate. This mixture was incubated for 2 hr at 37 °C, and then the pNA light emission was quantified using a microplate reader at 400- or 405- nm (BioTek, H1M). Comparison of the absorbance of pNA from an apoptotic sample with an uninduced control allowed determination of the fold increase in caspase-3 activity. The protein content was determined by the Bradford method using the bovine serum albumin as a standard.
Real-time RT-PCR analysis of apoptosis-related gene expression
Total RNA from PC12 cells were extracted using high pure isolation kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Quality and quantity of total RNA were assessed by spectrophoto-meter (NanoDrop 2000, USA) and samples were stored at −80 °C until use. The primers used in this study were selected from published studies)7). The performances of all primer pairs were tested by primer concentration to determine the optimal reaction conditions. Thermal cycler conditions were 15-min at 50 °C for cDNA synthesis, 10-min at 95 °C followed by 40 cycles of 15-sec at 95 °C to denature the DNA, and 45-sec at 60 °C to anneal and extend the template. Melting curve analysis was performed to ascertain specificity by continuous acquisition from 65 °C–95 °C with a temperature transient rate of 0.1 °C/sec. All reactions were performed in triplicate in a Corbett system (Australia). The values obtained for the target gene expression were normalized to β-actin and analyzed by the relative gene expression −ΔΔCT method where -ΔΔCT= (CT target−CT β-actin) Unknown − (CT target − CT β-actin) calibrator.
Statistical analysis
Each experiment was performed at least three times and the results were presented as mean±SEM (standard error of the mean). One-way analysis of variance (ANOVA) followed by Tukey’s test was used to compare the differences between means. A probability value of P<0.05 was considered to be statistically significant.
Results
Effect of isolated compounds on cytotoxicity induced by CIS
As a first step, the cytotoxicity of the compounds on PC12 cells was examined. We found that the compounds in the concentration range 5–100 μg/ml had no cytotoxic effect on PC12 cells (Figure 2a). Moreover, the viability of PC12 cells was evaluated after 24 hr of exposure to different concentrations of CIS using the MTT method. Our results showed that CIS induced cytotoxicity in a concentration-dependent manner. The mean IC50 value±SEM was 27.4±1.56 μM (Figure 2b). Using the concentrations of compounds that were nontoxic, another experiment was performed to evaluate the effects of these compounds on cytotoxicity induced by CIS. As shown in Figure 3, among compounds only OST (6.25 and 25 µg/ml) and QUE (6.25 µg/ml) have outstandingly protective effects against CIS-induced toxicity in PC12 cells. Compared with the group treated with CIS alone, the cell population increased in the groups that had been pretreated with OST and QUE.
Figure 2.
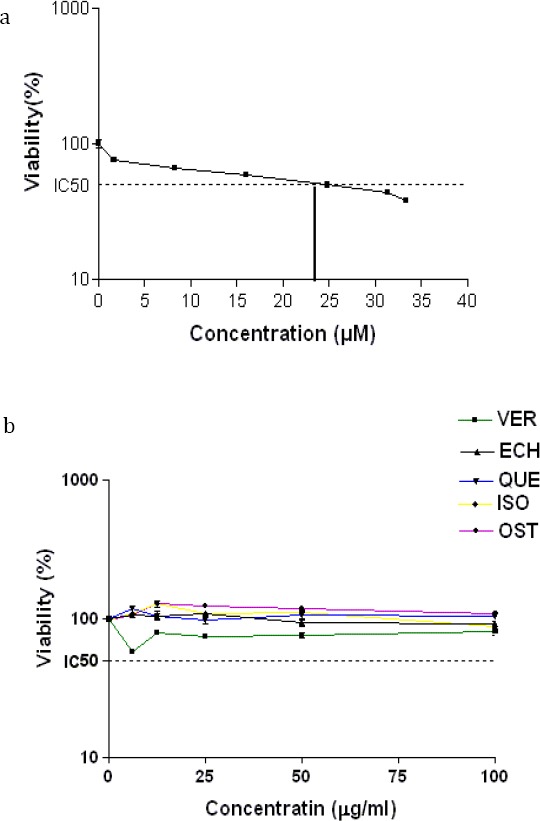
The effects of a) cisplatin(CIS) (0-33.3 µM), and b) isolated compounds (0-100) from Echinophora cinerea on the viability of the PC-12 cells. The cell viability was determined by MTT assay after 24 hr exposure as described in materials and methods. Data are expressed as the mean±SEM of three separate experiments. **P-value <0.01, ***P-value<0.001 vs Control
Figure 3.
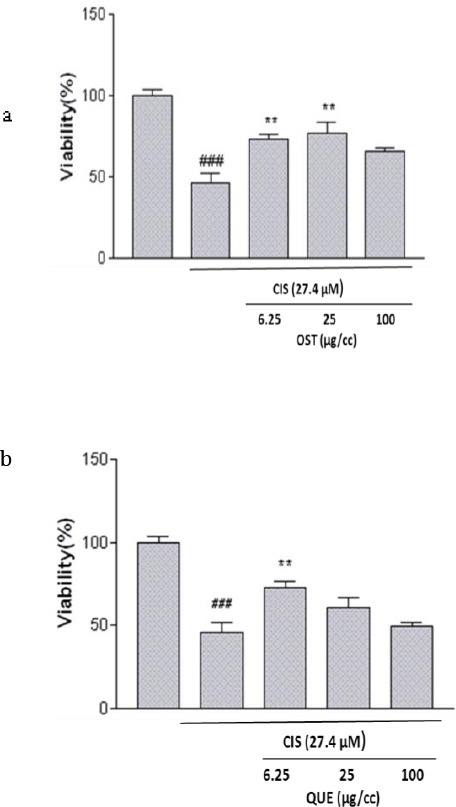
The effect of a) osthol (OST) and b) quercetin (QUE) on cisplatin-induced cytotoxicity in PC-12 cells. Cells were pretreated with osthol (25 µg/ml) and quercetin (6.26 µg/ml) 24 hr before exposure to 27.4 µM of CIS. Data are expressed as the mean±SEM of three separate experiments (n=5). ### P-value<0.01 vs control, ** P-value < 0.01 vs cisplatin-treated cells
Based on this information 24 hr pretreatment with both of the compounds was used for subsequent studies.
Effects of selective compounds on CIS-induced mitochondrial membrane potential (MMP) collapse
MMP was determined using a cell permeable cationic fluorescent dye. Depolarization of mitochondrial membrane potential caused by the CIS-induced damage of the outer membrane resulted in the loss of the dye from the mitochondria and a decrease in intracellular fluorescence so that CIS (27.4±1.56) significantly decreased MMP to 56.3±2.5 % of control in PC12 cells. Pretreatment of cells with the OST and QUE inhibit the reduction of MMP induced by CIS (Figure 4).
Figure 4.

The effect of osthol (OST) (25 µg/ml) and quercetin (QUE) (6.26 µg/ml) on cisplatin (CIS) (27.4 µM) –induced mitochondrial membrane potential (MMP) collapse as detected by rhodamine 123. Data are expressed as the mean±SEM of three separate experiments. ## P<0.01 vs Control,* P-value <0.01, ** P-value <0.05 vs cisplatin-treated cells
Effects of selective compounds on caspase– 3 activity
In the current study for improvement of MTT results and also characterizing the type of cell death involved in our experiments, the activity of caspase was evaluated. Activation of caspase is well-known to play an essential role in the initiation and progression of programmed cell death (23). The obtained results showed that CIS significantly increased caspase-3 activation in PC12 cells (173.5±6.2). Pretreatment with OST and QUE decreased significantly caspase-3 activation to 137.9±1.46 and 117.32±3.25 compared to CIS- treated cells, respectively (Figure 5).
Figure 5.
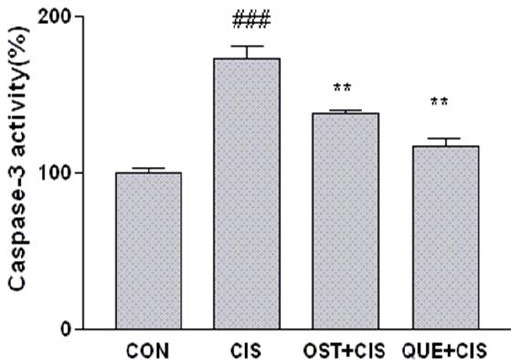
The effect of osthol (OST) and quercetin (QUE) on caspase-3 activity. Cells pretreated with osthol (25 µg/ml) and quercetin (6.26 µg/ml) 24 hr before exposure to 27.4 µM of CIS. Caspase-3 activity was measured by colorimetric detection of p-nitroaniline and expressed as percent of control. Data are expressed as the mean±SEM of three separate experiments. ## P<0.01 vs Control, ** P-value<0.02, * P-value<0.05 vs cisplatin-treated cells
Determination of the expression levels of apoptosis-regulatory genes
To approve the above results and also to investigate how the compound pretreatment decreases CIS-induced apoptosis, we examined the mRNA expression of the Bcl-2 protein family (Bcl2 and Bax) in PC12 cells. CIS significantly increased Bax mRNA expression to 2.95-fold of control and decreased Bcl2-mRNA expression to 0.316-fold of control. When cells were pretreated with OST and QUE, the levels of BAX mRNA expression decreased significantly. Moreover, pretreatment with QUE up-regulated Bcl2 compared to cells that had not been pretreated with QUE. OST had no effect on Bcl-2 mRNA expression (Figure 6a). Furthermore, the Bax/Bcl2 ratio increased 12.43-fold upon treatment with CIS, while in cells that had been pretreated with OST andQUE, the Bax/Bcl2 ratio markedly decreased to 2.50 and 1.81- fold, respectively (Figure 6b).
Figure 6.
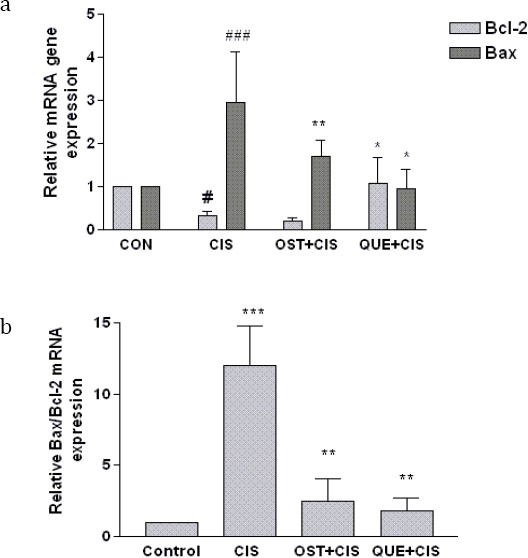
The effect of querctin (QUE) and osthol (OST) on (a) Bcl2, Bax and (b) Bax/Bcl2 mRNA expression in PC12 cells. Normalization relative to b-actin was performed. Levels of mRNA are expressed relative to control cells in the mean±SEM values derived from three independent experiments. # P-value<0.05, ### P-value<0.001 vs control, * P-value<0.05, ** P-value<0.01 vs cisplatin treated cells
Effect of the Compounds on CIS-induced ROS Generation
In order to measure oxidative stress induced by CIS, ROS accumulation was detected in PC12 cells by using the fluorescent probe DCF, which detects intracellular peroxides. Adding CIS to PC12 cells caused a significant increase in the ROS level. Then we evaluated whether the compounds protect PC12 cells from oxidative damage caused by CIS via lowering levels of ROS. As demonstrated in Figure 7, OST exerted excellent radical scavenging activity. Pretreatment of the cells with OST significantly reduced the ROS levels to 72.81±7.19%. It is interesting that QUE treatment did not show any notable effect on oxidative stress induced by CIS in the used concentration of QUE.
Figure 7.

The effect of osthol (OST) and querctin (QUE) on the cisplatin –induced ROS overproduction as detected by DCF. Cells were pretreated with osthol (OST) (25 µg/ml) and querctin (QUE) (6.26 µg/ml) 24 hr before exposure to 27.4 µM of cisplatin. Data is expressed as the mean±SEM of three separate experiments. ### P-value <0.0_1 vs Control, * P-value <0.05 vs cisplatin treated cells
Discussion
In recent years, several natural antioxidants have been studied as potential neuroprotective agents and have produced promising results in both in vivo and in vitro models of neurodegenerative diseases and neuronal toxicity (23). CIS is one of the broad range chemotherapy drugs. However, clinical use of CIS is severely limited by its adverse effect, including neurotoxicity (24). Oxidative damage contributes to CIS-induced neurotoxicity, but the exact mechanism remains unclear. The aim of the present study was to evaluate the possible protective effects of compounds isolated from E. cinerea, an antioxidant plant, against- CIS-induced cell injury in PC12 cells. The following six compounds were isolated: osthol (OST), quercetin glucoside (QUE), verbenone glucoside, kaempferol glucoside, echinophorin B, and isoimperatorin. Results showed that CIS decreases cell viability in PC12 cells. To gain an understanding of mechanisms by which CIS can induce cytotoxicity in PC12 cells, we examined some parameters involved in apoptosis. Mitochondria have a critical role in activating apoptosis in mammalian cells. The Bcl-2 family of proteins is able to regulate the permeability of the mitochondrial membrane (25). Permeabilization of the mitochondrial membrane causes bioenergetics failure and permits the release of a small hem protein, cytochrome c (Cyt c), to the cytosol, which results in, caspase-9 activation. (26). Mature caspase-9 activates additional caspase-9 molecules as well as caspase-3, and in turn, the execution of cell apoptosis. Caspase-3 activates downstream caspases in a proteolytic cascade (27). In the present study, a decrease in the expression of Bcl-2 and MMP and also increase in Bax mRNA expression and caspase-3 activation were observed after treating the PC12 cell line with CIS. Oxidative stress is proposed to be responsible for CIS-induced apoptosis in PC12 cells (28). Therefore, we decided to evaluate ROS production after treatment with CIS. The obtained results indicated that treatment with CIS leads to an increase in the ROS generation in PC12 cells. In the next stage of our experiment, we investigated the protective effects of isolated compounds on PC12 cells against CIS-induced neurotoxicity and explored the underlying mecha-nisms. Our results confirmed that among isolates OST and QUE showed the highest protective effects against the cytotoxicity induced by CIS whereas others failed to exhibit any protective effects. OST (7-methoxy -8-isopent epoxy-coumarin), is a natural coumarin derivative, which is extracted from many medicinal plants, such as Angelica, Prangos, etc. (18, 29). QUE (3,3’,4’,5,7-pentahydroxy flavone) belongs to flavonoids. It is present in many edible fruits and vegetables such as apples, grapes, lemons, onion, kale, and tomatoes (30). The results showed that OST and QUE are able to protect PC12 cells through inhibition of apoptosis, as proved by the attenuation of mRNA expression of proapoptotic gene Bax and caspases-3 activation. Since the Bax/Bcl-2 ratio is an important regulator of apoptosis (27), it is relevant to note that OST and QUE pretreatment increased the Bax/Bcl-2 ratio compared to CIS alone in PC12 cells. Moreover, both compounds increased MPP reduction induced by CIS. Mechanistically, OST significantly inhibited reactive oxygen species (ROS)-generation in CIS-treated PC12 cells. It is interesting that pretreatment with QUE did not show any significant effects on the ROS generation. This result identified that the cellular effects of QUE are the complex and anti-apoptotic effect of QUE, not mediated through the reduction of ROS levels. Accordingly, some other researchers have reported that QUE had a neuro-protective effect against rat brain ischemia/reperfusion (I/R) injury (31). In addition, a previously published study indicated that QUE prevents neuronal death induced by exposure to hydrogen peroxide (H2O2) in P19 neuron cells (32). Also, in C6 glioma cells, QUE attenuates oxidative stress induced by hydrogen peroxide or interleukin-1β (33). In recent years, numerous studies have focused on the neuroprotective potential of OST (34, 35). OST reportedly possesses neuroprotective effects by inhibiting oxidative stress and apoptosis. Recently, we reported that OST is able to protect PC12 cells from apoptosis induced by doxorubicin (7).
Conclusion
Our in vitro experiment showed that QUE and OST are apoptotic inhibitors and effectively blocked CIS-induced neurotoxicity, predicting their therapeutic potential in the prevention of chemotherapy-induced neurotoxicity.
Acknowledgment
The results presented here are extracted from the Pharm D thesis of Sh Khajouei. This study was financially supported by the Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran.
References
- 1.Gulec M, Oral E, Dursun OB, Yucel A, Hacimuftuoglu A, Akcay F. Mirtazapine protects against cisplatin-induced oxidative stress and DNA damage in the rat brain. Physical Ci Neuro Sci. 2013;67:50–8. doi: 10.1111/j.1440-1819.2012.02395.x. [DOI] [PubMed] [Google Scholar]
- 2.Cavaletti G, Pezzoni G, Pisano C, Oggioni N, Sala F, Zoia C. Cisplatin-induced peripheral neurotoxicity in rats reduces the circulating levels of nerve growth factor. Neuro Sci Lett. 2002;322:103–106. doi: 10.1016/s0304-3940(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 3.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neuro Biol Dis. 2011;41:661–668. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SA, Choi KS, Bang JH, Huh K, Kim SU. Cisplatin-Induced Apoptotic Cell Death in Mouse Hybrid Neurons Is Blocked by Antioxidants Through Suppression of Cisplatin-Mediated Accumulation of p53 but Not of Fas/Fas Ligand. J Neuro Chem. 2000;75:946–953. doi: 10.1046/j.1471-4159.2000.0750946.x. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Machado C, Mendonca LM, de Paula Venancio V, Bianchi MLP, Antunes LMG. Coenzyme Q10 protects Pc12 cells from cisplatin-induced DNA damage and neurotoxicity. Neuro Toxicol. 2013;36:10–16. doi: 10.1016/j.neuro.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Karimi G, Ramezani M, Tahoonian Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid-BasComplementary Alt Med. 2005;2:383–386. doi: 10.1093/ecam/neh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shokoohinia Y, Hosseinzadeh L, Moieni-Arya M, Mostafaie A, Mohammadi-Motlagh H-R. Osthole Attenuates Doxorubicin-Induced Apoptosis in PC12 Cells through Inhibition of Mitochondrial Dysfunction and ROS Production. Bio Med Res Int. 2014:383–386. doi: 10.1155/2014/156848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mojarrab M, Mehrabi M, Ahmadi F, Hosseinzadeh L. Protective effects of fractions from Artemisia biennis hydroethanolic extract against doxorubicin- induced oxidative stress and apoptosis in PC12 cells. Iran J Basic Med Sci. 2016;19:803–810. [PMC free article] [PubMed] [Google Scholar]
- 9.Mojarrab M, Nasseri S, Hosseinzadeh L, Farahani F. Evaluation of antioxidant and cytoprotective activities of Artemisia ciniformis extracts on PC12 cells. Iran J Basic Med Sci. 2016;19:430–438. [PMC free article] [PubMed] [Google Scholar]
- 10.Roleira FM, Tavares-da-Silva EJ, Varela CL, Costa SC, Silva T, Garrido J, Borges F. Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chem. 2015;183:235–258. doi: 10.1016/j.foodchem.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Sajjadi SE, Ghannad A. Composition of the Essential Oil of Echinophora cinerea (Boiss.) Hedge et Lamond. J Essen Oil Res. 2002;14:114–15. [Google Scholar]
- 12.GhasemiPirbalouti A, Gholipour Z. Chemical composition, antimicrobial and antioxidant activities of essential oil from Echinophora cinerea harvested at two phenological stages. J Essent Oil Res. 2016:1–11. [Google Scholar]
- 13.Gokbulut I, Bilenler T, Karabulut I. Determination of chemical composition, total phenolic, antimicrobial, and antioxidant activities of Echinophora tenuifolia essential oil. Int. J Food Prop. 2013;16:1442–1451. [Google Scholar]
- 14.Sharafati-chaleshtori R, Rafieian-kopaei M, Mortezaei S, Sharafati-chaleshtori A, Amini E. Antioxidant and antibacterial activity of the extracts of Echinophora platyloba DC. Afr J Pharm Pharmacol. 2012;6:2692–5. [Google Scholar]
- 15.Shafee-Zadeh F. Medicinal plants of Lorestan. 1st ed. Lorestan University of Med Sci; 2002. [Google Scholar]
- 16.Shokoohinia Rashidi M, Hosseinzadeh L, Jelodarian Z. Quercetin-3-O-β-D-glucopyranoside, a dietary flavonoid, protects PC12 cells from H2O2-induced cytotoxicity through inhibition of reactive oxygen species. Food Chem. 2015;167:162–7. doi: 10.1016/j.foodchem.2014.06.079. [DOI] [PubMed] [Google Scholar]
- 17.Yoo Y, Hong J, Hur KC, Oh E-S, Chung J. Iron enhances NGF-induced neurite outgrowth in PC12 cellsMol Cells2004; 17:340–6. [PubMed] [Google Scholar]
- 18.Jelodarian Z, Shokoohinia Y, Rashidi M, Ghiasvand N, Hosseinzadeh L, Iranshahi M. New polyacetylenes from Echinophora cinerea (Boiss.) Hedge et Lamond. Nat Prod Res. 2017:1–8. doi: 10.1080/14786419.2017.1300797. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. “Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays”. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Mojarrab M, Jamshidi M, Ahmadi F, Alizadeh E, Hosseinzadeh L. Extracts of Artemisia ciniformis protect cytotoxicity induced by hydrogen peroxide in H9c2 cardiac muscle cells through the inhibition of reactive oxygen species. Adv Pharm Sci. 2013;36:10–16. doi: 10.1155/2013/141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson M, Kurz T, Brunk B, Nilsson S E, Frennesson C.I. “What does the commonly used DCF test for oxidative stress really show?”. Biochem J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 22.Chandra G, Diana S, Beattie S. Coupling between electron transfer and proton pumping in respiratory chain complexes II+III of Rat Liver Mitochondria. ChinBio ChemJ. 1995;11:200–206. [Google Scholar]
- 23.Zhang L, Cheng X-R, Juan-Juan H, Lan S, Guan-Hua D. Neuroprotective effects of hyperoside on sodium azide-induced apoptosis in PC12 cells. ChinJ Nat Med. 2011;9:450–455. [Google Scholar]
- 24.Zeiss CJ. The apoptosis-necrosis continuum: Insights from genetically altered mice. Vet Pathol. 2003;40:481–495. doi: 10.1354/vp.40-5-481. [DOI] [PubMed] [Google Scholar]
- 25.Salakou S1, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos DS, Papapetropoulos T, Petsas T. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21:123–32. [PubMed] [Google Scholar]
- 26.Shokoohinia Y, Hosseinzadeh L, Alipour M, Mostafaei A, Mohammadi Motlagh HR. Comparative evaluation of cytotoxic and apoptogenic effects of several coumarins on human cancer cell lines: osthole induces apoptosis in p53-deficient H1299 cells. Adv Pharmacol Sci. 2014 doi: 10.1155/2014/847574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosseinzadeh L, Behravan J, Mosaffa F, Bahrami G, Bahrami A, Karimi G. Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food Chem Toxicol. 2011;49:1102–1109. doi: 10.1016/j.fct.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Li DW, Sun JY, Wang K, Zhang S, Hou YJ, Yang MF. Attenuation of Cisplatin-Induced Neurotoxicity by Cyanidin, a Natural Inhibitor of ROS-Mediated Apoptosis in PC12. Cells Mol Neur Bio. 2015;35:995–1001. doi: 10.1007/s10571-015-0194-6. [DOI] [PubMed] [Google Scholar]
- 29.Chao X, Zhou J, Chen T, Liu W, Dong W, Qu Y. Neuroprotective effect of osthole against acute ischemic stroke on middle cerebral ischemia occlusion in rats. Brain Res. 2010;1363:206–211. doi: 10.1016/j.brainres.2010.09.052. [DOI] [PubMed] [Google Scholar]
- 30.Hollman PC, van Trijp JM, Buysman MN, vd Gaag MS, Mengelers MJ, de Vries JH, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Let. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 31.Lei X1, Chao H, Zhang Z, Lv J, Li S, Wei H1, Xue R1, Li F1, Li Z. Neuroprotective effects of quercetin in a mouse model of brain ischemic/reperfusion injury via anti-apoptotic mechanisms based on the Akt pathway. Mol Med Rep. 2015;12:3688–3696. doi: 10.3892/mmr.2015.3857. [DOI] [PubMed] [Google Scholar]
- 32.Vuković L, Puhović J, Erhardt J, Oršolić N. Neuroprotective effect of quercetin against hydrogen peroxide-induced oxidative injury in P19 neurons. J Mol Neuro Sci. 2012;47:286–299. doi: 10.1007/s12031-012-9737-1. [DOI] [PubMed] [Google Scholar]
- 33.Chen TJ1, Jeng JY, Lin CW, Wu CY, Chen YC. Quercetin inhibition of ROS-dependent and -independent apoptosis in rat glioma C6 cells. Toxicol. 2006;223:113–126. doi: 10.1016/j.tox.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 34.He Y, Qu S, Wang J, He X, Lin W, Zhen H. Neuroprotective effects of osthole pretreatment against traumatic brain injury in rats. Brain Res. 2012;1433:127–136. doi: 10.1016/j.brainres.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Hai-Jie Ji, Jin-Feng Hu, Yong-Hui Wang, Xiao-Yu Chen, Ran Zhou, Nai-Hong Chen. Osthole improves chronic cerebral hypo-perfusion induced cognitive deficits and neuronal damage in hippocampus. Eur J Pharmacol. 2010;636:96–101. doi: 10.1016/j.ejphar.2010.03.038. [DOI] [PubMed] [Google Scholar]


