Abstract
Objective(s):
This study aimed to investigate the effect of zinc oxide nanoparticles (ZnO-np) on biofilm formation and expression of the flu gene in uropathogenic Escherichia coli (UPEC) strains.
Materials and Methods:
Minimum inhibitory concentration (MIC) of ZnO-np was determined by agar dilution method. The effect of MIC and sub-MIC concentrations of ZnO-np on biofilm formation were determined by microtiter plate assay. The expression level of the flu gene was assessed by Real-Time PCR assay.
Results:
MIC and sub-MIC ZnO-np concentrations reduced biofilm formation by 50% and 33.4%, respectively. Sub-MIC ZnO-np concentration significantly reduced the flu gene expression in the UPEC isolates (P<0.0001).
Conclusion:
The sub-MIC concentration of ZnO-np reduces biofilm formation and flu gene expression in UPEC isolates. It is suggested to use nanoparticles for coating medical devices to prevent bacterial colonization.
Keywords: Biofilm, Urinary tract infection, Uropathogenic Escherichia -coli, Zinc oxide nanoparticle
Introduction
Uropathogenic Escherichia coli (UPEC) expresses some adhesive factors such as type 1, P, S and f1c pili that cause urinary tract infection (UTI) by adherence to urinary tract epithelial cells (1). UPEC is the most common cause of UTI (2, 3). Treatment of UTI imposes a substantial economic burden on the healthcare systems around the world (4). Patients with urinary catheter have higher risk of acquiring UTI. Biofilm formation on catheter is one reason for this risk, bacterial biofilm is formed on after seven days of catheterization, which can eventually lead to UTI (5). Biofilm is a community of bacteria which embed in an exo-polysaccharide matrix. Bacteria within the biofilm are protected from environmental stresses such as dryness, host immune response, antimicrobial substances and antibiotics (6). Different surface structures such as type 1 fimbriae, curli, PGA polysaccharides, clavulanic acid, flagella and antigen 43 (Ag43) are involved in the process of biofilm formation. Ag43 is an important surface protein in E. coli which is encoded by flu gene. Receptors of Ag43 are present not only on human target cells but also on the surface of these bacteria that leads to adherence of bacteria to each other and microcolony formation by bacterial auto-aggregation. This process is essential in early stages and lead to increase biofilm formation in later stages. Deletion of the Ag43 interferes with biofilm formation (7, 8).
Due to increased resistance to antimicrobial agents, infectious disease remains a public health problem worldwide. These problems increase the need for high dose of antibiotics that often lead to intolerable toxicity. Hence, alternative strategies for treatment of bacterial infections were sought and nanostructures were introduced as new antimicro-bial agents. Although the exact mechanisms of action of nanoparticles is not yet fully understood, it may be dependent on factors such as composition, surface changes, inherent properties of nanoparticles, nanoparticle concentration and specie of bacteria. Zinc oxide nanoparticle (ZnO-np) were found as the most toxic against E. coli among the metal oxide nanoparticles. ZnO disrupts membrane integrity via production of reactive oxygen species that destroy bacteria (9-12). In addition, production of hydrogen peroxide and Zn2+ have shown a key role in the antibacterial activity of nanoparticles. ZnO nanoparticles have selective toxicity toward bacteria, with minimal effects on human and animal cells (13). The aim of this study was to evaluate the effect of ZnO-np on biofilm formation and flu gene expression in UPEC strains.
Materials and Methods
Bacterial strains
Twenty nine UPEC isolates that previously collected from urine samples of inpatients and outpatients in Gorgan, North of Iran were used in this study. Their antibiotic sensitivity profile, resistance to third generation cephalosporins and extended spectrum beta-lactamase (ESBLs) production were confirmed by standard methods, previously. E. coli PTCC 1399 strain was used as positive control for evaluation of biofilm formation and flu gene expression.
Preparation of ZnO-np
ZnO-np with approximate size of 10 to 30 nm was purchased from US Research Nanomaterials Co (USA, lot number: us3590). ZnO-np powder was mixed with propylene glycol and the solution was sonicated for 30 min at frequency of 20000 HZ to prevent agglutination of nanoparticles. Finally, six different concentrations of nanoparticles were prepared (156, 312 / 5, 625, 1250, 2500 and 5000 μg/ml).
Determination of minimum inhibitory concentration (MIC)
MIC of ZnO-np was determined by agar dilution method (14, 15). Different concentrations of ZnO nanoparticles were added to melted Mueller Hinton agar medium. Fifty μl of each UPEC isolates with concentration equal to 0.5 McFarland were added on the medium and the plates were incubated at 37 °C for 24 hr. MIC was determined as the lowest concentration at which nanoparticles completely inhibited growth of the isolates, and the 1/2 MIC was considered as sub-MIC.
Biofilm inhibition assay
The inhibitory activity of ZnO-np against UPEC biofilm formation was evaluated by microtiter plate method, according to Samet et al instructions (16). Briefly, to 190 µl of bacterial suspension (105 CFU/ml) in BHI broth containing 1% (w/v) sucrose were inoculated in 96 microtiter plate and 10 μl of MIC or sub-MIC concentrations of ZnO-np were added. Plates were incubated in a rotator at 200 rpm and 37 °C for 24 hr. The extent of biofilm in each well was determined by crystal violet dye which released by ethanol/acetone (80:20). Samples with optical density (OD490) of less than 0.1, 0.1-0.2, 0.2-0.3, and more than 0.3 were considered as non, weak, moderate and strong biofilm former (17). These experiments were repeated at least three times.
Expression of the flu gene
Three isolates which formed strong biofilm in media with and without sub MIC concentration of ZnO nanoparticles were chosen for studying expression of the flu gene. Suspension of 105 CFU/ml of each bacterial isolate was inoculated in brain-heart infusion broth, containing 1% sucrose and sub-MIC concentrations of ZnO-np. The control was prepared using the same medium without ZnO-np. After 24 hr of incubation at 37 °C with rotation, the supernatant was discarded and RNA was extracted from biofilms using RNX-Plus kit (SinaColon Co) according to the manufacturer’s instructions. Absorbance was assessed by a spectrophoto-meter, and the ratio of absorbance at 260 nm and 280 nm was used to assess the purity of the extracted RNA. The result within the 1.8 to 2 range was considered as acceptable purity. Quality of the extracted RNA was evaluated via electrophoresis on 1.2% agarose gel at 100 V for 60 min. Genomic DNA was removed by RNase-free DNase I (Thermo Scientific) according to the manufacturer’s instructions. One µg of the treated RNA was used for cDNA synthesis using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific), with the final volume of 20 µl and time period of 2 hr. Then, 5 ng of cDNA were used for measuring flu gene expression using specific primers (Table 1). Real-time PCR was performed by ABI-7300 system (Advanced Biosystems, Foster, California, USA) using SYBR Green kit (ampliqon, Denmark).
Table 1.
Primers used in this study for real time PCR of flu and gap A genes in uropathogenic escherichia coli
An initial activation phase was carried out at 95 °C for 15 min followed by 35 cycles at 95 °C (each cycle lasted 15 sec) and 60 °C for 1 min. Nucleotide sequences of the gapA gene that encodes glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, was considered as the independent control for gene expression. In this study, Ct (cycle threshold) obtained by Real-time PCR was used for calculating mRNA expression by the ΔΔCT method (20). The data analysis was done using one-sample T-test. All tests were repeated three times and the mean of results was calculated.
Results
MIC of ZnO nanoparticles on UPEC
The MIC ZnO–np on 18 (63.3%) UPEC isolates were 2500 μg /ml (Table 2) and the mean of MIC value for the tested isolates was 2791±1.2 µg/ml. The distribution MIC value among properties isolates was assessed and analyzed by statistical methods. (Properties such as: the origin of bacteria from inpatients or outpatients, production ESBL, history urinary catheterization, etc.). ESBLs producing UPEC isolates was higher MIC of ZnO-np than non-ESBL isolates (P<0.01). However, no significant correlation was found between the mean of MIC and other variable (Table 2).
Table 2.
MIC of ZnO-np on uropathogenic Escherichia coli isolates
| MIC Characters | 5000 | 2500 | 1250 | Mean (µg/ml) | P-value |
|---|---|---|---|---|---|
| ESBL | 5 | 7 | 0 | 3541 | P<0.01 |
| Non-ESBL | 1 | 11 | 5 | 2279 | |
| Cathetera | 4 | 9 | 3 | 2890 | P=0.2 |
| Without cathetera | 2 | 9 | 2 | 2692 | |
| Inpatientb | 3 | 9 | 3 | 2750 | P=0.8 |
| Outpatientb | 3 | 9 | 2 | 2857 |
The UPEC were collected from patient with urinary catheter or without catheter and
The UPEC isolates origin
The effects of ZnO-np on biofilm formation
Most UPEC isolates (60%) were produced weak biofilm in BHI broth containing 1% (w/v) sucrose. ZnO-np at MIC concentration fully inhibited biofilm formation in 6 (20%) of UPEC isolates and in 30% of isolates reduce the optical density of biofilm formation from moderate or strong level to weak level. In culture media which contained the sub-MIC of ZnO-np, reduction in biofilm formation were observed in 33.4% of isolates (all isolates with moderate and strong biofilm formation in media without ZnO-np), none of UPEC isolates showed fully inhibition of biofilm formation at this concentration of ZnO-np. Our results showed that the effect of ZnO-np is much greater on the isolates with strong biofilm compared to isolates with weak biofilm (Table 3).
Table 3.
The effects of ZnO-np on the biofilm formation of uropathogenic Escherichia coli according their biofilms
| Biofilms | No ZnO-np | After treatment with MIC of ZnO-np | After treatment with sub-MIC of ZnO-np | |||
|---|---|---|---|---|---|---|
| No biofilm | Weak | No biofilm | Weak | Moderate | ||
| Weak | 18 (60%) | 3 (16.6%) | 15 (83.4%) | - | 18 (100%) | - |
| Moderate | 7 (23.3%) | 1 (14.3%) | 6 (85.7%) | - | 5 (71.4%) | 2 (28.6%) |
| Strong | 5 (16.7%) | 2 (40%) | 3 (60%) | - | 5 (100%) | - |
| Total | 30 | 6 (20%) | 24 (80%) | - | 28 (93.3%) | 2 (6.7%) |
The effect of sub-MIC of ZnO-np on expression level of the flu gene
Three isolates of UPEC that formed biofilm in the negative control medium and medium containing sub-MIC of ZnO-np were chosen for studying expression of the flu gene by real-time PCR. The results showed a significant decrease in expression of the flu gene in the bacterial isolates (P<0.0001). The gene expression level decreased by 14.3, 243.9 and 8.6 -fold in isolates 1, 2 and the standard strain, respectively. On average, there was a 31.15-fold reduction in the expression level of flu gene (Figure 1). Results of gene expression for these bacterial isolates were consistent with the results of the phenotype stage.
Figure 1.
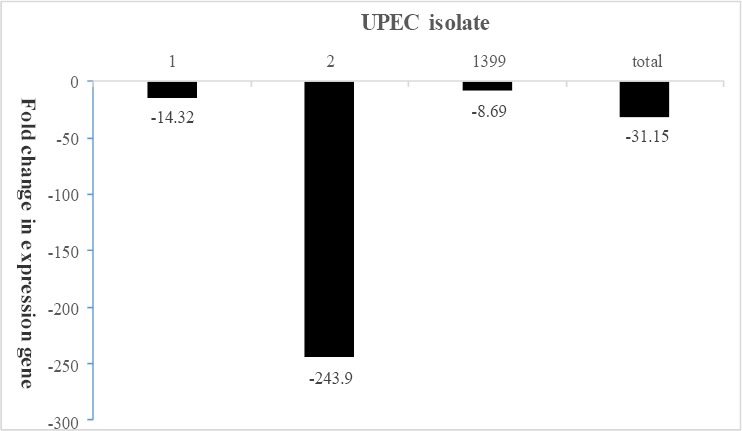
The effect of ZnO-np sub-MIC on flu gene expression
Discussion
The transition from planktonic lifestyle to adherent stage requires a very careful coordination in genes expression. Various environmental signals are essential for biofilm formation, including alteration in concentrations of nutrients (glucose, indole, polyamine), inorganic molecules (iron, phosphate), pH, antimicrobial substances, tempera-ture, oxygen concentration, and etc. (21). Schembri et al assessed expression of different genes in biofilms of E. coli k12 by the flow chambers method using DNA microarrays. Their study showed that Ag43 expression was significantly increased in E. coli biofilms compared to each stage of the planktonic growth. These findings were confirmed by assessing Ag43 gene expression in planktonic stages (exponential and stationary phase) and biofilm production via Western blotting (8).
This study showed that ZnO-np at MIC level have more powerfull effect on inhibition of biofilm formation in UPEC isolates which have stronger biofilm. The Biofilm formation was fully inhibited in about 20% of isolates with strong biofilm instead of 14% and 16% of isolates with moderate or weak biofilms, respectively.
We found that there is a good relation between level of biofilm formation and expression of flu gene, it means higher expression level was found in isolates with stronger biofilm but in isolates with weak biofilm the expression of this gene was low or undetectable (data were not shown).
Sub-MIC concentration of ZnO-np reduced expression of flu gene and therefore biofilm forma-tion was reduced. These results are consistent with findings of Islam, who showed that nitric oxide inhibits biofilm formation only in UPEC isolates that have the flu genes (coding Ag43)(22). Nevertheless, more studies are required to confirm the effects of nanoparticles on bacterial isolates that produce weaker biofilms.
In this study the lowest MIC of ZnO-np on UPEC was determined as 1250 μg/ml, which is similar to findings of Hosseinzadeh et al (1250 μg/ml) (23) and Emami and Chehrazi (1000 μg/ml) (24). However, this result was inconsistent with findings of Premanathan et al (500 μg/ml) (25) and Padmavathy and Vijayaraghavan (400 μg/ml) (26). This difference could be due to size of nanoparticles. In the study of Hosseinzadeh et al, the size of nanoparticles were 20 nm, which is similar to our study. However, the size of most nanoparticles were less than 10 nm in the studies of Padmavathy and Vijayaraghavan and Premanathan et al The nanoparticles in the mentioned studies also showed better effects, which could be attributed to small size of the nanoparticles.
There was a significant difference between the MIC of ESBL and non-ESBL isolates, which could be related to the following reasons: Nanoparticles bind to thiol group of surface proteins, and expression of ESBL proteins could inhibit the expression of these bacterial surface proteins and binding of nanoparticles, ESBL proteins change the bacterial cell surface charge and inhibit the attachment of nanoparticles or resistance genes for nanoparticles could be transferred by plasmids that contain ESBL enzymes. In addition to all the mentioned reasons, type of bacterial isolates and the size of particles could also be associated to this difference (27).
In our study, the effect of ZnO-np on biofilm formation was not as expected. This could be related to the following reasons: 1. Induced genes during biofilm formation may contain genes for resistance to heavy metals, oxygen-limited conditions and transfer and oxidoreductase proteins (8). 2. Hydrophobic proteins are replaced with hydrophilic proteins or vice versa after biofilm formation, which alter bacterial cell surface charge that leads to nanoparticles’ excretion (10). The findings of Applerot et al (28) and Musarrat et al (29) showed that ZnO-np can significantly inhibit biofilm formation in E. coli strains. The inhibitory effect of ZnO-np in their study was greater than in our study. This difference is because of the different methods that were used for evaluation of biofilm formation (continuous flow chamber method was used by Applerot et al). In addition, bacterial strain and size of nanoparticles are important factors that may affect the inhibitory activity of the nanoparticles. Smaller particles have greater surface area to volume ratio and induce stronger antibacterial effect.
Conclusion
ZnO-np showed inhibitory effects on biofilm formation in UPEC isolates which is depended to the concentration of this nanoparticles, and showed stronger inhibitory effects in MIC than sub-MIC concentrations. Sub-MIC concentration of ZnO-np significantly decreases expression of the flu gene in UPEC isolates with strong biofilm but can’t inhibit the biofilm formation.
Acknowledgment
This work was financially supported (Grant No.3564791) by the Golestan University of Medical Sciences, Gorgan, Iran. The results described in this paper were part of an MSc student thesis. Authors declare that there is no conflict of interests in this study. The authors would like to thank Maya Babai and Hanie Bagheri for sharing bacterial strains and Masood Bazori for technical advice. The authors also acknowledge Naeme Javid and Hanie Bagheri for their helpful comments and support in scientific discussions.
References
- 1.Soto SM, Guiral E, Marco F, Vila J. Biofilm Formation in Uropathogenic Escherichia coli Strains: Relationship with Urovirulence Factors and Antimicrobial Resistance. INTECH Open Access Publisher; 2011. [DOI] [PubMed] [Google Scholar]
- 2.Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85:11–19. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oelschlaeger T, Dobrindt U, Hacker J. Pathogenicity islands of uropathogenic E. coli and the evolution of virulence. Int J Antimicrob Agents. 2002;19:517–521. doi: 10.1016/s0924-8579(02)00092-4. [DOI] [PubMed] [Google Scholar]
- 4.nderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect Immun. 2010;78:963–975. doi: 10.1128/IAI.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanna A, Berg M, Stout V, Razatos A. Role of capsular colanic acid in adhesion of uropathogenic Escherichia coli. Appl Environ Microbiol. 2003;69:4474–4481. doi: 10.1128/AEM.69.8.4474-4481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solano C, Echeverz M, Lasa I. Biofilm dispersion and quorum sensing. Curr Opin Microbiol. 2014;18:96–104. doi: 10.1016/j.mib.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Van Houdt R, Michiels CW. Role of bacterial cell surface structures in Escherichia coli biofilm formation. Res Microbiol. 2005;156:626–633. doi: 10.1016/j.resmic.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Schembri MA, Kjærgaard K, Klemm P. Global gene expression in Escherichia coli biofilms. Mol Microbiol. 2003;48:253–267. doi: 10.1046/j.1365-2958.2003.03432.x. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha A, Zhilong S, Gee NK, Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J Endod. 2010;36:1030–1035. doi: 10.1016/j.joen.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, Rojo T, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Khameneh B, Diab R, Ghazvini K, Fazly Bazzaz BS. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog. 2016;95:32–42. doi: 10.1016/j.micpath.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Diab R, Khameneh B, Joubert O, Duval R. Insights in nanoparticle-bacterium interactions: new frontiers to bypass bacterial resistance to antibiotics. Curr Pharm Design. 2015;21:4095–4105. doi: 10.2174/138161282128150922175445. [DOI] [PubMed] [Google Scholar]
- 13.Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279:71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 15.Yousef JM, Danial EN. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nano-particle zinc oxide against pathogenic strains. J Health Sci. 2012;2:38–42. [Google Scholar]
- 16.Samet M, Ghaemi E, Jahanpur S, Jamalli A. Evaluation of biofilm-forming capabilities of urinary Escherichia coli isolates in microtiter plate using two different culture media. International Journal Of Molecular And Clinical Microbiology. 2013;3:244–247. [Google Scholar]
- 17.Naves P, Del Prado G, Huelves L, Gracia M, Ruiz V, Blanco J, et al. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J Appl Microbiol. 2008;105:585–590. doi: 10.1111/j.1365-2672.2008.03791.x. [DOI] [PubMed] [Google Scholar]
- 18.Wallecha A, Oreh H, van der Woude MW. Control of gene expression at a bacterial leader RNA, the agn43 gene encoding outer membrane protein Ag43 of escherichia coli. J Bacteriol. 2014;196:2728–2735. doi: 10.1128/JB.01680-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viveiros M, Dupont M, Rodrigues L, Couto I, Davin-Regli A, Martins M, et al. Antibiotic stress, genetic response and altered permeability of E. coli. PloS One. 2007;2:e365. doi: 10.1371/journal.pone.0000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Vadyvaloo V, Martínez L. Mechanisms of post-transcriptional gene regulation in bacterial biofilms. Front Cell Infect Microbiol. 2014;4:38. doi: 10.3389/fcimb.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam S. Effect of nitric oxide on biofilm formation by Escherichi a coli. Uppsalat Universitet. 2008;5:17. [Google Scholar]
- 23.Hoseinzadeh E, Alikhani M, Samarghandy M. Evaluation of synergistic effect of commercial zinc oxide and copper oxide nanoparticles against gram positive and gram negative bacteria by fraction inhibitory concentration index. ZUMS J. 2012;20:29–41. [Google Scholar]
- 24.Emami-Karvani Z, Chehrazi P. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr J Microbiol Res. 2011;5:1368–1373. [Google Scholar]
- 25.Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed Nanotechnol Biol Med. 2011;7:184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Materials Sci Technol Adv Mater. 2008;9:035004. doi: 10.1088/1468-6996/9/3/035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.nsari MA, Khan HM, Khan AA, Sultan A, Azam A. Synthesis and characterization of the antibacterial potential of ZnO nanoparticles against extended-spectrum β-lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated from a tertiary care hospital of North India. Appl Microbiol Biotechnol. 2012;94:467–477. doi: 10.1007/s00253-011-3733-1. [DOI] [PubMed] [Google Scholar]
- 28.pplerot G, Lellouche J, Perkas N, Nitzan Y, Gedanken A, Banin E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. Rsc Adv. 2012;2:2314–2321. [Google Scholar]
- 29.Musarrat J, Ali K, Ansari M, Saquib Q, Siddiqui M, Khan S, et al. Green Synthesis of nanoparticles and their role as nano-antibiotics and anti-biofilm agents. Planta Med. 2015;81:OA44. [Google Scholar]


