Abstract
Clozapine has proved to be an effective antipsychotic for the treatment of refractory schizophrenia – characterised by the persistence of symptoms despite optimal treatment trials with at least two different antipsychotics at adequate dose and duration – but its use is hampered by adverse effects. The development of clozapine-induced diabetes is commonly considered to arise as part of a metabolic syndrome, associated with weight gain, and thus evolves slowly. We present the case of an individual with refractory schizophrenia and metformin-controlled diabetes who developed rapid-onset insulin-dependent hyperglycaemia immediately after starting clozapine. Given the refractory nature of his illness, the decision was made to continue clozapine and manage the diabetes. This case supports the existence of a more direct mechanism by which clozapine alters glycaemic control, aside from the more routine slow development of a metabolic syndrome.
Declaration of interest
S.S.S. is supported by a European Research Council Consolidator Award (Grant Number 311686) and the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Copyright and usage
© The Royal College of Psychiatrists 2017. This is an open access article distributed under the terms of the Creative Commons Non-Commercial, No Derivatives (CC BY-NC-ND) license.
Clozapine is an atypical antipsychotic medication used in the treatment of refractory schizophrenia, a condition defined as the persistence of symptoms despite optimal treatment trials with at least two different antipsychotics at adequate dose and duration. Despite its unique benefits, it can also cause severe side-effects, most notably agranulocytosis, and as a consequence its use is strictly regulated in most countries and requires routine monitoring of full blood count.1
Although many atypical antipsychotics, such as quetiapine or olanzapine, can induce type 2 diabetes mellitus,2,3 clozapine is believed to pose a higher risk of developing this metabolic disorder.4 This diabetes is conventionally thought to be associated with the development of a broader metabolic syndrome accompanied by an increase in obesity. However, could there be a more directly acting mechanism influencing glycaemic control? There are some data that suggest that antagonism of M3 cholinergic receptors, a feature shared by clozapine and olanzapine, can cause beta-cell dysfunction5; whereas Smith et al6 have suggested clozapine could directly increase the secretion of glucagon, leading to a rapid elevation of glucose levels; and others have proposed direct mitochondrial damage to insulin-responsive cells.7 It is of note that there are instances of acute loss of glycaemic control reported with other atypical antipsychotic compounds.8–10
Nevertheless, the onset of diabetes mellitus poses a serious challenge to practitioners and is a significant reason for clozapine discontinuation11–13; however, given the lack of alternative treatments, there is an argument for aggressive treatment of the diabetes mellitus to allow clozapine to be continued or reinitiated.12
Here, we present the case of an individual with refractory schizophrenia who developed rapid-onset insulin dependence at the commencement of his clozapine therapy – too short a time frame for an acute worsening of a metabolic syndrome, implicating a direct mechanism for rapid loss of glycaemic control – and in whom diabetes was treated successfully without discontinuing clozapine.
Case presentation
Mr A is a 48-year-old male of African origin with a diagnosis of treatment-resistant paranoid schizophrenia. He was transferred to the National Psychosis Unit, a tertiary referral in-patient service, for re-titration of clozapine after cessation because of side-effects.
Mr A was first known to UK psychiatric services in 1993, the year of his arrival in this country, at the age of 25. He presented with paranoid and grandiose delusions, as well as auditory hallucinations. His symptoms persisted despite treatment and were often associated with violent behaviour, requiring several involuntary admissions to psychiatric wards over the years. Various medications were trialled, including olanzapine (15 mg daily for approximately 4 years), risperidone (up to 9 mg daily for approximately 5 years), paliperidone palmitate (150 mg monthly for approximately 2 years), flupentixol depot (40 mg three times a week for approximately 10 years) and clozapine (up to 400 mg a day for 2 non-consecutive years).
Clozapine proved to be the most effective treatment for symptom control, but was stopped on two occasions after a rapid loss of glycaemic control, requiring insulin treatment.
Shortly after both clozapine cessations, his diabetes had improved to the point that insulin administration was no longer necessary, and his glycaemic control was maintained with metformin 1 g twice daily. He also suffered from hypertension (average blood pressure: 165/101 mmHg) and hypercholesterolemia (low-density lipoprotein (LDL) levels: 270 mg/dL). He was a one-pack-per-day smoker and was overweight (body mass index (BMI) of 28.7 at the time of admission).
On admission to the unit, Mr A was irritable, often shouting and at times intimidating towards staff. He presented with paranoid and grandiose delusions and was grossly thought-disordered. Perceptual abnormalities were suspected, as he was often observed responding to unseen stimuli.
The antipsychotic treatment on admission – risperidone – was switched to lurasidone because of a more benign metabolic side-effect profile.14 However, this was not effective in controlling symptoms and was later changed to amisulpride, which also produced no improvement in his mental state. Finally, given the lack of any progress, it was decided to commence clozapine.
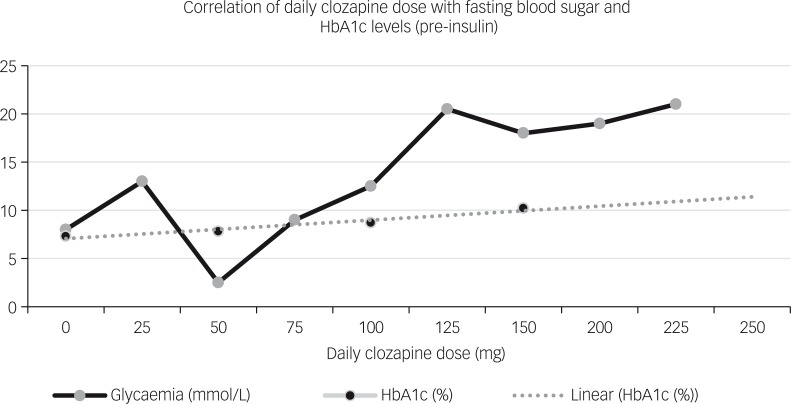
At this stage, it was decided to seek collaboration with the local endocrinologists to safely manage the risk of emergent loss of glycaemic control. The individual’s fasting blood glucose levels were measured before commencing clozapine to establish a baseline (4–6 mmol/L). Despite using a slow-titration approach, 6 days after commencing clozapine his glucose levels started to increase linearly (see Fig. 1). HbA1c values also increased over a short period of time (from 7.3 to 12.1% in the course of 2 months). Interestingly, clozapine dose was only 100 mg (serum levels: 65 ng/ml) at the time his glucose levels were becoming elevated. This elevation was associated with the increase of clozapine dose.
Fig. 1. Correlation of clozapine daily dose with fasting blood sugar and HbA1c levels, prior to the administration of insulin.
He was commenced on insulin therapy, at an initial dose of four units of fast-acting insulin once daily. Clozapine was slowly titrated upwards in increments of 25 mg, once the blood glucose levels had stabilised for 2–3 days, until a dose of 700 mg/day (serum levels: 460 ng/ml) was reached. The dose of insulin was increased slowly until he achieved glycaemic control while being treated with 52 units of medium-acting insulin twice daily. He continued taking metformin 1 g twice a day and commenced on a 2 mg weekly injection of long-acting exenatide – a glucagon-like peptide-1 agonist.
The individual’s mental state improved considerably during his admission. His thought and speech disorders were ameliorated, although still noticeable in a prolonged conversation. Perceptual abnormalities were no longer noted, although he continued to experience paranoid delusions to a lower degree. He started to engage in art activities, showing some talent in this area, and retained a calm demeanour for increasing periods of time. He gained partial insight, identifying the need for medications and follow-up. His weight increased slightly (from a BMI of 28.7 to 28.9) and his smoking was reduced by half.
After 5 months of treatment, the individual’s psychotic symptoms were significantly reduced and glycaemic control was stable. He was discharged from hospital, with his care taken over by his local mental health team; his diabetes care was managed by his general practitioner with specialist input from the endocrinologists.
Discussion
This case supports the existence of a direct mechanism by which clozapine may alter the glycaemic control pathways. Our patient developed rapid loss of glycaemic control within days of starting the medication, meaning it was unlikely to be caused by intermediate factors such as increased appetite, weight gain or insulin resistance. He was not taking any other medication likely to cause hyperglycaemia. Clozapine produced a linear dose-related increase in glycaemic levels during the first 2 months of treatment, which from 400 mg onwards seemed to reach a plateau. Although there is an apparent dose-dependence, treatment duration-dependence as a contributing factor cannot be excluded.
Given the lack of any effective symptom management with other antipsychotic medication and the complexity of the clinical presentation, it was decided to continue clozapine, but to manage the hyperglycaemia while continuing with the medication.
To do this, we followed the Maudsley prescribing guidelines’ recommendations for treatment of antipsychotic-related diabetes.15 Of equal importance was the collaboration with a clinical pharmacist specialising in mental health and with endocrinologists, who oversaw the individual’s treatment. This approach requires vigorous baseline and follow-up monitoring of glucose levels and HbA1c values, slower clozapine titration schedule, and full control of glycaemic levels before every dose increase of clozapine.
Clinicians should also monitor weight gain and lipid levels, as well as considering other potential risk factors for diabetes5; one such factor being African ethnicity, which some studies suggest is associated with a higher risk of diabetes mellitus.16 There are several pharmacological treatment options for diabetes mellitus; in this case, we used insulin and long-acting exenatide – a recently introduced glucagon-like peptide-1 (GLP-1) agonist that requires a weekly injection – added to his existing metformin. The reason for using exenatide is based on recent research, which suggests that hyposecretion of incretin hormone GLP-1 may partially account for the rapid diabetogenic effect of clozapine.17
Clearly, it may still be necessary to discontinue clozapine in the event of a severe diabetes mellitus complication, such as ketoacidosis.12
Our case illustrates the successful management of insulin dependence in an individual with refractory schizophrenia and diabetes mellitus previously controlled by metformin. In people with refractory schizophrenia, the effectiveness of clozapine must be balanced against the risks posed by side-effects. In such cases, a personalised plan is required to support the aggressive treatment of the iatrogenic side-effects while continuing clozapine treatment.
References
- 1.Nielsen J, Young C, Ifteni P, Kishimoto T, Xiang Y, Schulte P, et al. Worldwide differences in regulations of clozapine use. CNS Drugs 2016; 30: 149–61. [DOI] [PubMed] [Google Scholar]
- 2.Deng C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am 2013; 42: 545–63. [DOI] [PubMed] [Google Scholar]
- 3.Jin H, Meyer JM, Jeste DV. Atypical antipsychotics and glucose dysregulation: a systematic review. Schizophr Res 2004; 71: 195–212. [DOI] [PubMed] [Google Scholar]
- 4.Foley DL, Mackinnon A, Morgan VA, Watts GF, Castle DJ, Waterreus A, et al. Effect of age, family history of diabetes, and antipsychotic drug treatment on risk of diabetes in people with psychosis: a population-based cross-sectional study. Lancet Psychiatry 2015; 2: 1092–8. [DOI] [PubMed] [Google Scholar]
- 5.Stahl S, Mignon L, Meyer J. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand 2009; 119: 171–9. [DOI] [PubMed] [Google Scholar]
- 6.Smith GC, Zhang ZY, Mulvey T, Petersend N, Lach S, Xiuc P, et al. Clozapine directly increases insulin and glucagon secretion from islets: implications for impairment of glucose tolerance. Schizophr Res 2014; 157: 128–33. [DOI] [PubMed] [Google Scholar]
- 7.Contreras-Shannon V, Heart DL, Paredes RM, Navaira E, Catano G, Kaushal Maffi S, et al. Clozapine-induced mitochondria alterations and inflammation in brain and insulin-responsive cells. PLoS ONE 2013; 8 (suppl 3): e59012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duiverman ML, Cohen D, Van Oven W, Nioboer P. A patient treated with olanzapine developing diabetes de novo: proposal for hyperglycaemia screening. Neth J Med 2007; 65: 346–8. [PubMed] [Google Scholar]
- 9.Madsen K. Fatal hypertriglyceridaemia, acute pancreatitis and diabetic ketoacidosis possibly induced by quetiapine. Case Rep 2014; doi: 10.1136/bcr-2013-202039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi M, Ohishi S, Katsumi C, Moriya T, Miyaoka H. Rapid onset of quetiapine-induced diabetic ketoacidosis in an elderly patient. Pharmacopsychiatry 2005; 38: 183–4. [DOI] [PubMed] [Google Scholar]
- 11.Mustafa F, Burke J, Abukmeil S, Scanlon J, Cox M. ‘Schizophrenia past clozapine’: reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry 2014; 48: 11–4. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen J, Correll C, Manu P, Kane J. Termination of clozapine treatment due to medical reasons. J Clin Psychiatry 2013; 74: 603–13. [DOI] [PubMed] [Google Scholar]
- 13.Taylor D, Douglas-Hall P, Olofinjana B, Whiskey E, Thomas A. Reasons for discontinuing clozapine: matched, case-control comparison with risperidone long-acting injection. Br J Psychiatry 2009; 194: 165–7. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JM, Mao Y, Pikalov A, Cucchiaro J, Loebel A. Weight change during long-term treatment with lurasidone: pooled analysis of studies in patients with schizophrenia. Int Clin Psychopharmacol 2015; 30: 342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor D, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. Wiley, 2015. [Google Scholar]
- 16.Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double-blind clozapine, olanzapine or haloperidol. Schizophr Res 2009; 110: 95–102. [DOI] [PubMed] [Google Scholar]
- 17.Mayfield K, Siskind D, Winckel K, Russell AW, Kisel S, Smith G, et al. Glucagon-like peptide-1 agonists combating clozapine-associated obesity and diabetes. J Psychopharmacol 2016; 30 (suppl 3): 227–36. [DOI] [PubMed] [Google Scholar]