Abstract
Hypothesis
A review of the most recent literature will provide clinicians with an update of secondary endolymphatic hydrops, aiding in diagnosis and treatment of affected patients.
Background
Secondary endolymphatic hydrops is a pathologic finding of the inner ear resulting in episodic vertigo and intermittent hearing loss. It is a finding for which extensive research is being performed.
Methods
A review of the most recent literature on secondary endolymphatic hydrops was performed using PubMed literature search.
Results
Recent investigation of secondary endolymphatic hydrops has brought attention to traumatic and inflammatory insults as causes for secondary endolymphatic hydrops. Such etiologies, including post-surgical effects of cochlear implantation and endolymphatic sac ablation; otosclerosis and its operative intervention(s); acoustic and mechanical trauma; medications; and systemic inflammatory processes, have been determined as causes of secondary lymphatic hydrops. Histopathological slides for many of the etiologies of secondary endolymphatic hydrops are presented.
Conclusions
Through an understanding of the pathophysiology and etiologies of secondary endolymphatic hydrops, clinicians will gain a better understanding of this complex disease process, which will aid in treatment of patients with this disease process.
Keywords: Endolymphatic hydrops, trauma, vestibular disorder, inner ear pathology
INTRODUCTION
Endolymphatic hydrops (EH) is a pathologic finding of distended endolymphatic space by and enlargement of endolymphatic volume (1). EH has been associated with episodic vertigo and fluctuating hearing loss (2). Various disease processes are associated with findings of primary endolymphatic hydrops, including embryopathic process, such as Mondini dysplasia; and idiopathic process, such as Meniere’s disease (2).
Traumatic and inflammatory processes may result in secondary endolymphatic hydrops. Secondary endolymphatic hydrops (SEH) occurs if the endolymph resorption system results from a variety of causes, each of which is induced by either traumatic or inflammatory events (2). Other etiologies, including idiopathic versus iatrogenic etiologies, have been investigated in the literature, however, exact mechanisms of developing SEH remain under debate. Histopathologic findings of EH also does not always correlate with clinical symptoms of fluctuating hearing loss and/or vertigo, which further complicates the understanding of this process. Either traumatic or inflammatory processes could lead to resultant changes in cerebrospinal fluid pressure. Such changes in CSF pressure have been proposed as a cause for SEH due to the perilymph being in continuity with the CSF, however, the exact pathophysiology of this process is unknown (3).
Recently investigated reasons for SEH include traumatic injury, such as post-operative trauma following cochlear implantation (4–6) and stapedectomy for otosclerosis (7); ablation of the endolymphatic sac (8,9); and acoustic and mechanical trauma (2). Medications, including vasopressin (1,8), have also been associated with development of SEH. Medical illnesses, including inflammatory/vascular diseases and cerebrospinal fluid pressure changes, have also had reported associations (1,10).
In contrast, delayed endolymphatic hydrops, first described by Nadol et al. in 1975, is delayed onset vertigo, typically years in units of time, in the setting of profound sensorineural hearing loss (11,12). Causes other than profound deafness have also been described, including head injury, history of mumps/measles, prior mastoidectomy, labyrinthitis, and/or meningitis, however, symptoms have been reported as late as 20 years after the onset (12).
In this article, the aforementioned etiologies will be discussed, along with data regarding the pathophysiology underlying these processes. Review of the most recent literature will provide clinicians with an update on this topic and aid in their diagnosis and treatment of such patients.
METHODS
A review of the most recent literature on secondary endolymphatic hydrops was performed using PubMed literature search. A total of 169 papers were found by using the search term “secondary endolymphatic hydrops”, ultimately including 44 relevant papers in this review.
Post-mortem histopathology was reviewed for inclusion in the review. All included histopathologic figures are original images and have not been previously published in the literature. Of note, post-mortem histopathology of the presented cases compared the affected ear to the contralateral ear, allowing the nonaffected ear to act as a control.
RESULTS
Pathophysiology
Endolymphatic hydrops results when the endolymph volume inside the scala media is higher than usual (4). This higher endolymph volume results in bowing of Reissner’s membrane and the basilar membrane (1). Bowing the Reissner’s membrane and the basilar membrane results in bending of stereocilia away from their typical position, thereby decreasing their functional sensitivity to sound (13). In cases of persistent endolymphatic hydrops, loss of stereocilia and/or synaptic connections may result (14), which may result in permanent hearing loss. In some post-mortem temporal bones, pathology has shown rupture of Reissner’s membrane and/or basilar membrane as a result of the increased endolymph volume, which may also contribute to hearing loss and episodic vertigo (1,15).
Etiology
In regards to secondary endolymphatic hydrops, traumatic injury and inflammation have been found to result in similar pathophysiology as described above. Investigations of the impact of trauma and inflammation have been performed. Findings from these investigations will be presented here based on the etiology of SEH.
a) Post-operative trauma
With the rise in use of cochlear implants (CI), attention has been drawn toward the incidence of hearing loss following implantation. Prior studies had placed emphasis on hearing loss resulting from traumatic injury and/or inflammatory changes at the sensory hair cells, leading to their apoptosis (4). Weeks to months after cochlear implantation, some patients may experience decrease in hearing, which has been found unexplainable on the basis of traumatic injury and apoptosis of hair cells (16,17).
More recently, endolymphatic hydrops has been seen even up to three months post-operatively in cochlear implant patients (Figure 1). Secondary endolymphatic hydrops following cochlear implantation using conventional CI electrode arrays has been reported in 42–59% of patients in two recent studies (5,6). Both of these study populations, however, where quite small and only included 12 and 17 patients, respectively (5,6).
FIG. 1.
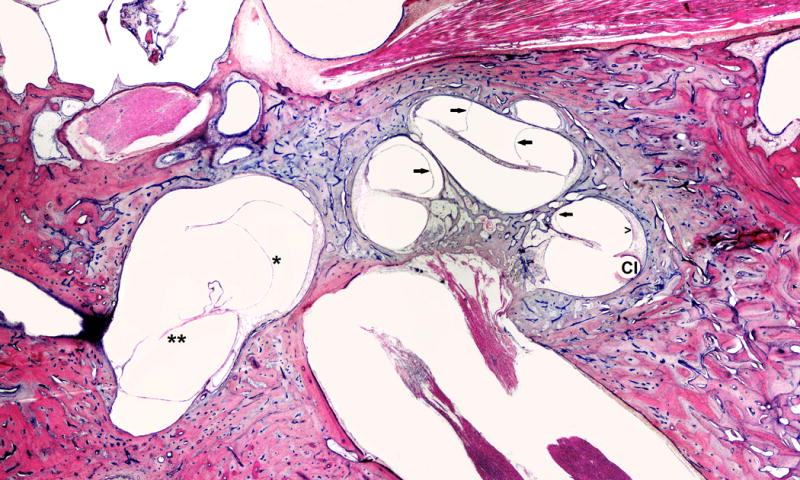
This is a 66-year-old white male with a clinical history of heart attack, occupational noise exposure, and left sensorineural hearing loss. He was treated with left cochlear implant (CI). Histopathology evaluation of the left ear showed: Simple mastoidectomy cavity, profound hydrops (arrows) in all turns of cochlea, saccular (*) and utricular hydrops (**), atrophy of stria vascularis (open arrow head) in lower basal turn, and hyalinization and fibrosis around electrode insertion site (CI).
These results brought into question whether thin, more flexible “hearing preserving” electrodes would be less traumatic and, therefore, result in a lower incidence of SEH. A recent study by Smeds et al. aimed to investigate the impact of hearing-preserving cochlear implant electrodes on the incidence of SEH (4). Twenty-one adult guinea pigs were used to assess the incidence of SEH, with findings revealing findings consistent with SEH within the first month after surgery (4). SP/AP ratios were used to track the impact of the CI on development of SEH, which resulted in reduced SP/AP levels by 28 days post-operative (4).
Several causes of SEH following CI placement have been proposed. One proposed cause includes blockage of the endolymphatic system secondary to the electrode penetrating the basilar membrane and causing trauma to the scala media or scala vestibule. This would ultimately result in fibrosis of the scala media and decrease outflow of endolymph from the cochlea (4). In the Smeds et al. study, however, the scala media was not entered, and this has not been found in other prior studies on post-mortem histopathology.
Other proposed mechanisms include cochlear inflammation secondary to the trauma to the cochlea with cochlear implantation. With direct cochlear injury, it has been found that post-capillary venules will increase permeability, allowing for intercellular adhesion molecule-1, or ICAM-1, to be expressed in higher number (18–20). Increased expression of ICAM-1 results in extravasate of leukocytes into the cochlea which causes increased inflammatory response (20). Whether this inflammatory response is enough to result in SEH has yet to be fully concluded but may certainly explain the late presentation of SEH following cochlear implantation.
Reports of SEH following stapedectomy have also been recently published. In a long-term review of patient outcomes, patients following stapedectomy for otosclerosis were evaluated for their incidence of SEH. Authors used low-frequency sensorineural hearing loss as a marker for SEH (7). Histologic specimens of temporal bones from these patients were also reviewed. Of note, it is uncertain if SEH was caused by otosclerosis, the surgical stapedectomy, or some other factor. The incidence of low-frequency sensorineural hearing loss was not found to be a true marker of SEH, however, these patients were found to have histologic evidence of SEH when compared to the study’s control group(7) (Figure 2).
FIG. 2.

This is a 78-year-old white female with a clinical history of bilateral profound hearing loss, as well as left tympanotomy and stapedectomy. She became extremely vertiginous in the post-operative period. Histopathologic examination showed: LEFT: stapedectomy, otosclerosis, dehiscent carotid artery and facial nerve, and profound saccular hydrops (*), decreased ganglion cells (SG).
Additional studies of patients with otosclerosis even prior to surgical intervention have been reviewed. MRI results of patients with otosclerosis have found varying degrees of both cochlear and vestibular endolymphatic hydrops in patients with a history of otosclerosis (21). Several of these patients had characteristic symptoms of SEH, including rotary vertigo and episodic sensorineural hearing loss, however, several patients with characterized severe SEH had no true symptoms associated with SEH (21).
b) Endolymphatic sac ablation
Post-operative effects of endolymphatic sac ablation have also been a widely investigated cause of SEH (Figure 3). Several studies have been performed on guinea pig models to assess the impact of surgical ablation on incidence of SEH (22,23). These studies have shown development of mild hydrops within days after surgery. Hydrops initially involved membranes of the saccule, endolymphatic sinus, Reissner’s membrane, and the utricle; however, over time, this progressed to severely distend Reissner’s membrane into scala vestibule within 3–4 months after ablation (22,23).
FIG. 3.
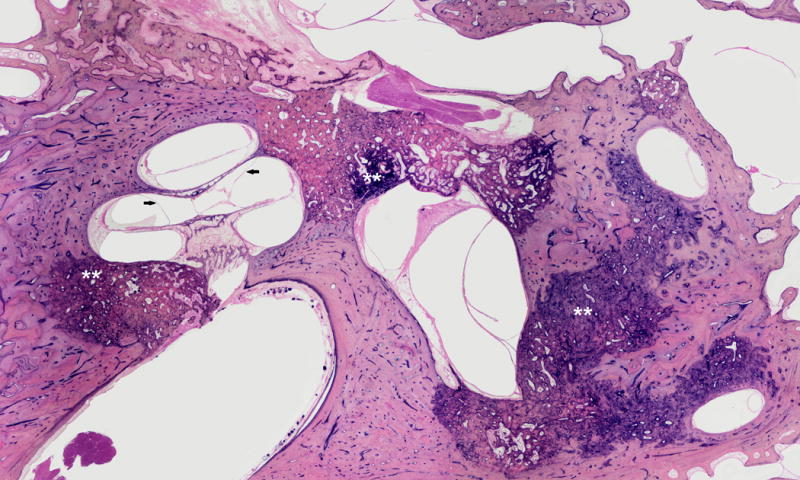
This is a 78-year-old female patient with a history of Meniere’s Disease and decreased hearing. Histopathologic examination showed otosclerosis, cochlear (arrows) and utricular hydrops. Note that otosclerosis blocks the endolymphatic duct.
More recent studies have investigated the role of aldosterone in causing worsening hydrops after endolymphatic sac ablation. In one study, partial ablation of the endolymphatic sac was performed in conjunction to giving aldosterone (24). Those who received aldosterone alone had slight hydrops as a result, while those who underwent both partial ablation of the endolymphatic sac in addition to receiving aldosterone had moderate to severe hydrops (24). Such findings have called into question the role of aldosterone in the pathophysiology of SEH.
c) Acoustic and mechanical trauma
The impact of acoustic trauma on the incidence of SEH has been long investigated. Since Tullio’s study in 1938, in which holes were drilled on the semicircular canals of pigeons, it has been noted that symptoms of dizziness, nausea, and/or nystagmus can result (25). More recent studies have aimed to further investigate the pathophysiology behind this constellation of symptoms, leading to findings of SEH (26). Specifically, pressure-striking force, as is seen on the membranous labyrinth following firearm sound exposure, has been found to result in SEH (27). Other noise exposure, including workplace exposure, typically causes damage to the cochlea secondary to metabolic stress rather than specifically damaging the membranous labyrinth and, therefore, has not been found to be a cause of SEH (28).
Mechanical trauma had also remained in question as a potential cause of SEH (Figure 4). With complaints of vertigo being common after minor head trauma, the duration of such symptoms is key in evaluation of such trauma patients. Recent studies have attempted to investigate causes of persistent vertigo following head trauma, finding that SEH is a less common cause of persistent vertigo (29). Other causes, such as benign paroxysmal positional vertigo, unilateral vestibular nerve injury, damage to the utricle and/or saccule, and perilymphatic fistula, were found to be more common in this population (29.
FIG. 4.
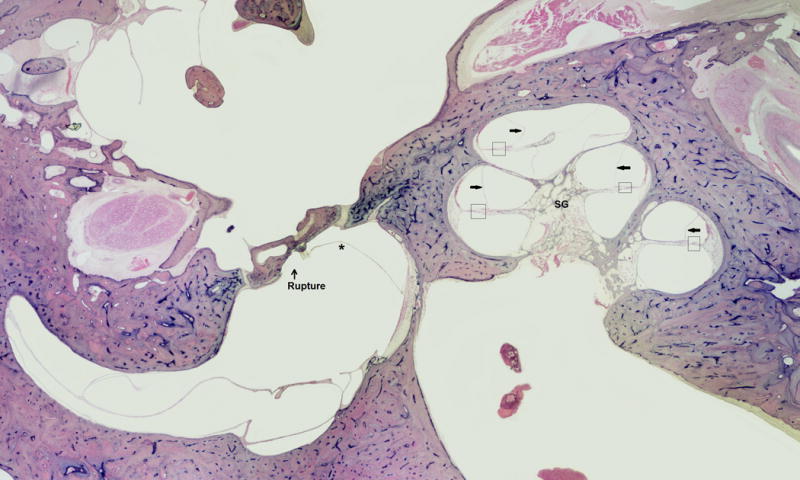
This is an 86-year-old male with a clinical history of Meniere’s disease and a Tack operation. Histopathology of the ear showed Tack hole seen in stapes footplate, severe endolymphatic hydrops, saccule hydropic and ruptured, severe loss ganglion cells, strial atrophy, and organ of Corti atrophy.
d) Effects of medications
Impact of volume-regulating medications has been of interest in light of the change in endolymph volume resulting in endolymphatic hydrops. As previously mentioned, aldosterone has been investigated for its role in SEH. Other medications, including vasopressin (also known as anti-diuretic hormone), have also been studied. Vasopressin (V2) receptors are known to be throughout the inner ear tissues, including the lateral wall and endolymphatic sac (1). Reports of vasopressin administration for one week in animal models have shown dose-dependent increases in endolymphatic volume, with average increase in volume by 17% (30).
Other studies with administration of OPC-31260, a V2 receptor antagonist, have resulted in decreased endolymphatic volume and, therefore, reversal of endolymphatic hydrops (31). Certainly, the V2 receptor plays a role in the inner ear fluid homeostasis and warrants additional investigation as a treatment modality for SEH.
e) Medical Etiologies
Other neurologic fluid imbalances, including changes in CSF pressure, have been associated with decrease in hearing sensitivity (1). Proposed mechanism for this change in hearing sensitivity has been a compensatory mechanism via expansion of the endolymphatic space but this has yet to be proven in the literature (32).
The impact of third window pathology, including large vestibular aqueduct and/or superior semicircular canal dehiscence syndromes, has been under recent investigation for their impact on perilymphatic pressure. A study by Sone et al. has recently been published, revealing varying degrees of endolymphatic hydrops as a result of underlying semicircular canal dehiscence (33.) There was, however, one patient with underlying semicircular canal dehiscence having no evidence of EH. Patients with large vestibular aqueduct syndrome had no specific relationship between severity of their syndrome and that of EH, but these patients had overall more severe EH compared to those with semicircular canal dehiscence (33). Findings of this study support SEH being a result of third window syndromes in affected patients, however, the paucity of other studies on this topic certainly requires further investigation of this topic.
Diagnosis of other middle and inner ear pathology has also been described. One case report discusses the impact of intracochlear schwannomas resulting in secondary endolymphatic hydrops, resulting in symptoms of unilateral tinnitus, vertigo, imbalance, and aural fullness (34–38) (Figure 5). Ultimately, such patients present with symptoms of disequilibrium and vertigo, which helps to lead to their diagnosis of schwannoma but also endolymphatic hydrops as a result (34).
FIG. 5.
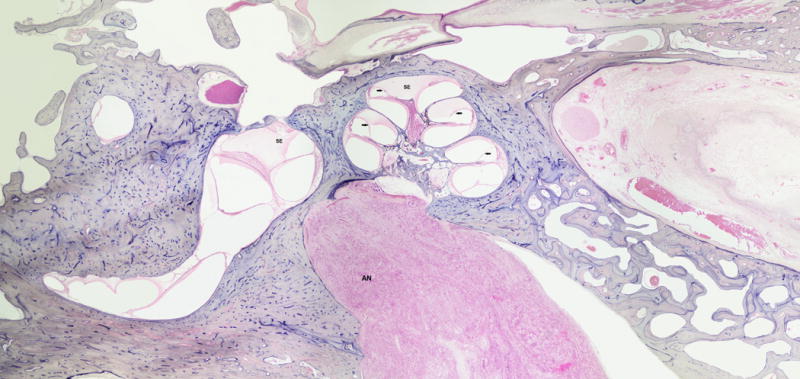
This is a 76-year-old black male with a clinical history of dizziness and severe sensorineural hearing loss. Histopathologic evaluation of the left ear showed intracanalicular vestibular schwannoma (AN), serous labyrinthitis (SE), and cochlear hydrops (arrows).
Those with history of sudden deafness have also been described to develop SEH. In one study, patients with history of sudden deafness who had developed vertiginous attacks years after the onset of their deafness were assessed. These patients typically presented with rotational vertigo about 8 years after the onset of their deafness (39). Compared to sudden deafness patients without SEH symptoms, those with SEH symptoms had abnormal caloric testing more frequently, but other vestibular testing results, such as cVEMP and oVEMP, did not differ (39).
Infectious etiologies, including otitis media and labyrinthitis, have been described with development of SEH (Figure 6). A recent study analyzing post-mortem human temporal bones found a significant increase in endolymphatic hydrops secondary to serous labyrinthitis and otitis media (40–42). Intralabyrinthine hemorrhage has also been shown to have an increase degree of endolymphatic hydrops in a similar human temporal bone study (40–42) (Figure 7). Those with otosyphilis have also been found to have higher rates of SEH on temporal bone histopathology (43). It is believed that this is secondary to some change in the endolymphatic system, although it is not clear if this change is the same and/or similar to that seen in Meniere’s disease (43).
FIG. 6.

This is a 29-year-old white male with a clinical history of Leukemia, dizziness, and left severe sensorineural hearing loss. His hearing loss was fluctuant. Histopathologic evaluation of the ear showed: hypertrophied middle ear mucosa, fibrous tissues in the posterior side of the middle ear, profound endolymphatic hydrops (arrows), severe loss of haircells, and strial atrophy (open arrow head).
FIG. 7.

This is a 16-year-old white, male with leukemia, dizziness and right hemotympanum. Histopathologic examination of the ear showed leukemic hemorrhage in the middle and inner ears with cochlear (arrows) and slight saccular hydrops (*).
Other medical illnesses, including those resulting in systemic vasculitis and/or inflammation, may also result in SEH. A case report of a patient with Cogan’s disease. Cogan’s disease is an autoimmune inflammatory disorder, resulting in sensorineural hearing loss, vertigo, and interstitial keratitis (44). In this case report, post-mortem temporal bone histopathology revealed bilateral endolymphatic hydrops, in addition to findings of necrotic organ of Corti and stria vascularis (44). Significant lymphocytic infiltration was found throughout the inner ear, bringing into question the role of autoimmune and systemic inflammatory disorders in the development of SEH (44).
CONCLUSION
Secondary endolymphatic hydrops is a disease process associated with episodic vertigo and fluctuating hearing loss. Certainly a greater understanding of the underlying etiologies and histopathology of SEH is essential to the care of patients with this disease process. More recent investigation of this disease process has pointed to traumatic and inflammatory causes; however, further investigation is needed to better understand SEH.
Acknowledgments
Source of Funding: None
Footnotes
Conflicts of Interest: None
References
- 1.Salt AN, Plontke SK. Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin North Am. 2010;43:971–83. doi: 10.1016/j.otc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YJ, Young YH. Secondary endolymphatic hydrops after acoustic trauma. Otol Neurol. 2016;36:1–6. doi: 10.1097/MAO.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 3.Fontaine N, Chapiot A, Debry C, Gentine A. A case of spontaneous intracranial hypotension: from Meniere-like syndrome to cerebral involvement. Euro Ann Otorhinolaryngol Head Neck Dis. 2012;129:153–6. doi: 10.1016/j.anorl.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Smeds H, Eastwood HT, Hapson AJ, Sale P, Campbell LJ, Arhatari BD, Mansour S, O’Leary SJ. Endolymphatic hydrops is prevalent in the first weeks following cochlear implantation. Hearing Res. 2015;327:48–57. doi: 10.1016/j.heares.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Handzel O, Burgess BJ, Nadol JB. Histopathology of the peripheral vestibular system after cochlear implantation in the human. Otol Neurotol. 2006;27:57 e64. doi: 10.1097/01.mao.0000188658.36327.8f. [DOI] [PubMed] [Google Scholar]
- 6.Richard C, Fayad JN, Doherty J, Linthicum FH. Round window versus cochleostomy technique in cochlear implantation: histologic findings. Otol Neurotol. 2012;33:1181–1187. doi: 10.1097/MAO.0b013e318263d56d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishai R, Halpin CF, McKenna MJ, Quesnel AM. How often does stapedectomy for otosclerosis result in endolymphatic hydrops? Otol Neurotol. 2016;37:984–90. doi: 10.1097/MAO.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egami N, Kakigi A, Takeda T, Yamasoba T. Dehydration effects ofa V2 antagonist on endolymphatic hydrops in guinea pigs. Hearing Res. 2016;332:151–9. doi: 10.1016/j.heares.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Egami N, Kakigi A, Sakamoto T, Takeda T, Hyodo M, Yamasoba T. Morphological and functional changes in a new animal model of Meniere’s disease. Lab Investig. 2013;93:1001–1011. doi: 10.1038/labinvest.2013.91. [DOI] [PubMed] [Google Scholar]
- 10.Jung DH, Nadol JB, Folkerth RD, Merola JF. Histopathology of the inner ear in a case with recent onset of Cogan’s syndrome: evidence for vasculitis. Ann Oto Rhinol Laryngol. 2016;125:20–4. doi: 10.1177/0003489415595426. [DOI] [PubMed] [Google Scholar]
- 11.Nadol NB, Jr, Weiss AD, Parker SM. Vertigo of delayed onset after sudden deafness. Ann Otol Rhinol Laryngol. 1975;84:841–6. doi: 10.1177/000348947508400617. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima M, Ito R, Miyaguchi S, Hirai T, Otami Y, Akahani S, Inohara H, Takeda N. Preceding profound deafness and co-factors promote development of endolymphatic hydrops in preliminary patients with delayed endolymphatic hydrops. Acta Otolaryngol. 2016 Jul;6:1–5. doi: 10.1080/00016489.2016.1203993. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Durrant JD, Dallos P. Modification of DIF summating potential components by stimulus biasing. J Acoust Soc Am. 1974;56:562–570. doi: 10.1121/1.1903291. [DOI] [PubMed] [Google Scholar]
- 14.Nadol JB, Thornton AR. Ultrastructural findings in a case of Meniere’s disease. Ann Otol Rhinol Laryngol. 1987;96:449–54. doi: 10.1177/000348948709600420. [DOI] [PubMed] [Google Scholar]
- 15.Schuknecht HF. Histopathology of Meniere’s disease. In: Harris JP, editor. Meniere’s Disease. Vol. 1999. Kugler; The Netherlands: pp. 41–52. [Google Scholar]
- 16.Gstoettner WK, Helbig S, Maier N, Kiefer J, Radeloff A, Adunka OF. Ipsilateral electric acoustic stimulation of the auditory system: results of long- term hearing preservation. Audiol Neurootol. 2006;11:49–56. doi: 10.1159/000095614. [DOI] [PubMed] [Google Scholar]
- 17.Woodson EA, Reiss LA, Turner CW, Gfeller K, Gantz BJ. The hybrid cochlear implant: a review. Adv Otorhinolaryngol. 2010;67:125–134. doi: 10.1159/000262604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M, Harris JP. Expression of intercellular adhesion molecule-1 during inner ear inflammation. Ann Otol Rhinol Laryngol. 2015;104:69–75. doi: 10.1177/000348949510400111. [DOI] [PubMed] [Google Scholar]
- 19.Kel GE, Tan J, Eastwood HT, Wongprasartsuk S, O’Leary SJ. Early cochlear response and icam-1 expression to cochlear implantation. Otol Neurotol. 2013;34:1595–1602. doi: 10.1097/MAO.0b013e31828f4929. [DOI] [PubMed] [Google Scholar]
- 20.Zou J, Pyykko I, Bjelke B, Toppila E. In vivo MRI visualization of endo- lymphatic hydrops induced by keyhole limpert hemocyanin round window immunization. Audiol Med. 2007;5:182–187. [Google Scholar]
- 21.Mukaida T, Sone M, Yoshida T, Kato K, Teranishi M, Naganawa S, Nakashima T. Magnetic resonance imaging evaluation of endolymphatic hydrops in cases with otosclerosis. Otol Neurotol. 2015;36:1146–50. doi: 10.1097/MAO.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 22.Kimura RS, Schuknecht HF. Membranous hydrops in the inner ear of the guinea pig after obliteration of the endolymphatic sac. Pract Otorhinolaryngol. 1965;27:343–54. [Google Scholar]
- 23.Kimura RS. Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. Ann Otorhinolaryngol. 1967;76:664–87. doi: 10.1177/000348946707600311. [DOI] [PubMed] [Google Scholar]
- 24.Dunnebier EA, Segenhout JM, et al. Two-phase endolymphatic hydrops: a new dynamic guinea pig model. Acta Otolaryngol. 1997;117:13–19. doi: 10.3109/00016489709117984. [DOI] [PubMed] [Google Scholar]
- 25.Tullio P. Demonstration des methodes pour la stimulation acoustique des caneaux semicirculaires. Acta Otolaryngol. 1938;26:267. [Google Scholar]
- 26.Lim DJ, Dunn DE, Johnson DL, et al. Trauma of the ear from infrasound. Acta Otolaryngol. 1982;94:213–31. doi: 10.3109/00016488209128907. [DOI] [PubMed] [Google Scholar]
- 27.Shea J, Jr, Ge X, Orchik DJ. Traumatic endolymphatic hydrops. Am J Otol. 1995;16:235–40. [PubMed] [Google Scholar]
- 28.Kopke RD, Coleman JK, Liu J, et al. Enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002;112:1515–32. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Fife TD, Giza C. Posttraumatic vertigo and dizziness. Semin Neurol. 2013;33:238–43. doi: 10.1055/s-0033-1354599. [DOI] [PubMed] [Google Scholar]
- 30.Takeda T, Takeda S, Kitano H, Okada T, Kakigi A. Endolymphatic hydrops induced by chronic administration of vasopressin. Hear Res. 2000;140:1–6. doi: 10.1016/s0378-5955(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 31.Takeda T, Sawada S, Takeda S, et al. The effects of V2 antagonist (OPC-31260) on endolymphatic hydrops. Hear Res. 2003;182:9–18. doi: 10.1016/s0378-5955(03)00135-7. [DOI] [PubMed] [Google Scholar]
- 32.Walsted A. Effects of cerebrospinal fluid loss on hearing. Acta Otolaryngol Suppl. 2000;543:95–8. doi: 10.1080/000164800454099. [DOI] [PubMed] [Google Scholar]
- 33.Sone M, Yoshida T, Morimoto K, Teranishi M, Nakashima T, Naganawa S. Endolymphatic hydrops in superior canal dehiscence and large vestibular aqueduct syndromes. Laryngoscope. 2016;126:1446–50. doi: 10.1002/lary.25747. [DOI] [PubMed] [Google Scholar]
- 34.Bittencourt AG, Alves RD, Ikari LS, Burke PR, Gebrim EMS, Bento RF. Intracochlear schwannoma: diagnosis and management. Int Arch Otorhinolaryngol. 2014;18:322–4. doi: 10.1055/s-0033-1364170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grayeli AB, Fond C, Kalamarides M, et al. Diagnosis and management of intracochlear schwannomas. Otol Neurotol. 2007;28:951–7. doi: 10.1097/MAO.0b013e3181514485. [DOI] [PubMed] [Google Scholar]
- 36.Jiang ZY, Kutz JW, Jr, Roland PS, Isaacson B. Intracochlear schwannomas confined to the otic capsule. Otol Neurotol. 2011;32:1175–9. doi: 10.1097/MAO.0b013e31822a20ea. [DOI] [PubMed] [Google Scholar]
- 37.Miller ME, Moriarty JM, Linetsky M, Lai C, Ishiyama A. Intracochlear schwannoma presenting as diffuse cochlear enhancement: diagnostic challenges of a rare cause of deafness. Ir J Med Sci. 2012;181:131–4. doi: 10.1007/s11845-010-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salzman KL, Childs AM, Davidson HC, Kennedy RJ, Shelton C, Harnsberger HR. Intralabyrinthine schwannomas: imaging diagnosis and classification. AJNR Am J Neuroradiol. 2012;33:104–9. doi: 10.3174/ajnr.A2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho TY, Cheng PW, Young YH. Secondary endolymphatic hydrops after sudden hearing loss. Acta Otolaryngol. 2013;133:1040–6. doi: 10.3109/00016489.2013.805432. [DOI] [PubMed] [Google Scholar]
- 40.Kaya S, Tsuprun V, Hizli O, Schachern PA, Paparella MM, Cureoglu S. Cochlear changes in serous labyrinthitis associated with silent otitis media: a human temporal bone study. Am J Otolaryngol. 2016;37:83–8. doi: 10.1016/j.amjoto.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaya S, Tsuprun V, Hizli O, Paparella MM, Cureoglu S. Quantitiative assessment of cochlear histopathologic findings in patients with suppurative labyrinthitis. JAMA Otolaryngol Head Neck Surg. 2016;142:364–9. doi: 10.1001/jamaoto.2015.3803. [DOI] [PubMed] [Google Scholar]
- 42.Kaya S, Hizli O, Schachern PA, Paparella MM, Cureoglu S. Effects of intralabyrinthine hemorrhage on cochlear elements: a human temporal bone study. Otol Neurotol. 2016;37:132–6. doi: 10.1097/MAO.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 43.Miller ME, Makary C, Lopez IA, Ishiyama A. Endolymphatic hydrops in otology syphilis: a temporal bone study. Otol Neurotol. 2010;31:681–6. doi: 10.1097/MAO.0b013e3181dbb7e4. [DOI] [PubMed] [Google Scholar]
- 44.Jung DH, Nadol JB, Folkerth RD, Merola JF. Histopathology of the inner ear in a case with recent onset of Cogan’s syndrome: evidence for vasculitis. Ann Oto Rhinol Laryngol. 2016;125:20–4. doi: 10.1177/0003489415595426. [DOI] [PubMed] [Google Scholar]


