INTRODUCTION
Despite being the focus of widespread public health efforts, childhood obesity remains an epidemic worldwide. The most recent US estimates show that 17.7% (95% confidence interval [CI], 14.5–21.4) of children 6 to 11 years old are obese (body mass index for age ≥ 95th Centers for Disease Control and Prevention [CDC] percentile), whereas a further 16.5% are overweight and at risk for becoming obese.1 Given the now well-documented consequences of obesity for childhood health and psychosocial functioning, as well as associated morbidity in adulthood, identifying novel, modifiable behaviors that can be targeted to improve weight control is imperative.
The observation that while obesity levels were increasing, the duration of children’s nighttime sleep was decreasing,2 accompanied by compelling evidence for the potential role of sleep in both intake and expenditure aspects of energy balance,3–7 suggests that nighttime sleep might be one such modifiable factor. Numerous cross-sectional and prospective observational studies have supported the association between sleep duration and obesity risk in children.8–10 A recent meta-analysis found that, across 22 prospective observational studies of children aged 6 months to 18 years at baseline, from diverse backgrounds, children with a shorter sleep duration had twice the risk of overweight/obesity (odds ratio [OR], 2.15; 95% CI, 1.64–2.81) compared with their longer-sleeping peers.10 The association was stronger among younger (OR, 1.88; 95% CI, 1.26–2.81) compared with older children (OR, 1.55; 95% CI, 1.22–1.97).10
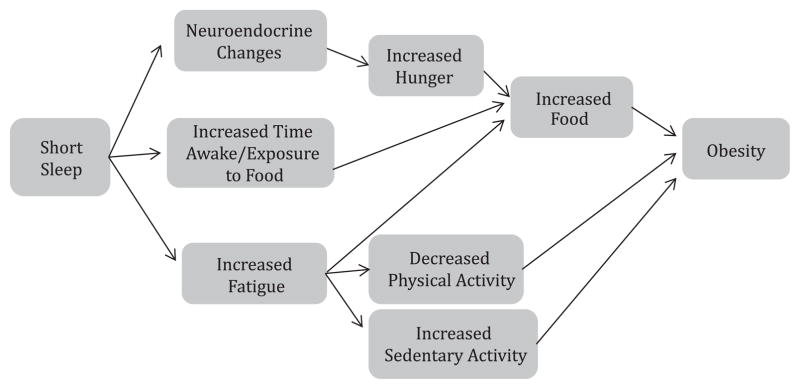
Several pathways have been suggested that may link short sleep with obesity risk,11 (Fig. 1). Experimental studies in healthy adults have provided evidence that sleep restriction or deprivation results in several neuroendocrine and inflammatory changes: impaired glucose metabolism, reduced insulin sensitivity, and increased levels of inflammatory mediators such as interleukin-6 and tumor necrosis factor.12–14 Of particular interest are the changes that occur in hormones related to hunger and appetite; sleep restriction has been shown to reduce levels of leptin, a hunger inhibitor, and increase levels of the hunger hormone ghrelin.6,7,15–17 Additional pathways proposed, and supported at least in part by adult experimental studies, include poorer food choices among those who are sleep deprived as well as reduced activity levels related to daytime tiredness.3–7 Pediatric observational studies18–23 are consistent with adult experimental studies, suggesting that similar pathways may be responsible for associations between short sleep and obesity risk in children as well. However, the pediatric literature remains limited by the observational nature of most of the existing studies.
Fig. 1.
Pathways through which sleep duration could affect obesity risk.
To build on previous work, our group developed Project SLEEP, a series of studies designed to determine whether changes in children’s sleep lead to changes in eating and activity habits and weight status. The approach was grounded in behavioral theory and informed by empirically supported treatment approaches for pediatric sleep disorders.24–28 Importantly, studies were designed to systematically build on each other, and to test hypotheses using 2 distinct approaches: (1) an experimental research design, and (2) randomized controlled trials to evaluate relative efficacy of behavioral interventions. The experimental study29 enabled careful manipulation of children’s sleep to create large discrepancies in sleep duration and thus optimize detection of the impact of sleep on eating pathways associated with obesity risk. Given the need for translation of epidemiologic findings for development of a novel approach for prevention and/or treatment of pediatric obesity, randomized controlled trials allowed for piloting of a novel behavioral intervention to enhance sleep. Findings from these studies, the process undertaken to move from one study to the next, and future directions for this work are discussed below.
Commonalities Across Studies
The studies described below were designed with an eye toward dissemination. As such, to enhance ecological validity, all studies were conducted with children sleeping at home and coming into the research center for assessment and intervention visits only. Further, all studies enrolled both children who were normal weight and overweight/obese given that epidemiologic studies have shown that long sleep is protective against subsequent change in weight status in both normal-weight and overweight/obese children.30,31 Thus inclusion of children drawn from both populations increased the ability to generalize findings for both prevention and treatment of obesity.
In addition, all studies enrolled children who reported sleeping approximately 9.5 h/ night (or less). This criterion was used because children in the United States report sleeping approximately 9.5 h/night on average,32,33 which is less than what has been recommended (ie, 10–11 h/night)34,35 for children 8 to 11 years old, which is the population of interest in these studies. Thus, as with the decision regarding enrollment by weight status, establishing a criterion for sleep at 9.5 h/night enabled greater ability to generalize findings. Importantly, in the experimental study, enrolling children who slept approximately 9.5 h/night allowed for both sleep extension and restriction by 1.5 h/night without reaching a ceiling for how much sleep children this age could achieve while also not sleep depriving them too much. In terms of the behavioral interventions, enrolling children who slept 9.5 hours or less allowed sufficient room to potentially enhance sleep using behavioral strategies.
In addition, across all studies the authors differentiated between 2 sleep constructs: time in bed (TIB) and the actigraph sleep period. Because children cannot be forced to sleep, but it is possible to prescribe when they should be in bed with the lights out and attempting to sleep, all prescriptions for changes in sleep were made by changing children’s TIB (ie, the time between their lights being turned off and the child trying to fall asleep and waking the next morning). However, the primary outcome of interest across all studies was change in the objective assessment of sleep: the actigraph sleep period (ie, the time between when the actigraph estimates sleep onset and offset).
Study 1 Development: Can Sleep Be Enhanced in Otherwise Healthy Children?
Several studies, primarily with preschool children, show that brief behavioral interventions can promote healthier sleep in children diagnosed with behavioral sleep disorders.25,26,36–39 However, there is limited evidence for the efficacy of behavioral intervention to enhance sleep in otherwise healthy school-aged children who are reported to have insufficient sleep. Thus the goal of study 1 was to determine whether a brief behavioral intervention could enhance sleep in short-sleeping children. Given that it was a first evaluation of the newly designed intervention (described later; Table 1) it focused on acute changes in sleep. With an eye toward obesity prevention/treatment, it also focused on whether changes in sleep affected children’s eating behaviors. We assessed the relative reinforcing value (RRV) of food, which provided an objective measure of motivation for an energy-dense food reward.
Table 1.
Behavioral intervention components employed in studies 1 and 3
| Behavioral Strategy | Operationalization |
|---|---|
| Goal setting | Families are prescribed a 1–1.5 h increase in children’s TIB, which is based on family reported TIB achieved at baseline and confirmed with actigraphy |
| Preplanning | Although the behavioral goal for TIB is prescriptive, interventionists preplan with families how best to achieve the goal given schedules and life circumstances. Flexibility is afforded on weekends (ie, children are allowed to stay up 1 h later as long as they can sleep in the next day for an additional hour) |
| Self-monitoring | Families are provided with sleep diaries in which they document the time lights are turned off and the child is trying to fall asleep, time the child wakes up, and time the child gets out of bed. Monitoring of mood, aberrations during the day (eg, vacation day from school, illness), and activities included in bedtime routines is also included |
| Problem solving | Both facilitators and barriers to achieving the behavioral goals are identified. Intervention staff work with families to help them identify strategies for maximizing facilitators and minimizing likelihood of barriers to behavior change |
| Positive reinforcement | Positive reinforcement is woven throughout the intervention. Intervention staff positively reinforce families throughout intervention sessions, parents are taught to do the same at home throughout the duration of the study, and a sticker chart with family-focused, nonmonetary (or minimally priced) rewards is used to encourage children to make changes in their TIB |
| Positive routine | To promote sleep onset, families are encouraged to develop a bedtime routine of approximately 20–30 min that includes the use of a routine set of behaviors that can serve as cues for sleep onset (eg, brushing teeth, getting pajamas on, reading a book together) |
| Sleep hygiene/stimulus control strategies | In addition to positive routines, several stimulus control strategies are reviewed and recommended to enhance the likelihood of adherence to the prescribed changes in TIB. These strategies include a consistent sleep schedule, no caffeine within at least 2 h of bedtime, no screen time as part of the bedtime routine, removing televisions and other light-emitting devices from bedrooms, and using beds only for sleeping (eg, no homework in bed) |
Fourteen children 8 to 11 years old who slept 9.5 h/night or less most nights of the week were enrolled into this 3-week pilot study. Following a 1-week baseline assessment, children were randomized to either increase their TIB by 1.5 h/night or continue with their current sleep habits. The 1.5-hour TIB increase was a prescription designed to maximize children’s ability to achieve an increase in the actigraph sleep period of at least 45 min/night (ie, estimating sleep onset latency of 20–25 minutes and not expecting children to have perfect adherence to the prescribed change in TIB). Intervention families were provided with effective behavioral strategies to increase TIB, and returned after 1 week to assess adherence and problem-solve regarding barriers. Table 1 details the effective behavioral strategies used in this study. A 2:1 (intervention/control) randomization scheme was used to ensure adequate samples to assess intervention efficacy. All children returned again 2 weeks after baseline for the follow-up assessment. Primary variables of interest were change in the actigraph sleep period and change in food reinforcement (ie, how motivated a child was for a food reward). The Behavioral Choice Task,40 a validated computer-based measure, was used to assess food reinforcement.41–43 In this pilot, we were specifically interested in the RRV of energy-dense snack foods compared with sedentary activities (that were equally liked).
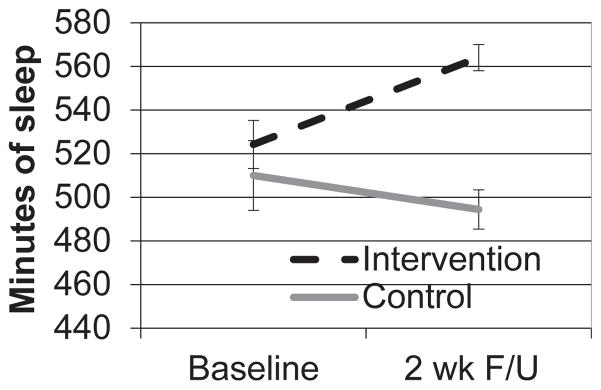
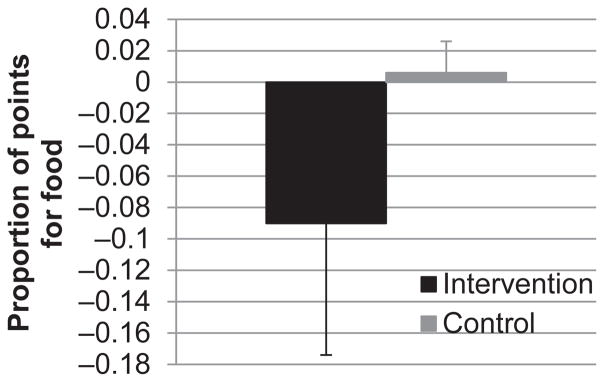
Twelve (86%) children had complete study data (1 child did not attend the 2-week assessment, and an actigraph malfunctioned in a second child). Children were 9.2 ± 1.1 years old with a mean body mass index (BMI) percentile (using CDC norms) of 71.7 ± 29.3 (58% overweight/obese); 75% were male, and 66% were non-Hispanic white. As shown in Fig. 2, children in the intervention condition increased their acti-graph sleep period by 40 ± 22 min/night versus a decrease (−16 ± 30 min/night) in control participants (t = 3.77; P = .004; d = 2.30). Children in the intervention tended to decrease the proportion of points earned for a food reward (−0.09 ± 0.21), whereas children in the control group showed no change (0.006 ± 0.04; t = −1.09; nonsignif-icant; d = 0.56) (Fig. 3). Although this change in the RRV of food was not significant, it represents a medium-sized effect.
Fig. 2.
Mean between-groups change in actigraph sleep period. F/U, follow-up.
Fig. 3.
Mean between-groups change in the RRV of food.
In summary, this study showed that our intervention was able to acutely enhance school-aged children’s sleep. Importantly, there were no observed or reported negative effects of intervention, such as longer sleep onset latency or greater percentage of time lying awake in bed. Further, there was also a signal that changes in sleep could lead to changes in children’s motivation for food. Thus these encouraging results suggested the need to continue to evaluate the potential utility of sleep in enhancing children’s weight-related behaviors. However, findings were limited by the focus on acute (2-week) changes in sleep in a small sample of children. Further, it was challenging reviewing with families all of the behavioral strategies (eg, goal setting, stimulus control/sleep hygiene, self-monitoring, and positive reinforcement) in a single visit of 45 to 60 minutes. Thus, to enhance potential efficacy, the intervention needed to be refined to deliver the behavioral content across greater than 1 treatment session. Further, to strengthen rationale it would be important to show that changes in sleep could be maintained over longer periods of time and with a larger sample of children. In addition, although the primary focus of study 1 was to determine whether sleep could be enhanced in otherwise healthy children, it was not designed to create large changes in sleep to maximize the ability to detect whether changes in sleep affect changes in several potential pathways through which sleep may affect obesity risk.
Study 2 Preliminary Testing: Experimental Changes in Sleep, Eating, and Weight
As study 1 was closing, study 2, which used an experimental design, was being launched. As noted earlier, this second study, a proof-of-concept study, was designed to maximize differences in children’s sleep to allow detection of how sleep may affect obesity risk, primarily through eating pathways. Primary findings have been previously reported; readers are referred to the main article for details.29 In brief, 39 children were enrolled into a 3-week study. During the first week, children were asked to sleep their typical amount. This week served 2 main purposes: to ensure final eligibility for the study based on reported sleep length (confirmed with actigraphy), and to establish a starting point from which to prescribe changes in TIB during the experimental weeks. After the baseline week, children were randomized to either increase or decrease TIB by 1.5 h/night (thus, the goal was to create a 3-hour TIB difference between experimental conditions). All changes in TIB were made by changing bedtimes; wake times remained constant across all study weeks. In order to achieve high levels of participant adherence, several strategies were used, including prescription of bedtimes and wake times (which were closely monitored by study staff through a twice-daily call-in system) as well as children being paid to adhere to the sleep schedule.29 Primary outcomes of interest were reported dietary intake (measured by 3-day 24-hour dietary recalls), fasting levels of leptin and ghrelin, and food reinforcement.
Thirty-seven of the 39 enrolled children completed the study. Findings showed high levels of adherence to the prescribed sleep schedule with a 141-minute difference in the actigraph sleep period between conditions.29 Importantly, when children decreased their sleep, they reported consuming 134 kcal/d more (based on 3 days of 24-hour dietary recalls) and weighed approximately 0.22 kg (0.5 lb) more at the end of the decreased week compared with their weight at the end of the increased week.29 Most of the additional caloric intake was reportedly consumed during the additional hours awake in the evening during the decreased sleep condition. Despite these changes in reported caloric intake and measured weight, there were no differences in food reinforcement or in fasting ghrelin levels. Further, findings regarding lep-tin were contrary to hypotheses, which may have been caused by several factors, including energy balance not being maintained (ie, children’s weight changed) and potential effects of the circadian timing system (given large shifts in bedtimes).29
Nonetheless, findings from this experimental study were encouraging. High levels of adherence to the prescribed changes in TIB and objective measures of sleep time allowed a valid comparison of how changes in sleep could affect changes in the study outcomes. Findings also provided a second signal that changes in sleep could affect changes in eating behaviors. Importantly, they also suggested that large changes in sleep could affect children’s weight status. However, limitations were observed, including the focus, again, on acute changes in sleep duration and a small study sample as well as a lack of a wash-out period between experimental conditions. To determine clinical significance of preliminary findings, a larger trial that assesses how prescribed changes in TIB affect children’s sleep, eating and activity behaviors, and weight status was needed.
Study 3 Efficacy: Does a Brief Behavioral Intervention Lead to Short-term Changes in Sleep, Eating and Activity Behaviors, and Weight Status?
To address limitations of the 2 previous studies, study 3, which is an ongoing, fully powered randomized controlled trial, was launched. Primary aims are to determine whether a brief behavioral intervention to increase sleep in school-aged children results in changes in the actigraph sleep period relative to control over a 2-month interval, and to determine the effect of intervention on eating and activity behaviors, and weight status. Specifically, we hypothesize that children randomized to the optimized sleep condition will show greater increases in the actigraph sleep period at 2 months than children in the control group. Second, children in the optimized sleep group will show a greater decrease in total caloric intake and percentage of their calories consumed as fat relative to controls. Additional secondary hypotheses are that the optimized sleep group will engage in more moderate-vigorous physical activity and less sedentary activity, that they will show a greater decrease in the RRV of food, and that they will show a greater decrease in BMI z-score compared with children in the control group.
One-hundred and four children aged 8 to 11 years who are reported by parents to sleep approximately 9.5 hours or less each night are being enrolled in the 2-month study. After completing a baseline assessment week during which eligibility based on reported TIB is confirmed with actigraphy, children are randomized in a 1:1 fashion to either the active behavioral intervention or a control for contact condition (ie, same number of visits as the intervention arm, but no discussion of enhancing sleep; just a focus on accurate completion of assessments and study procedures). As in study 1, children randomized to the intervention group are being asked to increase TIB by 1.5 h/night over the study period, and children in the control group are being asked to continue with current sleep behaviors. To extend findings from the first 2 studies, assessments are occurring at baseline, 2 weeks, and 2 months. At each assessment, sleep duration is being estimated using standard procedures for wrist-worn actigra-phy to establish the actigraph sleep period44,45; dietary intake is being assessed with 3 days of 24-hour dietary recalls (using multiple pass methodology), and physical activity is being assessed with hip-worn accelerometry. Height and weight are being assessed by study staff using standard procedures and with children in light clothing and without shoes. In addition, food reinforcement is being assessed as in studies 1 and 2 with the Behavioral Choice task. Importantly, given findings from study 2 suggesting that changes in food intake were observed later in the day, assessments were moved from the morning to the late afternoon/early evening in an effort to capture potential changes in eating behaviors that may result from changes in sleep.
The sleep intervention mirrors the one developed and tested in study 1, but is being delivered across 4 sessions. Given the evidence from the prior studies and the wider sleep literature that a brief behavioral intervention can produce large changes in sleep,25,26,36–39 2 in-person intervention sessions are being provided (60 minutes and 30 minutes, respectively) delivered by a trained behavioral interventionist in the first 2 weeks postrandomization: the first immediately following randomization and the second 1 week later. Given findings from behavioral weight control interventions that early success predicts overall success in behavioral programs, this was done intentionally to support rapid changes in TIB. As in study 1, effective behavioral strategies were used to promote changes in TIB (see Table 1). In-person sessions are followed by phone follow-ups at 4 and 6 weeks postrandomization. These sessions focus primarily on reinforcing progress toward sleep goals, identifying facilitators and barriers to enhancing TIB, and problem solving to maximize facilitators and minimize risk of continued barriers.
Findings from this ongoing trial will provide important information regarding the efficacy of a brief behavioral intervention to produce sustainable changes in sleep over a 2-month period. By focusing on additional outcomes such as children’s eating and activity behaviors and weight status, it will also allow assessment of whether enhancing children’s sleep could have important implications for prevention and treatment of pediatric obesity.
SUMMARY/DISCUSSION
In summary, findings from the studies described earlier show that enhancing children’s sleep may show promise in assisting with weight regulation. These findings are particularly encouraging given that different methodological approaches have been/are being used and that each study has built systematically on prior work. Although encouraging, several avenues of future study are warranted, including better delineation of the mechanisms through which sleep duration influences weight status, continued refinement of intervention targets, and evaluation of intervention efficacy over longer periods of time. Determination of the relative efficacy of sleep as an adjunct treatment approach for pediatric obesity may also be warranted.
Although several pathways through which sleep may affect obesity risk have been proposed, it will be important to more clearly specify potential mechanisms underlying this relationship. Doing so may not only help to strengthen the rationale for enhancing sleep as a means of affecting children’s weight status, it may also help identify ways to further enhance intervention efficacy. For example, findings from our experimental study suggest that the additional hours awake may, at least in part, account for additional caloric intake when children’s sleep is restricted.29 This finding is consistent with other emerging work with adolescents46 and adults,3 and may suggest that the increased exposure to food-rich environments is a primary pathway through which short sleep affects obesity risk. As such, interventions focused on stimulus control efforts to minimize less healthy food options and potentially decrease the variety of foods available in the home may be an important adjunct treatment target. Alternatively, given large shifts that were made in children’s bedtimes in study 2, the circadian timing system may also be influencing eating behaviors. Emerging work shows a potentially important role of the circadian system in eating behaviors, hormonal release and metabolism, and weight regulation.47–49 Thus it is possible that shifts in circadian timing could affect study outcomes.
In addition to previously identified mechanisms, other unique mechanisms could account for how changes in sleep affect obesity risk. For example, one mechanism that has been associated with both sleep and obesity risk is executive functions (EF). It is notable that the association between sleep and obesity risk is strongest during a period of rapid growth in EF.50,51 This observation is underscored by several studies that have shown independent associations between EF and both sleep52,53 and obesity risk.54,55 It has been suggested that sleep deprivation may lead to unhealthy eating behaviors and weight gain via changes in EF secondary to functional changes in the prefrontal cortex (PFC), specifically regions involved in reward and regulation of emotion and behavior. Imaging studies with adults support this hypothesis. When presented with food images, participants show, for example, increased activation in the right anterior cingulate cortex56 and orbitofrontal cortex57 when sleep is restricted (compared with a rested condition). These regions are involved in motivation, reinforcement, decision making, and self-control.57 An additional study showed decreased activation to food images in the ventromedial PFC in individuals who reported greater daytime sleepiness (compared with those who were less sleepy), suggesting possible decreases in inhibition related to energy-dense foods when fatigued.58 Thus insufficient sleep may predispose individuals to excessive caloric intake because the rewarding properties of food (particularly highly palatable foods)17 strengthen; the ability to inhibit responses to energy-dense foods is impaired; and the ability to sustain goal-directed, healthy eating behaviors is compromised. This hypothesis is consistent with behavioral findings. Experimental studies with adults suggest that increased caloric intake is not caused by greater homeostatic need for energy, but by hedonic processes (ie, greater appetitive drive).3,59,60 Enhancing sleep could therefore decrease excessive energy intake by decreasing the rewarding properties of food (an effect found in study 1), and enhancing individuals’ ability to resist food temptations within the context of our food-rich environment. At present these potential pathways are speculative. It will be important to conduct additional studies to more definitively delineate underlying processes that may be at work.
Beyond the importance of identifying mechanisms, future work could continue to extend the present findings in several ways. Although study 3 is ongoing, if findings are consistent with previous studies, demonstration of persistence of treatment effects over longer periods of time will be important. This finding will be particularly relevant given that enhancing children’s sleep for longer periods of time will likely be necessary to observe significant effects on weight-related outcomes. Given the need for translation of empirical findings to community settings, it will also be important to identify active treatment components. As noted earlier, our studies in this area affect both the duration of sleep and parameters that may optimize circadian functioning (ie, advancing bedtimes, promoting consistent sleep schedules). Thus it will be important to determine which component is driving the observed findings (or whether both are key) so that targeted messages can be developed to efficiently promote necessary behavior change. Given that this work has focused singularly on enhancing sleep duration, to maximize impact on weight outcomes, it will also be important to consider combining a brief behavioral sleep intervention with other established approaches and/or strategies for weight regulation (ie, as adjunct to standard behavioral weight control treatment and/or with targeted obesogenic behaviors). Such an approach may be key to understanding the utility of sleep at enhancing weight regulation, and maximizing the efficacy of brief approaches with high translation potential.
In conclusion, our developmental work related to the effect of enhancing sleep on weight-related outcomes suggests that sleep may play an important role in children’s weight regulation. Although additional work is needed to more definitively identify sleep as a key behavior to enhance in an effort to decrease pediatric obesity risk, these preliminary studies, along with emerging work with adolescents and adults, provide a strong foundation for continued exploration.
KEY POINTS.
Use of multiple methodological approaches for determining the potential efficacy of a novel approach for pediatric obesity prevention and treatment can provide a strong foundation and rationale for refining approaches and current treatment targets.
Systematic study of how sleep duration may affect eating and activity pathways suggests that sleep may be an important modifiable risk factor for obesity prevention and treatment.
Several future directions are warranted, including further refinement of behavioral interventions, and further delineation of the mechanisms through which sleep may affect obesity risk.
Acknowledgments
The authors acknowledge all of the collaborators involved in these studies, including Mary Carskadon, PhD; Hollie Raynor, PhD; Elissa Jelalian, PhD; Judith Owens, MD, MPH; Robert Considine, PhD; Joseph Fava, PhD; and Adam Davey, PhD. In addition, we thank the postdoctoral fellows and staff who ensured the success of each study, including Alyssa Cairns, PhD; Elizabeth Kuhl, PhD; Kathrin Osterholt Fedosov, MA; Brittany James; Jessica Lawton; Amanda Samuels; Victoria Mathieu; Zeely Denmat; Isabella Cassell; Risha Kheterpal; Ashley Greer; Heather Polonsky; and Andrew Pool. We are also indebted to all of the participating families in these studies, without whom reporting of these findings would not be possible.
Footnotes
Disclosures: None.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012;16(3):203–11. doi: 10.1016/j.smrv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(14):5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brondel L, Romer MA, Nougues PM, et al. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91(6):1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 5.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid SM, Hallschmid M, Jauch-Chara K, et al. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17(3):331–4. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 7.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93(6):1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart CN, Cairns A, Jelalian E. Sleep and obesity in children and adolescents. Pediatr Clin North Am. 2011;58(3):715–33. doi: 10.1016/j.pcl.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16(2):137–49. doi: 10.1111/obr.12245. [DOI] [PubMed] [Google Scholar]
- 11.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandner MA, Sands-Lincoln MR, Pak VM, et al. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nedeltcheva AV, Kessler L, Imperial J, et al. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94(9):3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflügers Arch. 2012;463(1):139–60. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullington JM, Haack M, Toth M, et al. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegel K, Leproult R, L’Hermite-Baleriaux M, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 18.Androutsos O, Moschonis G, Mavrogianni C, et al. Identification of lifestyle patterns, including sleep deprivation, associated with insulin resistance in children: the Healthy Growth Study. Eur J Clin Nutr. 2014;68(3):344–9. doi: 10.1038/ejcn.2013.280. [DOI] [PubMed] [Google Scholar]
- 19.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep duration and metabolic dys-regulation in overweight children and adolescents. Arch Dis Child. 2008;93(1):89–90. doi: 10.1136/adc.2007.124768. [DOI] [PubMed] [Google Scholar]
- 20.Javaheri S, Storfer-Isser A, Rosen CL, et al. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011;158(4):617–23. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews KA, Dahl RE, Owens JF, et al. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35(10):1353–8. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iglayreger HB, Peterson MD, Liu D, et al. Sleep duration predicts cardiometabolic risk in obese adolescents. J Pediatr. 2014;164(5):1085–90. e1. doi: 10.1016/j.jpeds.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke RV, Kuhn BR, Peterson JL. Brief report: a “storybook” ending to children’s bedtime problems–the use of a rewarding social story to reduce bedtime resistance and frequent night waking. J Pediatr Psychol. 2004;29(5):389–96. doi: 10.1093/jpepsy/jsh042. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn BR, Elliott AJ. Treatment efficacy in behavioral pediatric sleep medicine. J Psychosom Res. 2003;54(6):587–97. doi: 10.1016/s0022-3999(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 26.Mindell JA, Kuhn B, Lewin DS, et al. Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep. 2006;29(10):1263–76. [PubMed] [Google Scholar]
- 27.Sadeh A. Cognitive-behavioral treatment for childhood sleep disorders. Clin Psy-chol Rev. 2005;25(5):612–28. doi: 10.1016/j.cpr.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Mindell JA. Empirically supported treatments in pediatric psychology: bedtime refusal and night wakings in young children. J Pediatr Psychol. 1999;24(6):465–81. doi: 10.1093/jpepsy/24.6.465. [DOI] [PubMed] [Google Scholar]
- 29.Hart CN, Carskadon MA, Considine RV, et al. Changes in children’s sleep duration on food intake, weight, and leptin. Pediatrics. 2013;132(6):e1473–80. doi: 10.1542/peds.2013-1274. [DOI] [PubMed] [Google Scholar]
- 30.Agras WS, Hammer LD, McNicholas F, et al. Risk factors for childhood overweight: a prospective study from birth to 9. 5 years. J Pediatr. 2004;145(1):20–5. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78(1):309–23. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 32.Foundation NS. [Accessed March 5, 2006];Final report: 2004 Sleep in America poll. 2004 Available at: http://www.sleepfoundation.org/_content//hottopics/2004SleepPollFinalReport.pdf.
- 33.Spilsbury JC, Storfer-Isser A, Drotar D, et al. Sleep behavior in an urban US sample of school-aged children. Arch Pediatr Adolesc Med. 2004;158(10):988–94. doi: 10.1001/archpedi.158.10.988. [DOI] [PubMed] [Google Scholar]
- 34.Mindell JA, Owens J. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 35.Ferber R. Solve your child’s sleep problems: new, revised and expanded edition. New York: Fireside; 1996. [Google Scholar]
- 36.Friman PC, Hoff KE, Schnoes C, et al. The bedtime pass: an approach to bedtime crying and leaving the room. Arch Pediatr Adolesc Med. 1999;153(10):1027–9. doi: 10.1001/archpedi.153.10.1027. [DOI] [PubMed] [Google Scholar]
- 37.Glaze DG. Childhood insomnia: why Chris can’t sleep. Pediatr Clin North Am. 2004;51(1):33–50. vi. doi: 10.1016/s0031-3955(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 38.Owens JA, Palermo TM, Rosen CL. Overview of current management of sleep disturbance in children: II–behavioral interventions. Curr Ther Res. 2002;63:B38–52. [Google Scholar]
- 39.Stores G. Practitioner review: assessment and treatment of sleep disorders in children and adolescents. J Child Psychol Psychiatry. 1996;37(8):907–25. doi: 10.1111/j.1469-7610.1996.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 40.Epstein L. Behavioral choice task: for measuring absolute and relative reinforcing value and habituation of operant behavior [computer program] Buffalo, New York: State University of New York at Buffalo; 2007. [Google Scholar]
- 41.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40(1):15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 42.Epstein LH, Truesdale R, Wojcik A, et al. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav. 2003;78(2):221–7. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- 43.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27(1):41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 44.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin North Am. 2006;12(1):23–30. viii. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Sadeh A. Assessment of intervention for infant night waking: parental reports and activity-based home monitoring. J Consult Clin Psychol. 1994;62(1):63–8. doi: 10.1037//0022-006x.62.1.63. [DOI] [PubMed] [Google Scholar]
- 46.Beebe DW, Zhou A, Rausch J, et al. The impact of early bedtimes on adolescent caloric intake varies by chronotype. J Adolesc Health. 2015;57(1):120–2. doi: 10.1016/j.jadohealth.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–73. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 48.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–58. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nohara K, Yoo S-H, Chen ZJ. Manipulating the circadian and sleep cycles to protect against metabolic disease. Front Endocrinol. 2015;6:35. doi: 10.3389/fendo.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81(6):1641–60. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8(2):71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- 52.Randazzo AC, Muehlbach MJ, Schweitzer PK, et al. Cognitive function following acute sleep restriction in children ages 10–14. Sleep. 1998;21(8):861–8. [PubMed] [Google Scholar]
- 53.Fallone G, Acebo C, Seifer R, et al. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep. 2005;28(12):1561–7. doi: 10.1093/sleep/28.12.1561. [DOI] [PubMed] [Google Scholar]
- 54.Temple JL, Legierski CM, Giacomelli AM, et al. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87(5):1121–7. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonato DP, Boland FJ. Delay of gratification in obese children. Addict Behav. 1983;8(1):71–4. doi: 10.1016/0306-4603(83)90059-x. [DOI] [PubMed] [Google Scholar]
- 56.Benedict C, Brooks SJ, O’Daly OG, et al. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97(3):E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 57.St-Onge MP, McReynolds A, Trivedi ZB, et al. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95(4):818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Killgore WD, Schwab ZJ, Weber M, et al. Daytime sleepiness affects prefrontal regulation of food intake. Neuroimage. 2013;71:216–23. doi: 10.1016/j.neuroimage.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav. 2014;134:86–91. doi: 10.1016/j.physbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Chaput JP. Is sleeping more and working less a new way to control our appetite? Eur J Clin Nutr. 2010;64(9):1032–3. doi: 10.1038/ejcn.2010.90. [DOI] [PubMed] [Google Scholar]