Abstract
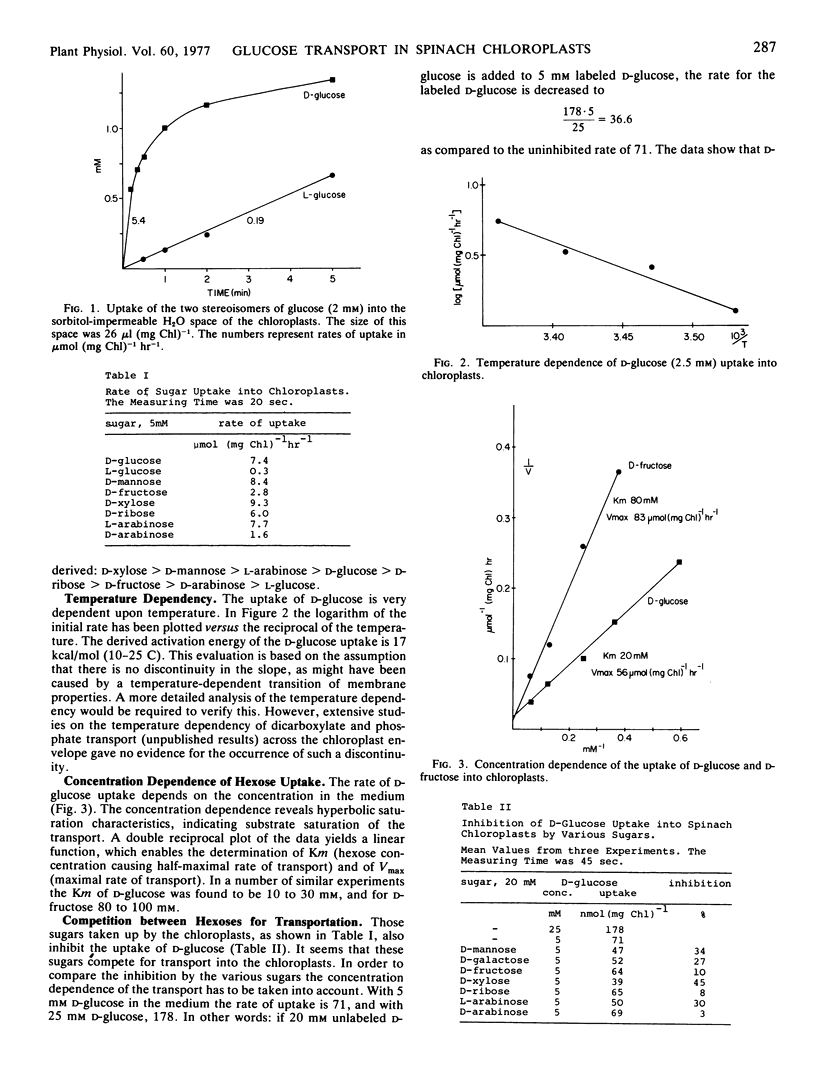
The uptake of radioactively labeled hexoses and pentoses into the sorbitol-impermeable 3H2O space (the space surrounded by the inner envelope membrane) of spinach (Spinacia oleracea L.) chloroplasts has been studied using silicone layer filtering centrifugation. Of the compounds tested, d-xylose, d-mannose, l-arabinose, and d-glucose are transported most rapidly, followed by d-fructose and l-arabinose. The rate of l-glucose uptake is only about 5% of that of d-glucose.
The transport of d-glucose and d-fructose shows saturation characteristics, the Km for d-glucose was found to be about 20 mm. All sugars transport and phloretin inhibit d-glucose transport. The temperature dependency of d-glucose transport appears to have an activation energy of 17 kcal/mol.
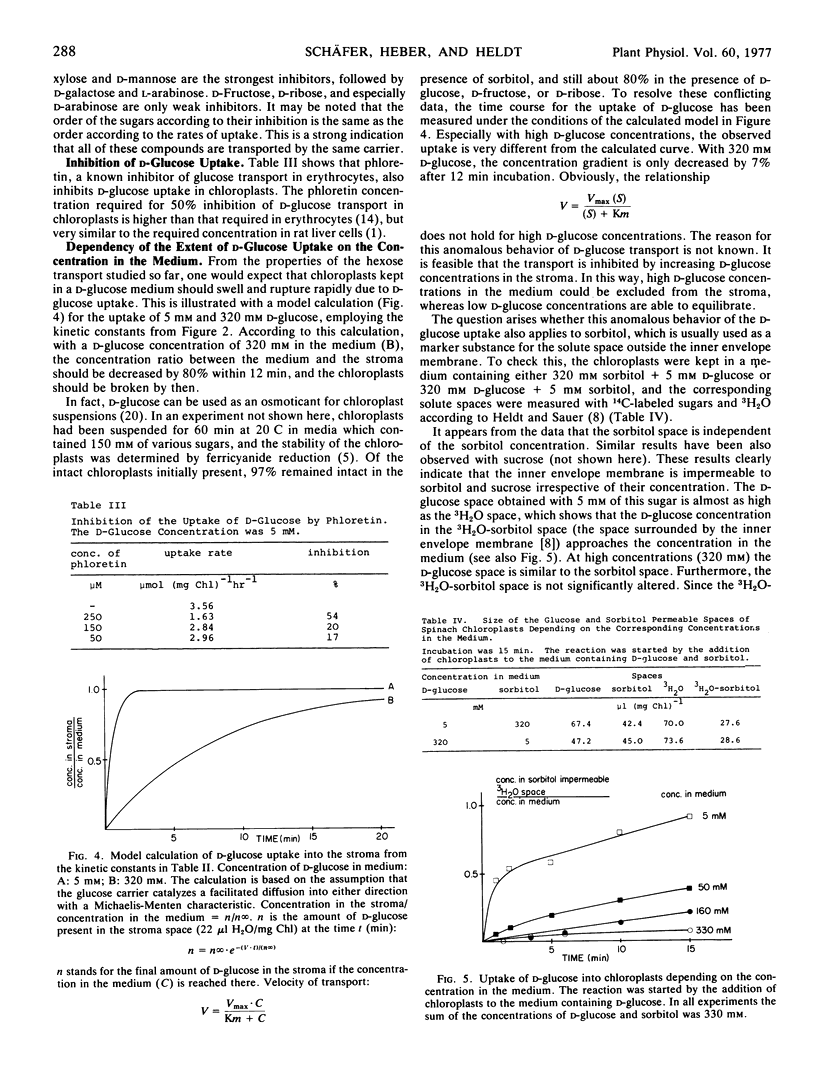
With low external concentrations of d-glucose the transport into the chloroplasts proceeds until nearly the external concentration is reached inside the chloroplasts.
d-glucose transport is inhibited by high d-glucose concentrations in the medium. It is concluded that d-glucose and other hexoses are transported by carrier-mediated diffusion across the inner envelope membrane. This transport is similar to the transport of d-glucose into erythrocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baur H., Heldt H. W. Transport of hexoses across the liver-cell membrane. Eur J Biochem. 1977 Apr 1;74(2):397–403. doi: 10.1111/j.1432-1033.1977.tb11404.x. [DOI] [PubMed] [Google Scholar]
- Bucke C., Walker D. A., Baldry C. W. Some effects of sugars and sugar phosphates on carbon dioxide fixation by isolated chloroplasts. Biochem J. 1966 Dec;101(3):636–641. doi: 10.1042/bj1010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDLER O., GIBBS M. Untersuchungen über den Einfluss der Photosynthese auf die Austauschvorgänge innerhalb des Hexosemoleküls. Z Naturforsch B. 1959 Jan;14B(1):8–13. [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotyk A., Höfer M. Uphill transport of sugars in the yeast Rhodotorula gracilis. Biochim Biophys Acta. 1965 Jul 22;102(2):410–422. doi: 10.1016/0926-6585(65)90131-7. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. Conformational specificity in a biological sugar transport system. Am J Physiol. 1958 Aug;194(2):333–337. doi: 10.1152/ajplegacy.1958.194.2.333. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G. Molecular structural factors in competitive inhibition of sugar transport. Science. 1959 Jul 10;130(3367):104–105. doi: 10.1126/science.130.3367.104. [DOI] [PubMed] [Google Scholar]
- Levi C., Gibbs M. Starch degradation in isolated spinach chloroplasts. Plant Physiol. 1976 Jun;57(6):933–935. doi: 10.1104/pp.57.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- MACLACHLAN G. A., PORTER H. K. Replacement of oxidation by light as the energy source for glucose metabolism in tobacco leaf. Proc R Soc Lond B Biol Sci. 1959 Sep 1;150:460–473. doi: 10.1098/rspb.1959.0035. [DOI] [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Determination of the temperature and pH dependence of glucose transfer across the human erythrocyte membrane measured by glucose exit. J Physiol. 1962 Mar;160:392–403. doi: 10.1113/jphysiol.1962.sp006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. T., Nobel P. S. Permeability of pea chloroplasts to alcohols and aldoses as measured by reflection coefficients. Biochim Biophys Acta. 1971 Jul 6;241(1):200–212. doi: 10.1016/0005-2736(71)90317-8. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]



