Abstract
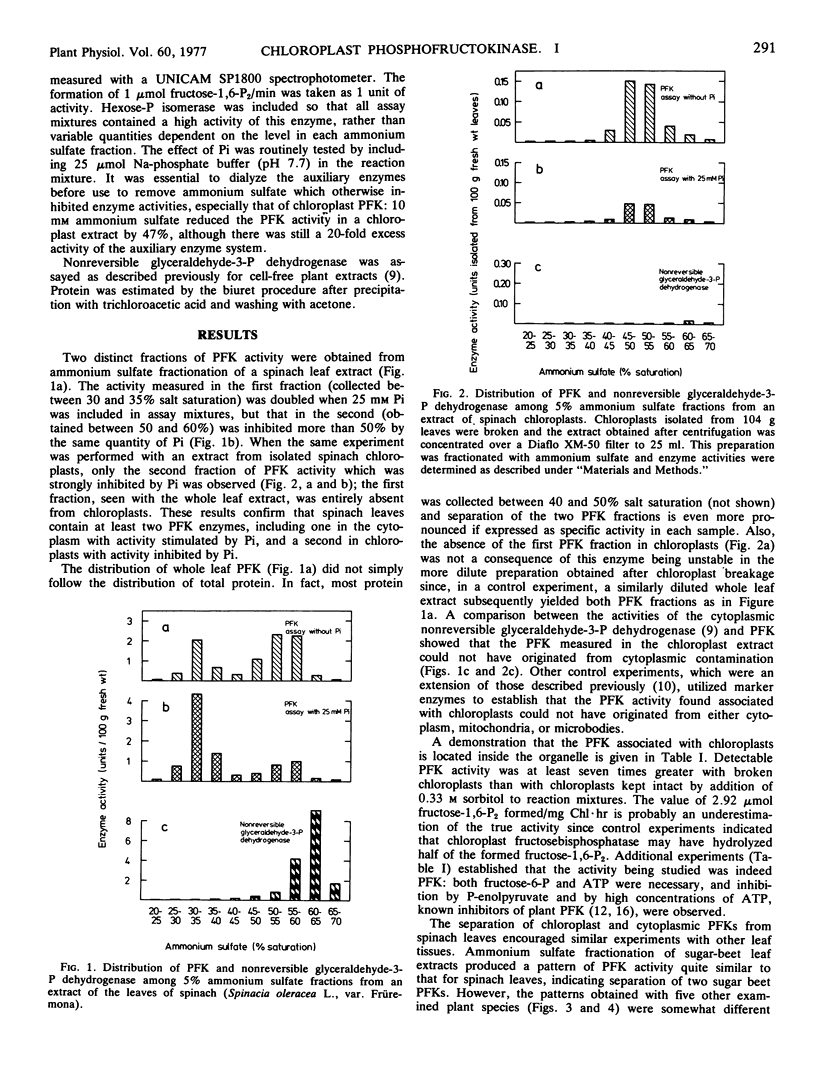
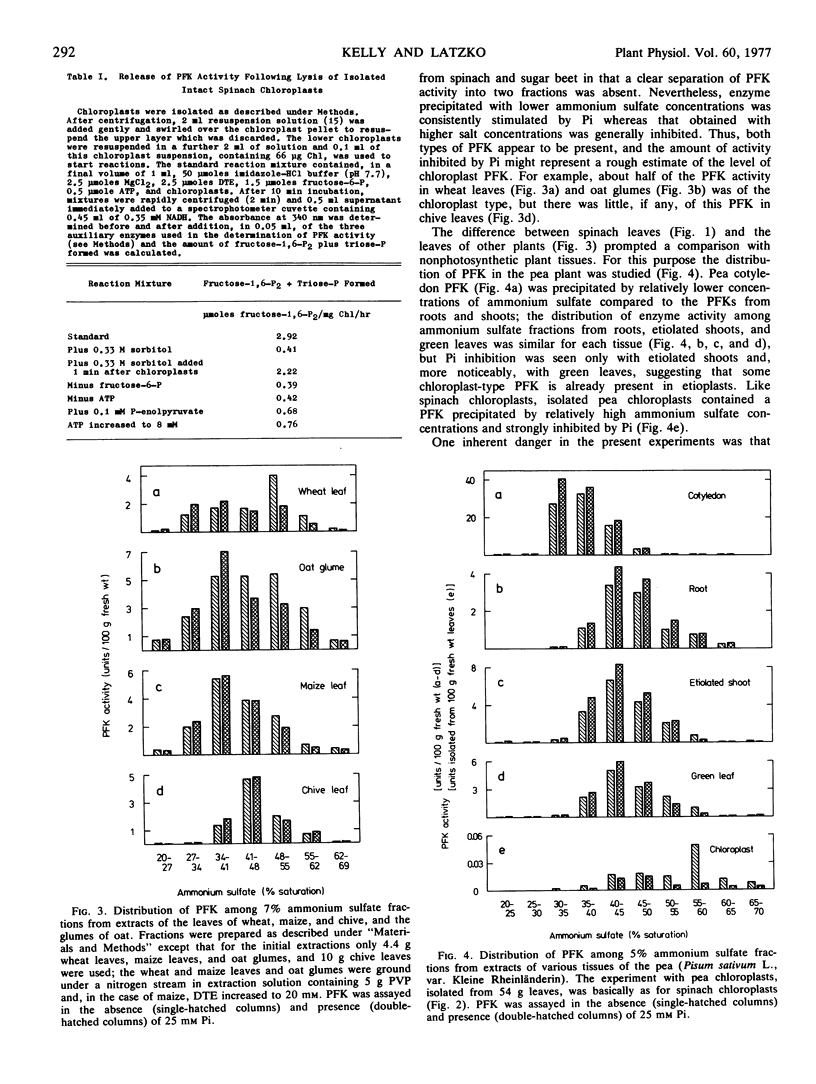
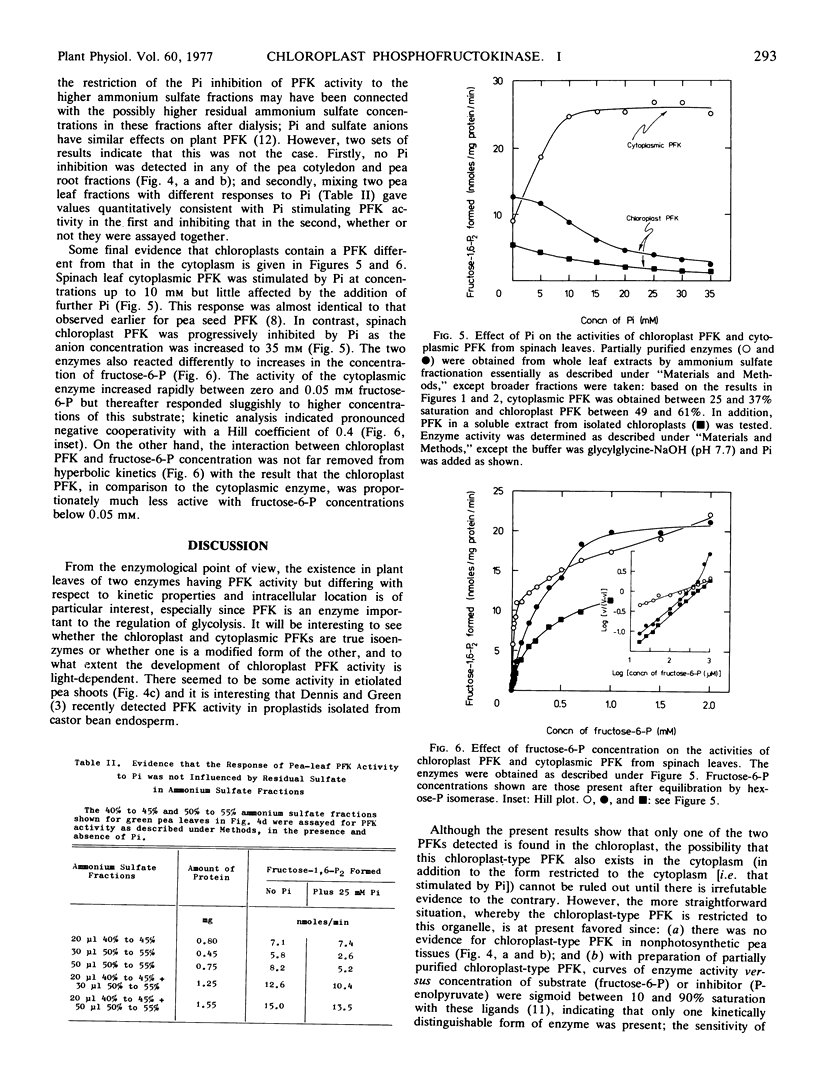
Ammonium sulfate fractionation of an extract from the leaves of spinach (Spinacia oleracea L.) produced two fractions of phosphofructokinase activity, the first stimulated by inorganic phosphate and the second inhibited by inorganic phosphate. Only the second fraction was obtained from similar treatment of an extract of isolated spinach chloroplasts. The two fractions differed distinctly with respect to kinetics for the substrate fructose 6-phosphate. Evidence for these two types of phosphofructokinase was also obtained with extracts from the leaves of wheat (Triticum aestivum L.), pea (Pisum sativum L.), and maize (Zea mays L.), and the glumes of oat (Avena sativa L.), but not from chive (Allium schoenoprasum L.) leaves, pea cotyledons, or pea roots. It was concluded that most leaves contain phosphofructokinase activity in chloroplasts as well as in the cytoplasm. Spinach chloroplast phosphofructokinase activity, which was at least 2.5 μmoles fructose 1,6-bisphosphate formed per mg chlorophyll per hour, did not result from contamination by cytoplasm or by other cellular organelles, and was not detected until after chloroplasts were broken.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., SALTMAN P., BANDURSKI R. S., BAKER R. S. Phosphonexokinase in higher plants. J Biol Chem. 1952 May;197(1):89–96. [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. The regulatory properties of a plant phosphofructokinase during leaf development. Biochim Biophys Acta. 1967 Sep 12;146(1):129–137. doi: 10.1016/0005-2744(67)90079-4. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Green T. R. Soluble and particulate glycolysis in developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 2;64(3):970–975. doi: 10.1016/0006-291x(75)90142-4. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Kachru R. B., Anderson L. E. Inactivation of pea leaf phosphofructokinase by light and dithiothreitol. Plant Physiol. 1975 Feb;55(2):199–202. doi: 10.1104/pp.55.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Gibbs M. Nonreversible d-Glyceraldehyde 3-Phosphate Dehydrogenase of Plant Tissues. Plant Physiol. 1973 Aug;52(2):111–118. doi: 10.1104/pp.52.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast Phosphofructokinase: II. Partial Purification, Kinetic and Regulatory Properties. Plant Physiol. 1977 Aug;60(2):295–299. doi: 10.1104/pp.60.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. The regulation of pea-seed phosphofructokinase by phosphoenolpyruvate. Biochem J. 1969 Nov;115(3):481–487. doi: 10.1042/bj1150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. A COMPARISON OF THE KINETIC PROPERTIES OF PHOSPHOFRUCTOKINASE FROM BACTERIAL, PLANT AND ANIMAL SOURCES. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 May 11;248:185–194. doi: 10.1007/BF00246673. [DOI] [PubMed] [Google Scholar]
- Levi C., Gibbs M. Starch degradation in isolated spinach chloroplasts. Plant Physiol. 1976 Jun;57(6):933–935. doi: 10.1104/pp.57.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Macdonald P. W., Strobel G. A. Adenosine Diphosphate-Glucose Pyrophosphorylase Control of Starch Accumulation in Rust-infected Wheat Leaves. Plant Physiol. 1970 Jul;46(1):126–135. doi: 10.1104/pp.46.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Jepsen N. M., Christeller J. T. Plants under Climatic Stress: III. Low Temperature, High Light Effects on Photosynthetic Products. Plant Physiol. 1972 May;49(5):798–802. doi: 10.1104/pp.49.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Koller H. R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974 Aug;54(2):201–207. doi: 10.1104/pp.54.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]


