Abstract
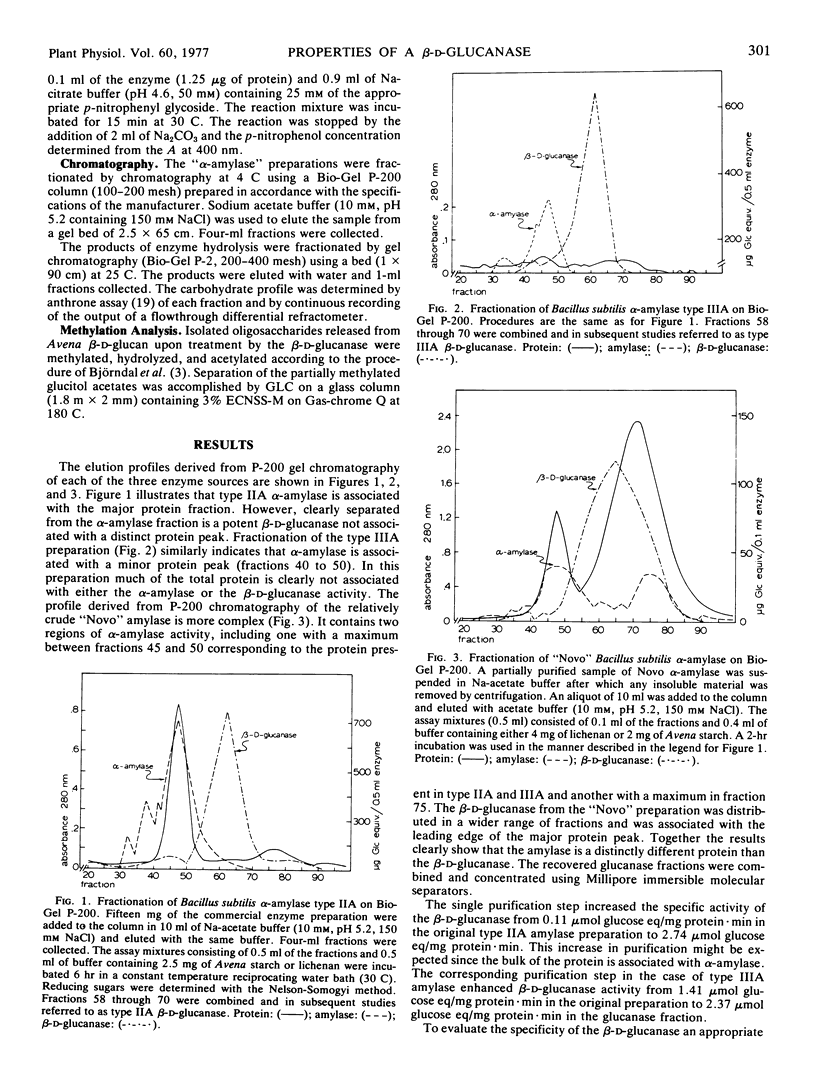
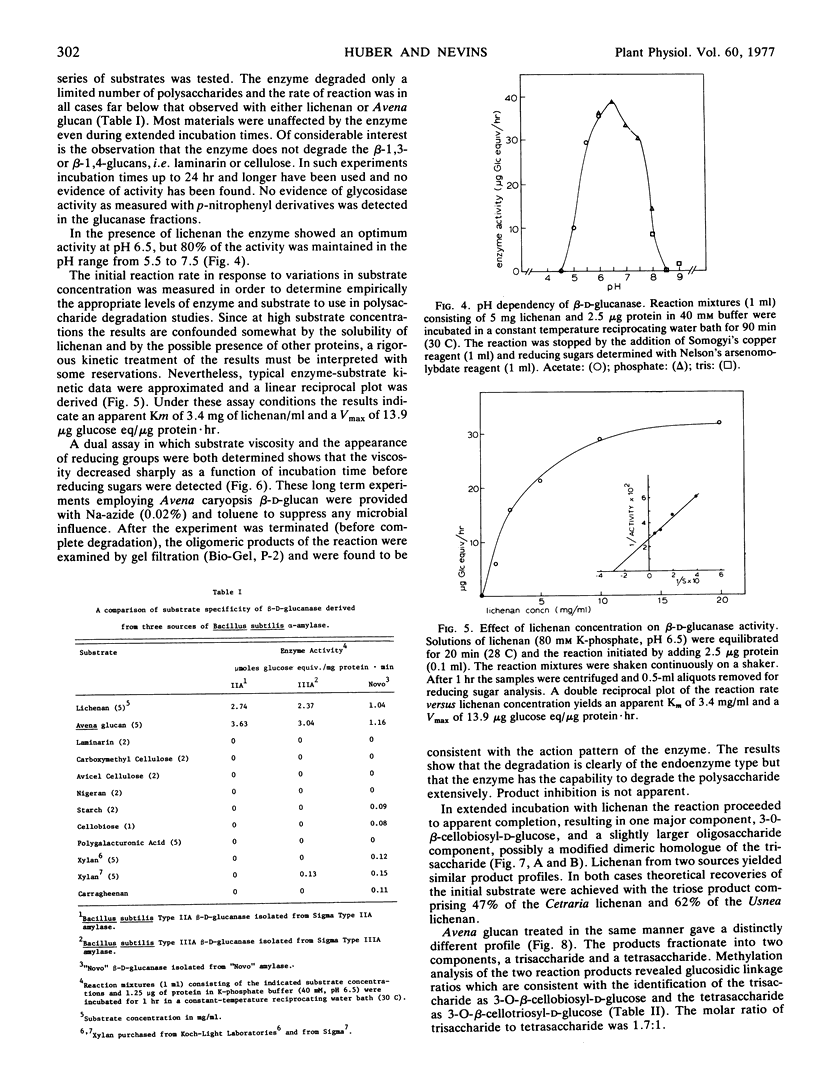
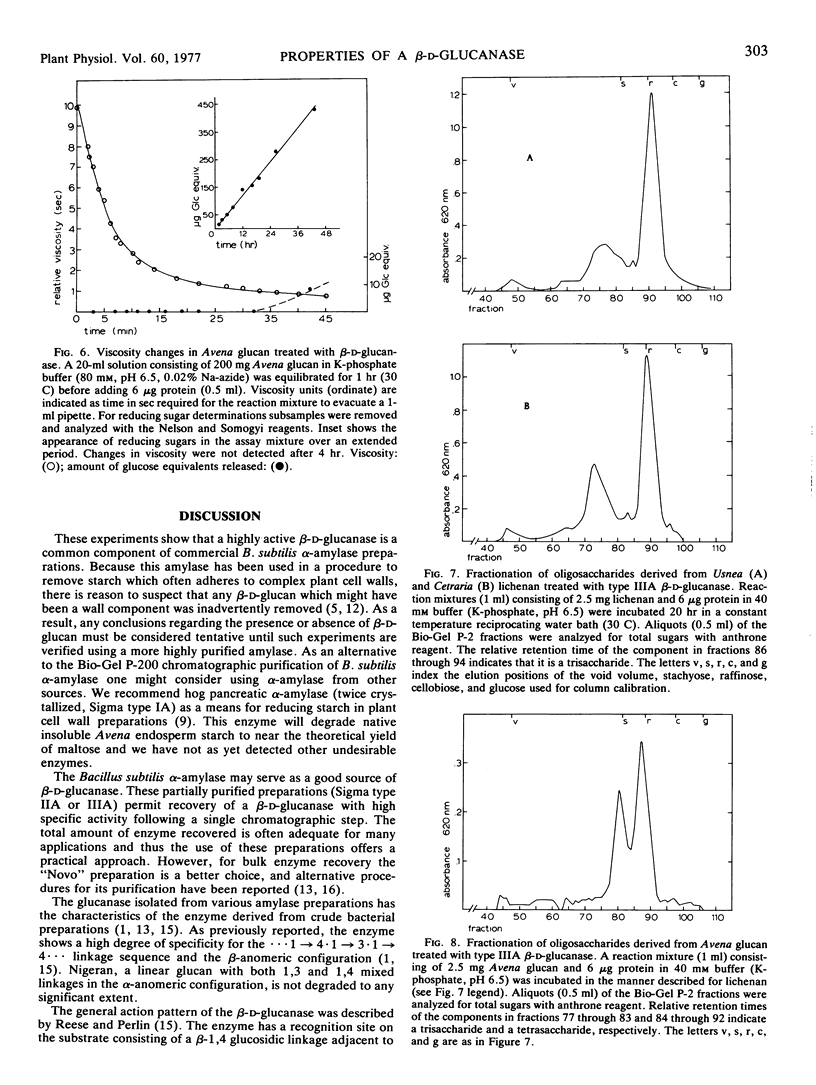
A β-d-glucanase highly specific for glucans containing a linkage sequence ··· Glc 1 → 4 Glc 1 → 3 Glc 1 → 4 Glc ··· has been isolated from several commercial preparations of Bacillus subtilis α-amylase including one purified by repeated crystallization. The β-d-glucanase will not hydrolyze cellulose or laminarin. Gel filtration on a Bio-Gel P-200 column results in separation of the glucanase from the α-amylase. The enzyme is of the endo type as changes in the substrate viscosity appear long before the appearance of detectable reducing sugars. No evidence of product inhibition was revealed and appropriate substrates were converted to oligosaccharides, the quantity of which approaches theoretical yields. The products of the reaction were separated according to molecular size by use of Bio-Gel P-2 gel filtration and found to be consistent with the action pattern of the enzyme. Kinetic studies show that the enzyme has an optimum activity at pH 6.5, a Vmax of 13.9 μg glucose equivalent released/μg protein·hour, and an apparent Km of 3.4 mg of lichenan per ml. Potential application of this enzyme for the structural characterization of plant cell wall glucans is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Stone B. A. A new substrate for investigating the specificity of beta-glucan hydrolases. FEBS Lett. 1975 Apr 1;52(2):202–207. doi: 10.1016/0014-5793(75)80806-4. [DOI] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivilaan A., Bandurski R. S., Schulze A. A partial characterization of an autolytically solubilized cell wall glucan. Plant Physiol. 1971 Oct;48(4):389–393. doi: 10.1104/pp.48.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSCATELLI E. A., HAM E. A., RICKES E. L. Enzymatic properties of a beta-glucanase from Bacillus subtilis. J Biol Chem. 1961 Nov;236:2858–2862. [PubMed] [Google Scholar]
- Manners D. J., Wilson G. Purification of malted-barley endo-beta-D-glucanases by ion-exchange chromatography: some properties of an endo-barley-beta-D-glucanase. Carbohydr Res. 1976 Jun;48(2):255–264. doi: 10.1016/s0008-6215(00)83221-8. [DOI] [PubMed] [Google Scholar]
- McNeil M., Albersheim P. The Structure of Plant Cell Walls: VII. Barley Aleurone Cells. Plant Physiol. 1975 Jan;55(1):64–68. doi: 10.1104/pp.55.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., PERLIN A. S. Enzymic preparation of 3-O-beta-cellobiosyl D-glucose. Biochem Biophys Res Commun. 1963 Jul 26;12:194–197. doi: 10.1016/0006-291x(63)90188-8. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Stone B. A. Beta-glucan synthesis by cell-free extracts from Lolium multiflorum endosperm. Biochim Biophys Acta. 1973 Jun 20;313(1):72–94. doi: 10.1016/0304-4165(73)90189-x. [DOI] [PubMed] [Google Scholar]
- Wood T. M. The cellulase of Fusarium solani. Purification and specificity of the -(1-4)-glucanase and the -D-glucosidase components. Biochem J. 1971 Feb;121(3):353–362. doi: 10.1042/bj1210353. [DOI] [PMC free article] [PubMed] [Google Scholar]


