Abstract
Red-meat lipid peroxidation in the stomach results in postprandial oxidative stress (POS) which is characterized by the generation of a variety of reactive cytotoxic aldehydes including malondialdehyde (MDA). MDA is absorbed in the blood system reacts with cell proteins to form adducts resulting in advanced lipid peroxidation end products (ALEs), producing dysfunctional proteins and cellular responses. The pathological consequences of ALEs tissue damage include inflammation and increased risk for many chronic diseases that are associated with a Western-type diet. In earlier studies we used the simulated gastric fluid (SGF) condition to show that the in vitro generation of MDA from red meat closely resembles that in human blood after consumption the same amount of meat. In vivo and in vitro MDA generations were similarly suppressed by polyphenol-rich beverages (red wine and coffee) consumed with the meal. The present study uses the in vitro SGF to assess the capacity of more than 50 foods of plant origin to suppress red meat peroxidation and formation of MDA. The results were calculated as reducing POS index (rPOSI) which represents the capacity in percent of 100 g of the food used to inhibit lipid peroxidation of 200 g red-meat a POSI enhancer (ePOSI). The index permitted to extrapolate the need of rPOSI from a food alone or in ensemble such Greek salad, to neutralize an ePOSI in stomach medium, (ePOS–rPOSI=0). The correlation between the rPOSI and polyphenols in the tested foods was R2=0.75. The Index was validated by comparison of the predicted rPOSI for a portion of Greek salad or red-wine to real inhibition of POS enhancers. The POS Index permit to better balancing nutrition for human health.
Abbreviations: POS, postprandial oxidative stress; MDA, malondialdehyde; ALEs, advanced lipid peroxidation end products; SGF, simulated gastric fluid; rPOSI, reducing POS index; ePOSI, POSI enhancer; AGEs, advanced glycation end-products; RAGE, receptor for AGE; ROO, refined olive oil; AA, l-ascorbic acid
Keywords: Stomach, Red-meat, Lipid-peroxidation, Malondialdehyde – MDA, Postprandial, Polyphenols
Graphical Abstract
Highlights
-
•
Absorption of diet MDA and ALEs in blood could induce risk factors for CVD and other diseases.
-
•
Red-meat generated MDA and ALEs in SGF are defined as ePOSI.
-
•
Reducing agents present in plant foods, reduced MDA and ALEs in SGF, are defined as rPOSI.
-
•
Calculated plant reducing agents by rPOSI was found to highly predict the reducing of ePOSI.
-
•
The POS index would help to quantify nutrition for promoting human health.
1. Introduction
Postprandial oxidative stress (POS) is characterized by an increase in susceptibility of the organism toward oxidative damage after consumption of a meal rich in lipids. The POS affected by high-fat diet is typically accompanied by transient endothelial dysfunction, inflammation and cellular oxidative stress which are established as an important risk factor for CVD [1], [2], [3]. Several epidemiological studies as well as experimental data suggest that population on diets characterized by the Western pattern, with high intake of high-fat red-meat, processed meat, fried foods, refined grains, but low in fruits and vegetables, are at high risk for the development of cardiovascular diseases (CVD), diabetes, colon cancer and other degenerative diseases [4], [5], [6], [7], [8].
The stomach acts as a bioreactor and is an excellent medium for enhancing lipid peroxidation, generation of free radicals, secondary lipid peroxidation products and co-oxidation of vitamins [4]. Many of these products generated from the break-down of lipid hydroperoxides such as: reactive aldehydes, ketones, and epoxides are cytotoxic. Once formed in the stomach they are readily absorbed by the intestine to subsequently interact with proteins and lipids to form advanced lipid oxidation end-products (ALEs) [5], [6]. Animal and human, after ingestion of peroxidized foods, have been shown to absorb and excrete increased amounts of malondialdehyde (MDA) and other carbonyls [7], [8]. Reactive aldehydes play critical roles in the pathogenesis of atherosclerosis [9], [10], and in many other chronic diseases such as diabetes, kidney diseases, retinopathy, neuropathy and cancer [11], [12]. The pathological effects of reactive aldehydes are related to their ability to modify reactive proteins or DNA by cross-linking, protein oligomerization, immune responses and to bind to RAGE (receptor for AGEs- advanced glycation end-products) activating the NADPH oxidase and generating reactive oxygen species [11], [13], [14], [15]. Such interaction with proteins and receptors could promote cellular oxidative stress, inflammatory mediators, and chronic diseases [11].
Modified-LDL or MDA-LDL are recognized as key factors in the initiation and acceleration of atherosclerosis [16], as markers of coronary artery disease severity [17] and others cardio-vascular diseases [18], [19]. To assess the stomach bioreactor capability, for further food lipid peroxidation, we have developed a randomized crossover study, in human, by which a meal of turkey red-meat was administered to volunteers and the levels of plasma and urine MDA were determined. After the meal of meat cutlets, plasma MDA levels rose and caused postprandial modification of LDL to MDA-LDL. Modification of LDL by MDA was found to be directly dependent on the increase of plasma MDA level following the meal [8], [20], [21]. Absorption of reactive carbonyls from a consumed meal that contains AGEs was also found in humans [9], [22]. However, most of the AGEs of selected popular foods are derived from those containing meat and high-fat [9].
Polyphenols compounds in foods have attracted a great deal of interest since the 1990s due to growing evidences on their potential beneficial effect on human health. Our early report that beverages such as red-wine which contains high concentration of polyphenol substances could inhibit LDL oxidation stimulated huge interest in this field [23], [24]. The LDL oxidation hypothesis of atherosclerosis triggered extensive investigation on the role of antioxidants including polyphenols against the onset and development of oxidized-LDL induced atherosclerosis [24], [25], [26]. Polyphenols containing foods and beverages exert potent antioxidant effects in-vitro, but not as efficient in-vivo, primarily due to low bioavailability and high metabolism which decrease their effectiveness [27]. We demonstrated, with human volunteers, that simultaneous consumption of red-turkey meat with foods rich in polyphenols (red-wine or black-coffee) prevents both lipid peroxidation and generation of MDA in the stomach and these lead to inhibited increase in plasma MDA and MDA-LDL [4], [8], [21]. This system was recently adopted by other investigators who used red-meat or hamburger during the meal for increasing lipid oxidation end-products in the blood system and preventing it by consuming with the meal food rich in polyphenols [28], [29], [30]. Most recently the PREDIMED group suggested that the health benefit of the Mediterranean diet should be attributed to the high consumption of foods containing polyphenols [31], [32]. We suggest that the main benefit of consuming plant polyphenols and other redox compounds in the human diet, as an integral part of the meal, arises mainly from the ability to prevent in the stomach lipid peroxidation and absorption of cytotoxic aldehydes and other ALEs into the blood system [4].
The simulated gastric fluid (SGF) assay is an established method that mimics the conditions in the stomach [8], [19], [20] to determine the extent of lipid peroxidation of various meats based on the release of MDA. To validate this system we conducted studies that compared the lipid peroxidation under these conditions with the appearance of MDA in human blood after consumption of the same meats. The results showed a very high correlation (R2=0.913) between concentrations of MDA, which were generated under stomach SGF conditions, and MDA in the blood with human volunteers for various food combinations [21], [33], [34].
The aim of this study was to determine the reducing capability of various foods (fruits, vegetables, beverages and spices) to inhibit lipid peroxidation in stomach medium by a very active pro-oxidant foods (red-turkey meat), and to calculate the Postprandial Oxidative Stress Index (POSI) which could help evaluating diets for improving human health.
2. Material and methods
2.1. Chemicals
Fresh meat (turkey, chicken and beef), frozen fish (tuna, salmon, halibut and tilapia), refined olive oil (ROO) containing 16 mg/L polyphenols, (BORGES, Spain), ω–3 supplement fish oil “Alsepa MAX” (Ocean-Nutrition, Canada) were obtained from a local stores. Metmyoglobin (metMb, from horse skeletal muscle), ß-carotene, Tween 20, butylated hydroxytoluene (BHT), catechin, pepsin (A, from porcine stomach mucosa), ferrous ammonium sulfate, xylenol orange, and triphenylphosphine (TPP) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Sodium chloride, hydrogen peroxide (30%) and l-ascorbic acid (AA) were obtained from Merck (Darmstadt, Germany). Ferric chloride (Fe) was obtained from Riedel-de-Haen (Hannover, Germany). Sodium borohydride was from BDH (Poole, Dorset, England). Solvents were all HPLC grade (J. T. Baker, Phillipsburg, NJ, USA). Soybean oil, red wine (Israeli Cabernet Sauvignon), and grilled turkey meat (shawarma) were bought at commercial stores in Israel. Simulated gastric fluid (SGF) was freshly prepared accordingly to the U.S. Pharmacopeia (the United States Pharmacopeia Inc. Rockville, MD, 2000). The SGF contained NaCl (200 mg), pepsin (320 mg) and HCl (700 µL of 36%) to DDW (100 ml) [35].
2.2. Malondialdehyde determination in meat after incubation in simulated gastric fluid (SGF)
Turkey red-meat, in the form of small slices, was heated for 1 min in a microwave, chilled, divided into portions and kept at −80 °C pending its use [32]. This frozen meat (10g) was ground with 30 ml of SGF for 60 s in a laboratory blender (Waring, New Hartford, CT) and adjusted to pH 3.0. The meat-liquid mixture (homogenate), in each treatment, was divided among several tubes and incubated in a shaking bath at 37 °C for 180 min. At four time points (0, 30, 90,180 min), lipid peroxidation was determined as MDA by the TBA method described by Kanner et al. [32] as follows: each sample was mixed immediately with 15%TCA at 1:1 ratio and centrifuged for 10 min at 20,800g. The supernatant was then treated with TBA (2.8 mg/ml) at 1:1 (v/v). The samples were heated in boiling water bath for 20 min and read at 532 nm (Synergy HT, BIO-TEK). MDA concentration was calculated according to 1 µmol/L=0.156 absorbance [36].
2.3. Determination of lipid hydroperoxides
Lipid peroxides were determined according to FOX-2 assay [33]. Red meat was treated as described for malondialdehyde. After incubation at 37 °C, 1 ml of the homogenate was mixed with 9 ml of methanol containing BHT (4.4 mM) for peroxide extraction and then centrifuged at 14,500g for 3.5 min. The supernatant (100 µL) was added (triplicates) to tubes containing 10 µL of methanol (or methanol+TPP) and incubated at 25 °C for 30 min. After incubation FOX-2 reagent (890 µL) which contains BHT (4.4 mM) was added and incubated for additional 30 min. The samples were measured by a spectrophotometer at 560 nm. Lipid hydroperoxides were calculated taking into account reduction of the results of methanol alone from samples of methanol +TPP. A standard curve was performed using hydrogen-peroxide of pure high grade. Determination of H2O2 in oils was done with FOX-2 reagent with and without catalase for determining the presence of H2O2. (1000 µM of hydroperoxides are the same as 1 mmol equivalent peroxides/kg oil).
2.4. Cooxidation of ß-carotene in meat homogenate
Turkey red-meat in SGF after homogenization was mixed with ß-carotene at a final concentration of 15 µM. The preparation of ß-carotene stock solution in water was as published previously. Briefly, ß-carotene (25 mg) and Tween 20 (0.9 ml) were solubilized in chloroform (25 ml) [37]. The solution (1 ml) was evaporated to dryness and solubilized with H2O (10 ml) to a stock solution. The reaction tubes were incubated in duplicate in a shaking bath at 37 °C for 90 min. The reaction was stopped by mixing with one volume of hexane and one volume of ethanol, and the mixture was left for another 5 min for phase separation. The ß-carotene was extracted with the hexane upper phase and determined by spectral analysis at 460 nm. The extinction coefficient of ß-carotene in hexane at 460 nm (for 1%, A=2550) was used for calculation the carotenoid concentration [37].
2.5. Determination of polyphenols in foods and beverages
The polyphenols content in foods was determined with Folin-Ciocalteau reagent and calculated as catechin equivalent after correction for ascorbate content in each food. The latter was determined by HPLC (see below). The results presented are the means of triplicates, and in the figures, each indicated error denotes the standard deviation. Ascorbic acid as an efficient reducing agent interacts with Folin-Ciocalteau reagent and affects by this the results of food polyphenols. Ascorbic acid was used to generate a standard calibration with the Folin-Ciocalteau reagent. The amount of ascorbic acid determined by HPLC method was normalized with those from Folin-Ciocalteau reagent and reduced from the results accepted for polyphenols, to better evaluate the total amount of the polyphenols in foods [37].
2.6. Vitamin C measurement
The homogenate samples were mixed with TCA (11.3%), (1:1 v/v) and centrifuged for 3 min at 20,800g. The supernatant was filtered through a 0.2 µm membrane, and a 20 µL aliquot was injected into a HPLC (Merck-Hitachi L-6200A) and separated with a Merck Lichrocart column RP-18, 125-4, eluted with an isocratic mobile phase of KH2PO4 (10 mM):MeOH (97:3 v/v) and tetrabutylammonium hydroxide 0.75 mM, at a flow rate of 1 ml/min and detected with a diode array detector (Shimatzu, Kyoto) at 268 nm. Ascorbic acid (Merck) was used to generate a standard calibration curve [37].
2.7. Plant material preparation for the POS index
Fresh plant material was cut into small slices of ~1 cm3 and immediately blanched by heating for 1 min in a microwave (Dow, S-Korea, at 800 W), chilled, and kept in vacuum packet, at −80 °C until used. The plant material was grounded and homogenized for 60 s with simulated gastric fluid (at a ratio of 1/1 w/v) adjusted to pH 3, in a laboratory blender (Warring, New Hartford, CT). The blanching was performed for inactivation of enzymes in particular polyphenol-oxidase, ascorbate oxidase and in general peroxidases which could oxidize polyphenols, ascorbic acid and other reducing agents. Beverages were prepared with hot-water as described previously [33], [38].
2.8. Statistical analysis
Results (means±SD) are expressed as percentage or weight or molar basis. Statistical significance was determined using one-way analysis of variance, following by a ranking procedure using Student-Newman-Keuls test (SAS software, SAS Institute Inc., Cary, NC). Results are the means of triplicates, and in the figures, each error bar (I) denotes the standard deviations.
3. Results
3.1. The basis of enhanced Postprandial Oxidative Stress Index (ePOSI) calculation
Red-turkey meat (200 g) lipid peroxidation in SGF was determined by extend of TBA-RS and expressed as MDA accumulation (nmole/g meat) at 37 °C, 180 min. These amounts of MDA (120±6.1 nmole/g meat) generated by 200 g meat in 1000 ml of SGF or in equivalent smaller model system was calculated as ePOSI=100. Red-turkey meat, the highest enhancer of lipid peroxidation in stomach medium between terrestrial animals [36], was chosen to be the meat referent for calculation of the inhibitory effect of different plant homogenates. Turkey red-meat in SGF and in the presence of olive oil (25 ml) or ω-3 oil (25 ml) was found to have ePOSI ~70 and ~300, respectively. POSI could be affected by many foods but especially by muscle foods, (Table 1).
Table 1.
Muscle food enhancers of POSI (ePOSI).
| Muscle food | MDA nmole/g | ePOSI/200 g FW |
|---|---|---|
| Turkey – Thigh | 120±11.2 | 100 |
| Turkey – Breast | 29±0.5 | 24 |
| Chicken – Thigh | 40±0.4 | 34 |
| Chicken – Breast | 20±0.2 | 17 |
| Beef - Shoulder (pH 4.6) | 65±0.6 | 54 |
| Pork – Leg | 45±0.4 | 38 |
| Tuna | 152±7.5 | 126 |
| Salmon | 122±3.3 | 102 |
| Halibut | 25±0.5 | 21 |
| Tilapia | 7±0.0 | 6 |
| Turkey+olive oil | 86±2.4 | 72 |
| Turkey+ω-3 | 367±6.3 | 306 |
| Turkey+tuna oil | 583±3.0 | 486 |
| Beef+ω-3 (pH 4.6) | 139±3.5 | 116 |
3.2. The basis of reduced Postprandial Oxidative Stress Index (rPOSI) calculation
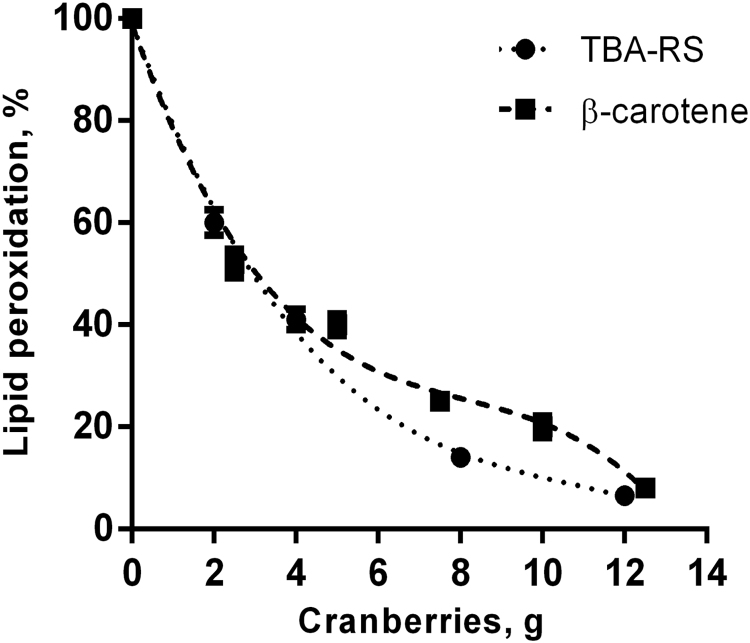
The reduced Postprandial Oxidative Stress Index (rPOSI) is defined as the capacity of a plant derived food in grams to completely (100%) inhibit MDA formation from 200 g turkey meat incubated in SGF for 180 min at 37 °C. Lipid peroxidation in SGF was determined by the extent of MDA formation measured as TBA-RS adduction product or oxidized ß-carotene. Fig. 1 shows red-meat lipid peroxidation determined by both methods by cranberries. The IC50 for cranberries to inhibit lipid peroxidation of 100 g meat was ~2.7 g, and 10.8 g for 100% inhibition (IC100). However, because most individuals consume 200 g meat per meal, rPOSI was calculated on these bases. For 200 g meat the IC100 is 21.8 g=rPOSI 100. The amount in g of each plant homogenate for IC100/200 g=rPOSI 100. In order to evaluate the differences between the plant rPOSI, we calculated the Index on plant 100 g basis. By using this method of calculation the rPOSI of 100 g cranberries is (100 g/21.6 g)×100=462. By using the same procedures the rPOSI of various foods are presented in Table 2, Table 3, Table 4, Table 5, Table 6.
Fig. 1.
Dose response curve of Turkey red-meat lipid peroxidation inhibited by Cranberries (IC50/100 g) determined by the methods of ß-carotene oxidation and MDA accumulation, in SGF, pH 3.0, 37 °C, t=180 min.
Table 2.
Edible fruits of high rPOSI (625-96).
| Food-fruits (var.) | Polyphenols/100 g FW (mg) | Ascorbic Acid/100 g FW (mg) | IC50/100 g meat (g) | IC100/200 g meata (g) | rPOSI/100 g FW |
|---|---|---|---|---|---|
| Blackberriesbc | 667±4.5 | 23±0.8 | 2.0 | 16.0 | 625 |
| Blackcurrantsbc | 680±7.9 | 170±2.2 | 2.1 | 16.8 | 588 |
| Quince (Portugal) | 460±6.3 | 69±1.8 | 2.2 | 17.6 | 568 |
| Cranberriesbc | 405±5.6 | 13±6.8 | 2.7 | 21.6 | 462 |
| Red – Plum (Red- heart)c | 432±10.6 | 10±0.5 | 3.9 | 31.2 | 320 |
| Blueberriesbc | 310±6.7 | 12±0.7 | 5.2 | 41.6 | 240 |
| Olive (Black – Manzanillo) | 338±5.8 | – | 6.8 | 54.4 | 180 |
| Guava (Ben-Dov) | 198±0.3 | 109±1.9 | 7.0 | 56.0 | 178 |
| Red –Plum (Sagiv) | 318±4.2 | 8±0.3 | 8.0 | 64.0 | 153 |
| Raspberriesbc | 289±2.5 | 12±0.5 | 8.2 | 65.6 | 152 |
| Pomegranate (Wonderful) | 145±3.5 | 23±0.7 | 9.0 | 72.0 | 138 |
| Pear (Spadona) | 100±3.8 | 22±0.8 | 13.0 | 104.0 | 96 |
The amount of fruit equals to rPOSI=100.
Frozen fruit.
Determined by two methods MDA, bleaching of β-carotene.
Table 3.
Edible fruits of rPOSI (74-13).
| Food- fruits (var.) | Polyphenols/100 g FW (mg) | Ascorbic acid/100 g FW (mg) | IC50/100 g meat (g) | IC100/200 g meatc (g) | rPOSI/100 g FW |
|---|---|---|---|---|---|
| Strawberries ( Tamar)b | 190±4.7 | 50±1.8 | 17.1 | 136.8 | 74 |
| Purple Grapes ( Red Globe)b | 150±1.5 | 2±0.3 | 20.0 | 160.0 | 63 |
| Green Grapes (Thomson) | 109±1.1 | 5±0.2 | 22.0 | 176.0 | 59 |
| Apple ( Gala) | 183±6.3 | 10±0.5 | 25.0 | 200.0 | 50 |
| Banana ( Ziv) | 114±4.2 | 10±0.4 | 26.5 | 212.0 | 47 |
| Date( Barhi – Fresh) | 150±5.0 | 5±0.2 | 27.2 | 217.6 | 46 |
| Peach ( White Lady) | 168±6.2 | 8±0.2 | 36.1 | 288.8 | 35 |
| Cherry ab | 221±7.8 | 7±0.4 | 40.3 | 322.4 | 31 |
| Fig ( Brazil) | 69±2.1 | 5±0.2 | 42.5 | 340.0 | 29 |
| Persimmon ( Triumph) | 67±2.5 | 31±1.4 | 52.3 | 341.8 | 24 |
| Orange ( Shamuti) | 93±4.1 | 57±2.2 | 60.4 | 483.2 | 21 |
| Date ( Medjool – dry) | 316±7.2 | 0 | 88.0 | 704.0 | 13 |
Frozen fruit.
Determined by two methods MDA, bleaching of β-carotene.
The amount of fruit equals to rPOSI=100.
Table 4.
Edible vegetables rPOSI (96-16).
| Food- vegetables (var.) | Polyphenols/100 g FW (mg) | Ascorbic Acid/100 g FW (mg) | IC50/100 g meat (g) | IC100/200 g meatb(g) | rPOSI/100 g FW |
|---|---|---|---|---|---|
| Spinach (Winter) | 105±2.0 | 80±2.3 | 13.1 | 104.8 | 96 |
| Broccoli (Monaco) | 85±3.5 | 233±19 | 20.2 | 161.6 | 62 |
| Purple cabbage (Robi King) | 230±6.2 | 98±2.3 | 22.0 | 176.0 | 57 |
| Red Pepper (Mazorka) | 47±5.6 | 205±4.1 | 24.4 | 193.6 | 53 |
| Green cabbage | 24±0.5 | 62±2.2 | 30.0 | 240.0 | 42 |
| Onion (Purple) | 120±1.9 | 16±0.8 | 35.1 | 280.8 | 36 |
| Carrot (Cello) | 11±0.5 | 8±0.5 | 40.0 | 320.0 | 31 |
| Red-Beeta | 196±6.0 | 2±0.1 | 47.0 | 370.0 | 27 |
| Onion (Yellow-Ori) | 80±3.2 | 12±0.2 | 50.2 | 401.6 | 25 |
| Eggplant (Black) | 171±2.5 | 2.2±0.1 | 57.5 | 460.0 | 22 |
| Cauliflower (White Corrona) | 42±2.1 | 65±2.3 | 70.1 | 560.0 | 18 |
| Tomato (Ravid) | 29±1.7 | 12±0.4 | 78.0 | 625.0 | 16 |
Determined by two methods MDA, bleaching of β-carotene.
The amount of vegetables equals to rPOSI=100.
Table 5.
Common beverages rPOSI (60-13).
| Food- beverages | Polyphenols/100 g FW (mg) | IC50/100 g meat (g) | IC100/200 g meatb(g) | rPOSI/100 g FW |
|---|---|---|---|---|
| Red-Wine (Petite-Sirah)a | 237±5.1 | 21.0 | 168.0 | 60 |
| Coffee Black (Turkish roast-ground) | 225±6.3 | 22.1 | 169.6 | 59 |
| Coffee (Spray-dried) | 255±7.2 | 31.9 | 255.2 | 39 |
| Tea (Black) | 113±5.1 | 52.1 | 416.8 | 24 |
| Tea (Green) | 125±2.3 | 82.2 | 657.6 | 15 |
| Cocoa | 97±4.1 | 93.3 | 746.4 | 14 |
| Coffee (Freeze-dried) | 200±5.3 | 94.1 | 752.8 | 13 |
Determined by two methods MDA, bleaching of β-carotene.
The amount of beverage equals to rPOSI=100.
Table 6.
The Mediterranean Greek salad rPOSI.
| Food- items/100 g | Polyphenols/100 g FW (mg) | Ascorbic Acid/100 g FW (mg) | rPOSI |
|---|---|---|---|
| Greek-Salad | |||
| Tomato | 29 | 12 | 16 |
| Cucumber | 0 | 0 | 0 |
| Red-Pepper | 47 | 205 | 53 |
| Green-Cabbage | 24 | 62 | 42 |
| Onion (Purple) | 120 | 16 | 36 |
| Olive (Black–Manzanillo 25 g) | 80 | 0 | 45 |
| Total/525 g salad | 300 | 295 | 192 |
| A Salad portion of 274 g | 156 | 153 | 100 |
3.3. The rPOSI of Fruit items
The rPOSI of several fruits is presented in Table 2, Table 3. The fruits (on basis of 100 g) with rPOSI higher than 74 is presented in Table 2. The most active fruits with high rPOSI were blackberry, black-currants, quince, cranberry, red-plums, blueberry, olive-black, guava, raspberry and pomegranate. All of these are rich in polyphenols, between 100 and 680 mg catechin equivalent/100 g FW. Fruits with rPOSI lower than 96 are presented in Table 3. It is important to note that different varieties of the same fruit or vegetable may have different rPOSI. For example the rPOSI of Red-heart plum is 320 where the Sagiv plum is 153. For dates Barhi fresh rPOSI is 46 and 13 for Medjool-dried. It is known that during ripening and dehydration a high part of the polyphenols are oxidized especially by polyphenol-oxidase [38] producing quinones and other oxidizing compounds which could act pro-oxidative.
3.4. The rPOSI of vegetables items
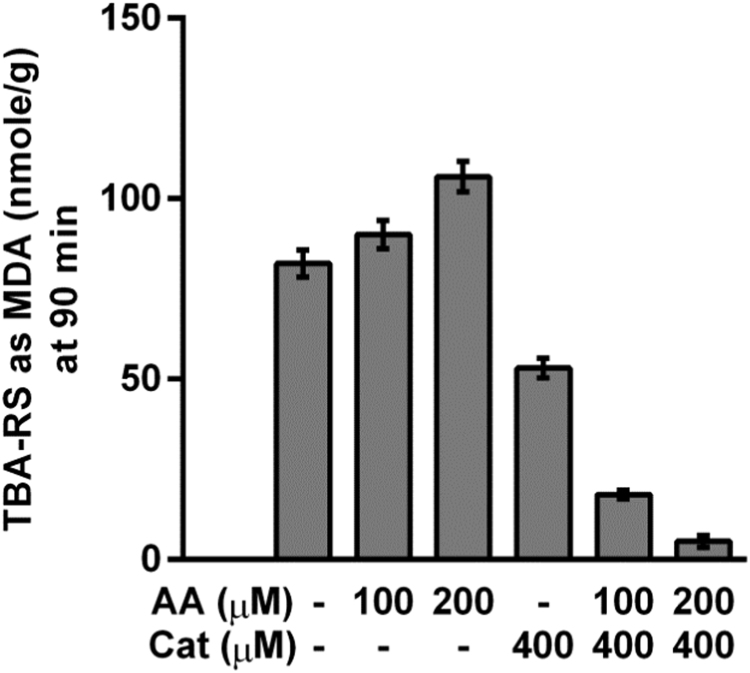
The inhibition of red-meat lipid peroxidation by various vegetables is presented in Table 4. The active vegetables with rPOSI between 100 and 40 were; spinach, broccoli, purple or green cabbage and red-pepper. These vegetables contain a cocktail of reducing agents, electron donors and acceptors such as polyphenols and tocopherols, thiocyanates, ascorbic-acid, carotenoids and chlorophylls. Onion (purple and yellow), red-beet and carrot are of medium activity with rPOSI of 40 to 25. The less active vegetables were tomato and green-pepper. Green-pepper which contain a relatively high concentration of ascorbic-acid but low in polyphenols, act pro-oxidative in stomach medium. From all the fruits and vegetables determined for POSI only green pepper was found to act as a pro-oxidant at 100 g Food Weight (FW) (results not shown). Fig. 2 presents results of Turkey red-meat lipid peroxidation as affected by ascorbic acid which act as a pro-oxidant and catechin which is an antioxidant. However when combined they exhibit an antioxidant synergistic effect. The relative high rPOSI of red-pepper seems to be because the presence of a natural cocktail of antioxidants at relative high critical concentration of ascorbic-acid, tocopherols, polyphenols and carotenoids [37], [39].
Fig. 2.
Turkey red-meat lipid peroxidation as affected by ascorbic acid (AA) and catechin determined by MDA accumulation in stomach medium at pH 3.0, 37 °C, t=90 min.
3.5. The rPOSI of beverages
Red-wine and black Turkish-coffee (roast-ground) were the beverages with the highest antioxidant activity with an IC100/200 g of 168 and 167 ml and rPOSI of 60 and 59, respectively. The amount of red-wine and black Turkish-coffee consumed during a meal is between this range (150–200 ml), was found by us to prevent lipid peroxidation of red-meat in 3 human studies [8], [21], [33]. The technology of preparing coffee products affects the antioxidant properties of the beverage. For example rPOSI for black Turkish-coffee drink is ~4.0 fold higher than the drink from freeze-dried coffee. Black-tea was found to have rPOSI almost 1.5 fold higher than the green-tea and cacao, (Table 5).
3.6. rPOSI of various foods
Some fruits after harvesting are very astringent on taste and hard on texture, they need a period of ripening. Such fruits include Persimmon, Date, Pear, Guava, Avocado, Mango and many more. We evaluated the effect of ripening on fruits such Persimmon and Dates and found a great decrease upon ripening in rPOSI, from 22 to 2 and 46 to 13, respectively. Fresh onion (yellow) after cutting to small pieces and blanched has rPOSI of 25, however if the cut pieces are not blanched and stored at room for 60 min, the rPOSI drops to 0. Sage and Rosemary fresh leaves show the highest rPOSI of 1785 and 1388, respectively. These leaves are known to contain high concentration of polyphenols an amount of 5–7 g could prevent totally lipid peroxidation of 200 g meat in stomach conditions, (results not-shown).
3.7. The use of rPOSI to estimate the impact of food combination to predict inhibition of MDA formation from 200 g meat
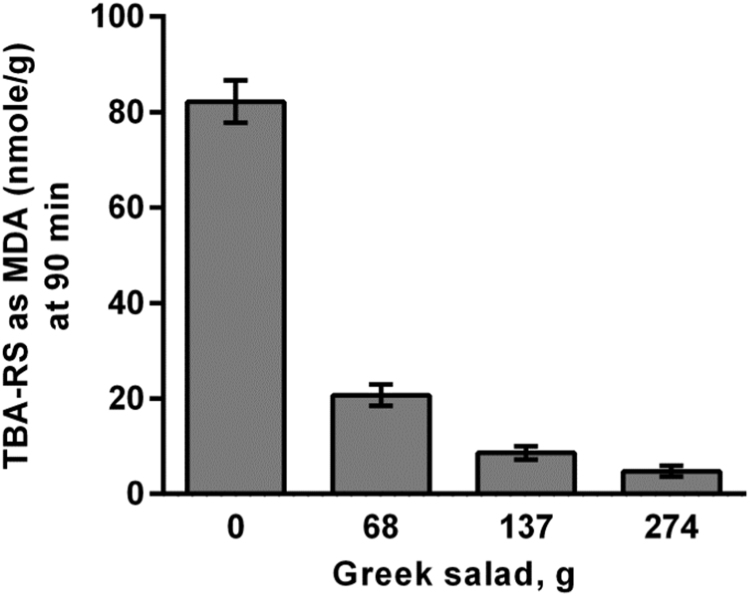
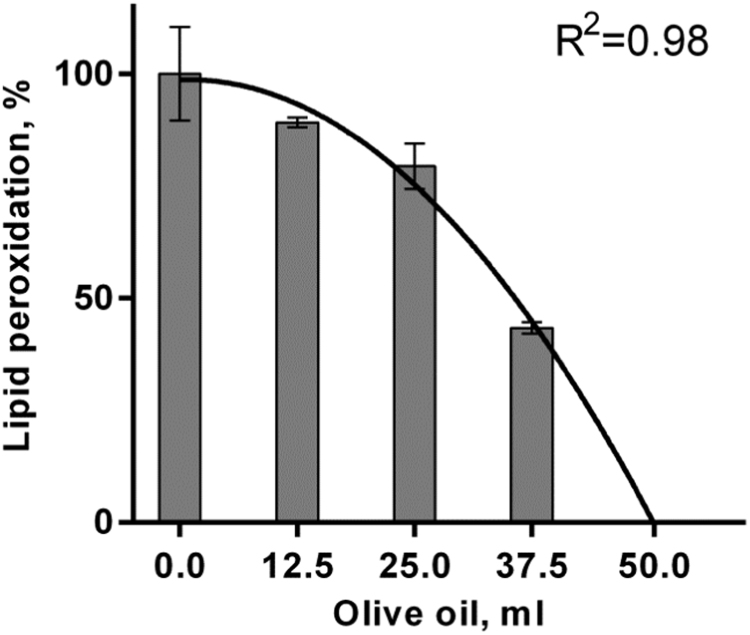
The calculation of rPOSI for a traditional Greek salad is presented in Table 6. The salad contains 100 g of each; tomato, cucumber, red-pepper, cabbage (green), onion (purple) and olives (25 g) in total 525 g with calculated rPOSI 192. Thus a homogenate of Greek salad weighing 274 g have rPOSI of 100, is high enough to eliminate ePOS of 100, or to inhibit by 100% lipid peroxidation of 200 g meat, in stomach condition. Data in Fig. 3 show that MDA production from 200 g meat incubated in SGF is greatly reduced by including increasing amounts of the salad mixture and is ~ totally inhibited by a mixture weighing 274 g as predicted. Dose response curve of the inhibition by olive oil of Turkey red-meat lipid peroxidation is presented in Fig. 4.
Fig. 3.
Inhibition by Mediterranean Greek salad of Turkey red-meat lipid peroxidation, determined by MDA accumulation in stomach medium at pH 3.0, 37 °C, t=90 min.
Fig. 4.
Dose response curve of the inhibition by olive oil of Turkey red-meat lipid peroxidation incubated in SGF, at pH 3.0, 37 °C, t=180 min.
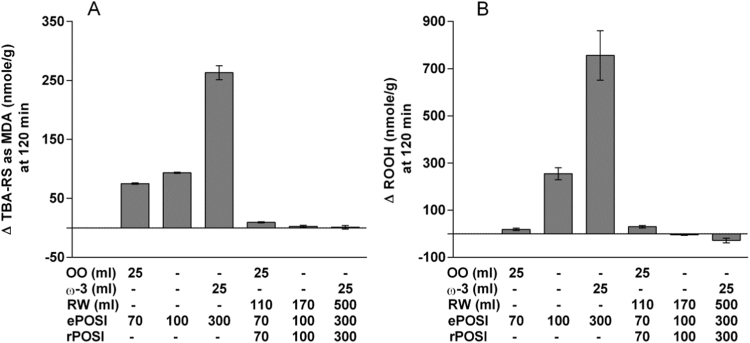
Another example is depicted in Fig. 5A and B. Here we calculated the ePOSi and rPOSI of 200 g turkey meat with and without various other additions. These include 25 g olive oil (antioxidant), 25 ml ω–3 oil (prooxidant) and red wine at various volumes (antioxidant). These combinations are listed at the abscissa of these two figures which also include corresponding predicted values of ePOSi and rPOSI. The ordinates in these figures show the actual MDA (5A) and lipid hydroperoxides (5B) formed after incubation in SGF. As shown there is an excellent correlation between predicted rPOSI/ePOSI combination values and actually measured MDA or lipid peroxidation following incubation in SGF. There was a 30% decrease in MDA and lipid peroxidation when olive oil was included, sharp increase in these valued when ω–3 oil (25 ml) was included and abolition of these increases when red wine was included.
Fig. 5.
Turkey red-meat lipid peroxidation, control (ePOSI=100), in the presence of olive oil (25 ml) (ePOSI=70) or ω-3 oil (25 ml) (ePOSI=300) inhibited by different amount of red-wine (predicted for rPOSI=70, rPOSI=100 and rPOSI=300), at stomach medium pH 3.0, 37 °C, t=120 min. A. MDA. B. Hydroperoxides.
4. Discussion
Chronic diseases often affecting Western population such as diabetes, CVD, neurodegenerative diseases, and several kind of cancer are characterized by elevated oxidative stress and inflammation [1], [3], [11]. Postprandial oxidative stress (POS) is a typical consequence of consumption of Western style high fat diet which results in enhanced lipid peroxidation, generation of free radicals, secondary lipid peroxidation products and co-oxidation of vitamins [4]. A considerable contributor of these reactions occurs in the stomach because of the acid milieu and exposure to air oxygen. Previous studies from our group have consistently shown that consumption of turkey meat by human volunteers was associated with rise in malonyldialdehye (MDA) and MDA-LDL in both plasma and urine and these increases were closely corresponded (r2=0.91) to production MDA and lipid peroxides when the same amount of meat was incubated in vitro in simulated gastric fluid (SGF) [33], [34]. Same studies have also shown that MDA production both in vivo and in vitro was attenuated and virtually abolished when the same meat was consumed or incubated with foods (olive oil, coffee, red wine) containing antioxidants [8], [21], [33], [34].
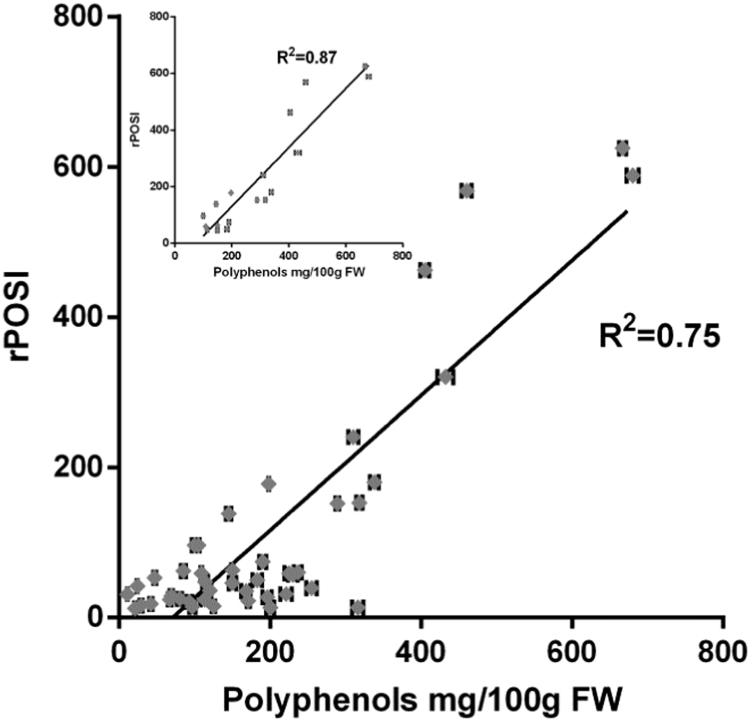
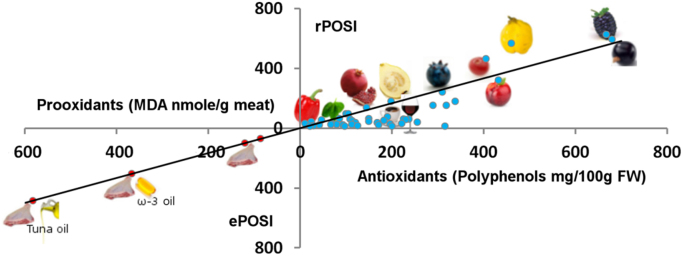
The present study relied on these early studies showing high correlation between in vivo and in vitro productions of MDA and lipid peroxidation to test the capacity of various foods including fruits and vegetables to inhibit these productions under in vitro conditions using incubation of the equivalent of 200 g turkey meat in 1000 ml SGF. In order to compare various foods and allow prediction of multi components meal to inhibit lipid peroxidation we have assigned to each food item the term rPOSI (reductive Postprandial Oxidative Stress Index) which is defined as the capacity in percent of 100 g product to inhibit the production of MDA (or lipid peroxidation) of 200 g turkey incubated for 180 min in SGF. The data obtained that both fruits and vegetables can each be divided into two categories those with high and those with lower rPOSI. It is interesting that fruits with particularly high rPOSI (625-94, Table 2) exhibit strong correlation with their polyphenol content ( Fig. 6, and abstract demonstrated in Fig. 7). This correlation is no longer significant with fruits and vegetables with lower levels of polyphenols. The inhibition of meat-lipid oxidation within lower (50-10) rPOSI is affected less by polyphenols content and more by possible presence of compounds with a mixed action of pro-oxidant and antioxidant effects on lipid peroxidation, catalyzed by iron-ions. Reducing compounds such as ascorbic acid or polyphenols at low concentrations in the presence of iron-ions act in paradox as prooxidant reaction to enhance propagation of lipid peroxidation. However, at high concentrations they react also very efficiently with free radicals to inhibit lipid peroxidation and production of ALEs [33], [40]. Such effects in in-vitro and in-vivo human studies were published recently by us on coffee polyphenols [33]. Red-meat contains relatively high concentration of myoglobin. The involvement of heme proteins such as myoglobin in lipid peroxidation is well described [41], [42]. At foods containing a critical high concentration of redox compounds, (better polyphenols), metmyoglobin acts as a hydroperoxidase (like glutathione peroxidase), breakdown the hydroperoxides mostly to non-toxic lipid hydroxides (LOH) [43]. This effect is synergistic and takes place only if the system contains a high critical concentration of antioxidants or food redox compounds, which prevent lipid peroxidation by 100%. The synergism effect of food compounds such: metMb, catechin, ascorbic acid or red-wine was previously demonstrated [37] and presented in red-meat (Fig. 2). The rPOSI index was calculated to reach this point of synergism with various foods in stomach medium.
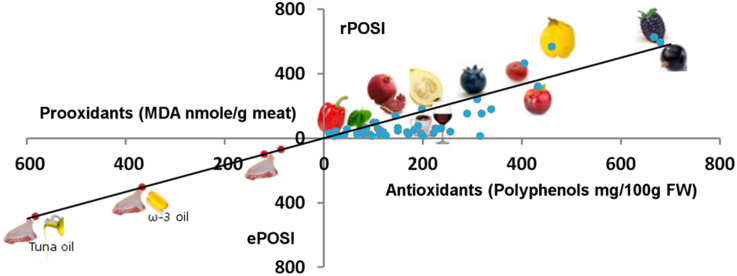
Fig. 6.
Correlation between the concentration of polyphenols in fruits, vegetables and beverages (mg/100 g FW) and rPOSI of 100 g food. In the small figure: correlation between 625 and 46 rPOSI (of 100 g FW) and polyphenols (mg/100 g FW) of fruits.
Fig. 7.
Abstract figure.
We have also assigned the term ePOSI or enhanced postprandial oxidative stress (Table 1) for various meat products in comparison to that of 200 g turkey meat which was assigned a value of 100 or to a combination of turkey meat with anti-(olive oil) and pro-(ω–3 oil) oxidants to account for other modifiers of lipid peroxidation. We applied these indices (r and e POSI) to accurately predict MDA production and lipid peroxidation of combinations of Greek salads or turkey meat consumed with various pro-(ω–3 oil) and anti-(olive oil, red wine). These predictions were corroborated by actual data obtained after incubation with SGF (Fig. 5A, B) which support the idea that this approach could be useful in the design of healthy meals even if they contain Western style ingredients. Based on these data we propose that the recent notion by the PREDIMED group that the health benefit of the Mediterranean diet should be attributed to the high consumption of foods containing polyphenol antioxidants (535–1170 mg/d) [31], [32] is likely to reflect at least in part the antioxidant actions that occur in the stomach. An important aspect of this paradigm that these interactions in the stomach are not linked to the bioavailability of the polyphenols.
Food-derived dALEs and advanced glycation end-products (dAGEs) are absorbed in human as AGE-peptides or AGE- lipids [22]. However, most of the dAGEs in foods are formed in muscle foods by interaction between oxidized lipids and proteins, reactions that gave rise to compounds such carboxy-methyl lysine (CML), and reactive aldehydes such glyoxal, methyl-glyoxal, acrolein, MDA and 4-hydroxy-nonenal (4-HNE) all of them are absorbed from the gut into the blood system [9], [44], [45]. Dietary reactive aldehydes, similar to those which are generated in stomach medium during a meal of red-meat, are important contributors to serum AGEs and ALEs in patients with atherosclerosis, diabetes, chronic kidney diseases (CKD), dementia and metabolic syndrome [9]. Clinical studies in patients with diabetes, CKD, showed that reducing dietary AGEs may lower oxidative stress and inflammation, restore AGE receptor1 (AGER1), sirtuin (SIRT1) levels, and protect against innate immune dysfunction [11]. Vlassara and her group suggested that dietary AGEs restriction is an effective, feasible, and economic method to reduce the level of toxic AGEs-reactive aldehydes and possibly, the associated diabetic, kidney and cardiovascular mortality [9], [46]. We suggest that our method to calculate POS Index of foods in diets will help to prevent generation of toxic reactive aldehydes and other toxic compounds in the gastrointestinal tract and act similar to dietary AGEs restriction. The POSI will help turning meat in general, including red-meat, into a high biological-value proteins and important source of micronutrients such as iron, zinc, B-vitamins and other vitamins which their co-oxidation in the stomach will be prevented [4], [37]. Ursini and co-authors [47] suggested that scavenging of free radicals by antioxidants for a beneficial health effect, in our nutrition, is only a wishful thinking. They propose that the most important effect of polyphenols and other phytochemicals in nutrition is the activation of the signaling factor Nrf2, which preserves the nucleophilic tone of the cells/organism, a very important effect described by many more authors. We consider also that the stomach is an important organ in our body which acts as a bio-reactor generating with some foods and especially muscle-foods free radicals and toxic compounds, inducing pathologic pathways in human metabolism. These pathologic pathways could be prevented during the meal by food antioxidants and mostly by polyphenols. Many large scale human intervention studies on the effect of dietary antioxidant supplements did not demonstrate preventative or therapeutic effects of the antioxidants [48]. We suggest that these null effects of the antioxidants were achieved mostly because of inappropriate in timing and in dosage supplementation. We believe that the lipid peroxidation inhibitory properties of polyphenols and other redox compounds in the stomach medium, during the meal, play a key role in the health benefit of the Mediterranean diet. Keeping the redox homeostasis in stomach medium by foods, during the meal, seems to be the golden mean of healthy living. On an equal caloric basis and a right timing and dosage consumption the POS Index should help to turn the Western diet pattern to a Mediterranean one for better balancing nutrition and human health.
Acknowledgments
We thank very much Dr Peri for the editing of this manuscript.
References
- 1.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A., Genovese S. Atherogenicity of postprandial hyperglycemia and lipotoxicity. Rev. Endocr. Metab. Disord. 2016;17:111–116. doi: 10.1007/s11154-016-9341-8. [DOI] [PubMed] [Google Scholar]
- 3.Kanner J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007;51:1094–1101. doi: 10.1002/mnfr.200600303. [DOI] [PubMed] [Google Scholar]
- 4.Kanner J., Gorelik S., Roman S., Kohen R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: the stomach as a bioreactor. J. Agric. Food Chem. 2012;60:8790–8796. doi: 10.1021/jf300193g. [DOI] [PubMed] [Google Scholar]
- 5.Draper H.H., Polensek L., Hadley M., McGirr L.G. Urinary malondialdehyde as an indicator of lipid peroxidation in the diet and in the tissues. Lipids. 1984;19:836–843. doi: 10.1007/BF02534512. [DOI] [PubMed] [Google Scholar]
- 6.Suomela J.-P., Ahotupa M., Kallio H. Triacylglycerol oxidation in pig lipoproteins after a diet rich in oxidized sunflower seed oil. Lipids. 2005;40:437–444. doi: 10.1007/s11745-005-1402-4. [DOI] [PubMed] [Google Scholar]
- 7.Grootveld M., Atherton M.D., Sheerin A.N., Hawkes J., Blake D.R., Richens T.E., Silwood C.J., Lynch E., Claxson A.W. In vivo absorption, metabolism, and urinary excretion of alpha, beta-unsaturated aldehydes in experimental animals. relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J. Clin. Investig. 1998;101:1210–1218. doi: 10.1172/JCI1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorelik S., Ligumsky M., Kohen R., Kanner J. A novel function of red wine polyphenols in humans: prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008;22:41–46. doi: 10.1096/fj.07-9041com. [DOI] [PubMed] [Google Scholar]
- 9.Uribarri J., del Castillo M.D., de la Maza M.P., Filip R., Gugliucci A., Luevano-Contreras C., Macias-Cervantes M.H., Markowicz Bastos D.H., Medrano A., Menini T., Portero-Otin M., Rojas A., Sampaio G.R., Wrobel K., Wrobel K., Garay-Sevilla M.E. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015;6:461–473. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Turco S., Basta G. An update on advanced glycation endproducts and atherosclerosis. BioFactors. 2012;38:266–274. doi: 10.1002/biof.1018. [DOI] [PubMed] [Google Scholar]
- 11.Stinghen A.E.M., Massy Z.A., Vlassara H., Striker G.E., Boullier A. Uremic toxicity of advanced glycation end products in CKD. J. Am. Soc. Nephrol. 2016;27:354–370. doi: 10.1681/ASN.2014101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita H., Tanaka K., Tanaka T., Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7:11018–11032. doi: 10.18632/oncotarget.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X., Yin H. Covalent modification of DNA by alpha, beta-unsaturated aldehydes derived from lipid peroxidation: recent progress and challenges. Free Radic. Res. 2015;49:905–917. doi: 10.3109/10715762.2015.1040009. [DOI] [PubMed] [Google Scholar]
- 14.Uchida K. Aldehyde adducts generated during lipid peroxidation modification of proteins. Free Radic. Res. 2015;49:896–904. doi: 10.3109/10715762.2015.1036052. [DOI] [PubMed] [Google Scholar]
- 15.Rabbani N., Thornalley P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015;458:221–226. doi: 10.1016/j.bbrc.2015.01.140. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg D., Parthasarathy S., Carew T.E., Khoo J.C., Witztum J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 17.Amaki T., Suzuki T., Nakamura F., Hayashi D., Imai Y., Morita H., Fukino K., Nojiri T., Kitano S., Hibi N., Yamazaki T., Nagai R. Circulating malondialdehyde modified LDL is a biochemical risk marker for coronary artery disease. Heart (Br. Card. Soc.) 2004;90:1211–1213. doi: 10.1136/hrt.2003.018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito T., Fujita H., Tani T., Ohte N. Malondialdehyde-modified low-density lipoprotein is a predictor of cardiac events in patients with stable angina on lipid-lowering therapy after percutaneous coronary intervention using drug-eluting stent. Atherosclerosis. 2015;239:311–317. doi: 10.1016/j.atherosclerosis.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Kadiiska M.B., Gladen B.C., Baird D.D., Germolec D., Graham L.B., Parker C.E., Nyska A., Wachsman J.T., Ames B.N., Basu S., Brot N., Fitzgerald G.A., Floyd R.A., George M., Heinecke J.W., Hatch G.E., Hensley K., Lawson J.A., Marnett L.J., Morrow J.D., Murray D.M., Plastaras J., Roberts L.J., 2nd, Rokach J., Shigenaga M.K., Sohal R.S., Sun J., Tice R.R., Van Thiel D.H., Wellner D., Walter P.B., Tomer K.B., Mason R.P., Barrett J.C. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Gorelik S., Ligumsky M., Kohen R., Kanner J. The stomach as a "bioreactor": when red meat meets red wine. J. Agric. Food Chem. 2008;56:5002–5007. doi: 10.1021/jf703700d. [DOI] [PubMed] [Google Scholar]
- 21.Gorelik S., Kanner J., Schurr D., Kohen R. A rational approach to prevent postprandial modification of LDL by dietary polyphenols. J. Funct. Foods. 2013;5:163–169. [Google Scholar]
- 22.Koschinsky T., He C.J., Mitsuhashi T., Bucala R., Liu C., Buenting C., Heitmann K., Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankel E.N., Kanner J., German J.B., Parks E., Kinsella J.E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 24.Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc. Res. 2007;73:341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Croft K.D. Dietary polyphenols: antioxidants or not? Arch. Biochem. Biophys. 2016;595:120–124. doi: 10.1016/j.abb.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Hollman P.C., Cassidy A., Comte B., Heinonen M., Richelle M., Richling E., Serafini M., Scalbert A., Sies H., Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011;141:989S–1009S. doi: 10.3945/jn.110.131490. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Henning S.M., Zhang Y., Rahnama N., Zerlin A., Thames G., Tseng C.H., Heber D. Decrease of postprandial endothelial dysfunction by spice mix added to high-fat hamburger meat in men with Type2 diabetes mellitus. Diabet. Med. 2013;30:590–595. doi: 10.1111/dme.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urquiaga I., Avila F., Echeverria G., Perez D., Trejo S., Leighton F. A Chilean berry concentrate protects against postprandial oxidative stress and increases plasma antioxidant activity in healthy humans. Oxid. Med. Cell Long. 2017;2017:8361493. doi: 10.1155/2017/8361493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gobert M., Remond D., Loonis M., Buffiere C., Sante-Lhoutellier V., Dufour C. Fruits, vegetables and their polyphenols protect dietary lipids from oxidation during gastric digestion. Food Funct. 2014;5:2166–2174. doi: 10.1039/c4fo00269e. [DOI] [PubMed] [Google Scholar]
- 31.Tresserra-Rimbau A., Rimm E.B., Medina-Remon A., Martinez-Gonzalez M.A., de la Torre R., Corella D., Salas-Salvado J., Gomez-Gracia E., Lapetra J., Aros F., Fiol M., Ros E., Serra-Majem L., Pinto X., Saez G.T., Basora J., Sorli J.V., Martinez J.A., Vinyoles E., Ruiz-Gutierrez V., Estruch R., Lamuela-Raventos R.M., Investigators P.S. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014;24:639–647. doi: 10.1016/j.numecd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Tresserra-Rimbau A., Rimm E.B., Medina-Remon A., Martinez-Gonzalez M.A., Lopez-Sabater M.C., Covas M.I., Corella D., Salas-Salvado J., Gomez-Gracia E., Lapetra J., Aros F., Fiol M., Ros E., Serra-Majem L., Pinto X., Munoz M.A., Gea A., Ruiz-Gutierrez V., Estruch R., Lamuela-Raventos R.M., Investigators P.S. Polyphenol intake and mortality risk: a re-analysis of the PREDIMED trial. BMC Med. 2014;12:77. doi: 10.1186/1741-7015-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirota R., Gorelik S., Harris R., Kohen R., Kanner J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol. Nutr. Food Res. 2013;57:916–919. doi: 10.1002/mnfr.201200557. [DOI] [PubMed] [Google Scholar]
- 34.Tirosh O., Shpaizer A., Kanner J. Lipid peroxidation in a stomach medium is affected by dietary oils (olive/fish) and antioxidants: the Mediterranean versus Western Diet. J. Agric. Food Chem. 2015;63:7016–7023. doi: 10.1021/acs.jafc.5b02149. [DOI] [PubMed] [Google Scholar]
- 35.Kanner J., Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic. Biol. Med. 2001;31:1388–1395. doi: 10.1016/s0891-5849(01)00718-3. [DOI] [PubMed] [Google Scholar]
- 36.Kanner J., Harel S. Initiation of membranal lipid-peroxidation by activated metmyoglobin and methemoglobin. Arch. Biochem. Biophys. 1985;237:314–321. doi: 10.1016/0003-9861(85)90282-6. [DOI] [PubMed] [Google Scholar]
- 37.Gorelik S., Lapidot T., Shaham I., Granit R., Ligumsky M., Kohen R., Kanner J. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: health implications. J. Agric. Food Chem. 2005;53:3397–3402. doi: 10.1021/jf040401o. [DOI] [PubMed] [Google Scholar]
- 38.Kanner J., Elmaleh H., Reuveni O., Bengera I. Invertase (Beta-Fructofuranosidase) Activity in 3 Date Cultivars. J. Agric. Food Chem. 1978;26:1238–1240. [Google Scholar]
- 39.Kanner J., Harel S., Mendel H. Content and stability of Alpha-tocopherol in fresh and dehydrated pepper fruits (Capsicum-Annuum-L) J. Agric. Food Chem. 1979;27:1316–1318. doi: 10.1021/jf60226a057. [DOI] [PubMed] [Google Scholar]
- 40.Lapidot T., Granit R., Kanner J. Lipid peroxidation by "free" iron ions and myoglobin as affected by dietary antioxidants in simulated gastric fluids. J. Agric. Food Chem. 2005;53:3383–3390. doi: 10.1021/jf040402g. [DOI] [PubMed] [Google Scholar]
- 41.Kanner J., German J.B., Kinsella J.E. Initiation of lipid peroxidation in biological systems. Crit. Rev. Food Sci. Nutr. 1987;25:317–364. doi: 10.1080/10408398709527457. [DOI] [PubMed] [Google Scholar]
- 42.Kanner J. Oxidative processes in meat and meat-products - quality implications. Meat Sci. 1994;36:169–189. doi: 10.1016/0309-1740(94)90040-X. [DOI] [PubMed] [Google Scholar]
- 43.Lapidot T., Granit R., Kanner J. Lipid hydroperoxidase activity of myoglobin and phenolic antioxidants in simulated gastric fluid. J. Agric. Food Chem. 2005;53:3391–3396. doi: 10.1021/jf040400w. [DOI] [PubMed] [Google Scholar]
- 44.Hull G.L.J., Woodside J.V., Ames J.M., Cuskelly G.J. N-epsilon-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012;131:170–174. [Google Scholar]
- 45.Fu M.X., Requena J.R., Jenkins A.J., Lyons T.J., Baynes J.W., Thorpe S.R. The advanced glycation end product, N-(epsilon)(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 46.Uribarri J., Cai W.J., Ramdas M., Goodman S., Pyzik R., Chen X., Zhu L., Striker G.E., Vlassara H. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes potential role of AGER1 and SIRT1. Diabetes Care. 2011;34:1610–1616. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ursini F., Maiorino M., Forman H.J. Redox homeostasis: the golden mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halliwell B. The antioxidant paradox: less paradoxical now? Br. J. Clin. Pharmacol. 2013;75:637–644. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]