Abstract
Acute oxygen (O2) sensing is essential for individuals to survive under hypoxic conditions. The carotid body (CB) is the main peripheral chemoreceptor, which contains excitable and O2-sensitive glomus cells with O2-regulated ion channels. Upon exposure to acute hypoxia, inhibition of K+ channels is the signal that triggers cell depolarization, transmitter release and activation of sensory fibers that stimulate the brainstem respiratory center to produce hyperventilation. The molecular mechanisms underlying O2 sensing by glomus cells have, however, remained elusive. Here we discuss recent data demonstrating that ablation of mitochondrial Ndufs2 gene selectively abolishes sensitivity of glomus cells to hypoxia, maintaining responsiveness to hypercapnia or hypoglycemia. These data suggest that reactive oxygen species and NADH generated in mitochondrial complex I during hypoxia are signaling molecules that modulate membrane K+ channels. We propose that the structural substrates for acute O2 sensing in CB glomus cells are “O2-sensing microdomains” formed by mitochondria and neighboring K+ channels in the plasma membrane.
Abbreviations: AM, adrenal medulla; CB, carotid body; ETC, electron transport chain; H2O2, hydrogen peroxide; MCI, mitochondrial complex I; MCII, mitochondrial complex II; MCIII, mitochondrial complex III; MCIV, Mitochondrial complex IV; MPP+, 1-methyl-4-phenylpyridinium; O2, oxygen; PO2, oxygen tension; QH2, ubiquinol; ROS, reactive oxygen species; TH, tyrosine hydroxylase
Keywords: Hypoxia, Acute oxygen sensing, Peripheral chemoreceptors, Carotid body, Adrenal medulla, Mitochondrial complex I, Reactive oxygen species (ROS), Pyridine nucleotides
Graphical abstract
Highlights
-
•
Acute O2 sensing by peripheral chemoreceptors depends on K+ channels.
-
•
Mitochondrial complex I function is required for acute O2 sensing.
-
•
Reactive oxygen species inhibits background K+ channels during acute hypoxia.
-
•
Pyridine nucleotides may signal voltage-gated K+ channels during acute hypoxia.
1. Introduction
Oxygen (O2) is necessary for oxidative phosphorylation, the major source of energy for the cells; therefore, the provision of sufficient O2 to the tissues is a fundamental physiological challenge. Deficiency of O2 (hypoxia), even if only transient, can have detrimental effects and critically contribute to the pathogenesis of severe and highly prevalent diseases in the human population. Adaptive responses, which can be acute or chronic, have evolved to minimize the effect of hypoxia on cells. During chronic (sustained) hypoxia, the hypoxia inducible factor-proline hydroxylase pathway is activated to regulate the transcription of numerous “O2-sensitive” genes. As a result of this process, in the time course of hours or days cell aerobic metabolism is switched to non-aerobic, to decrease cellular needs of O2, and the number of red blood cells and blood vessels are increased to improve O2 transport and distribution to the tissues [54], [57]. In mammals, hypoxia also triggers fast (in seconds) life-saving cardiorespiratory reflexes (hyperventilation and sympathetic activation) (Fig. 1A) to increase gas exchange in the lungs and rapid delivery of O2 to critical organs, such as the brain and heart. These acute responses to hypoxia are mediated by the carotid body (CB), the main arterial chemoreceptor, which is strategically located in the carotid bifurcation (Fig. 1B) [35]. In addition to the CB, there are other peripheral chemoreceptor tissues/organs acutely sensing hypoxia (e.g. pulmonary arteries, ductus arteriosus, adrenal medulla (AM) or neuroepithelial bodies in the lung), which, together, constitute a “homeostatic acute O2-sensing system” of fundamental biological and medical relevance (see [71]).
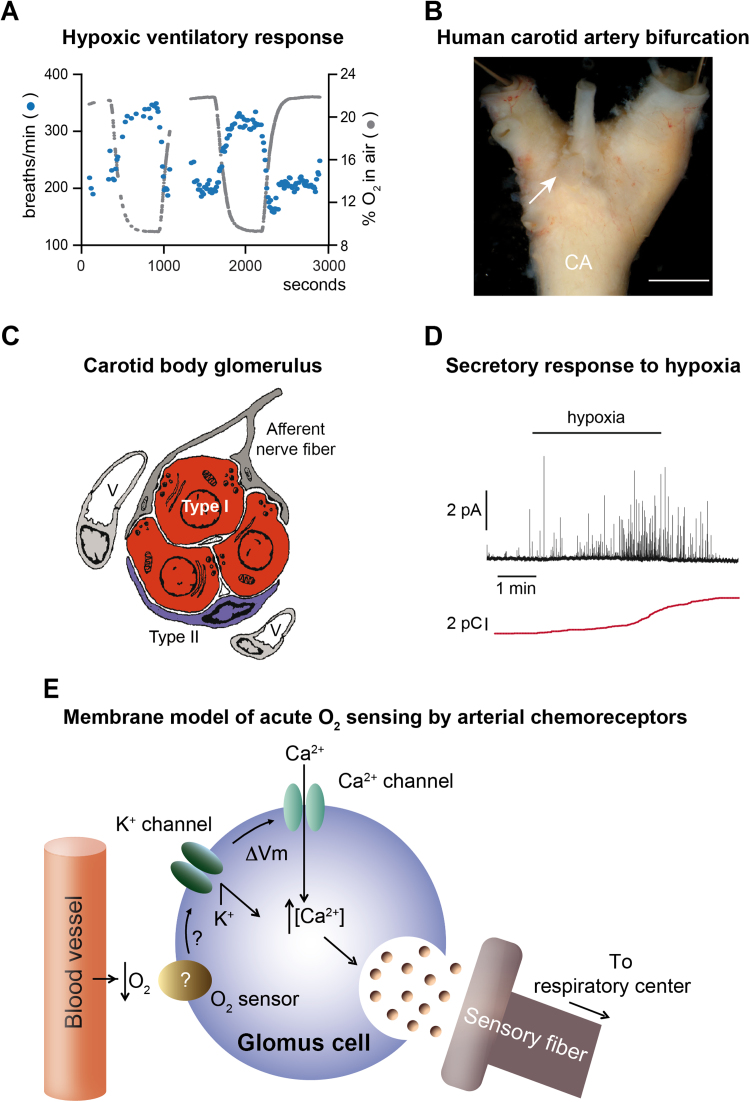
Fig. 1.
Membrane model of acute O2 sensing by arterial chemoreceptor cells. A. Plethysmographic recording of hypoxic ventilatory response from a wildtype mouse. Note the increase in breathing frequency (breaths/min) as the % O2 in air decreases. B. Photograph of the human carotid artery (CA) bifurcation after cleaning the surrounding fat and connective tissue. The arrow indicates the carotid body (scale bar: 1cm). C. Carotid body glomerulus with indication of glomus (type I) cells and sustentacular (type II) cells in contact with capillaries and afferent sensory fibers. V. vessel. D. Amperometric recording of the secretory response to hypoxia from carotid body glomus cells of a wildtype mouse. Each individual spike-like signal indicates a quantum of dopamine released from a secretory granule (pA, picoAmperes). The red color trace, in picoCoulombs (pC), represents the cumulative secretion signal (time integral of the recordings show at the top). E. Membrane model of acute O2 sensing by arterial chemoreceptor cells. See text for details. (Modified from [14], [34], [46]).
2. Membrane model of CB oxygen sensing
The CB is composed of clusters of cells (glomeruli), which contain neural crest-derived O2-sensitive glomus cells (also called type I cells) in close contact with capillaries and afferent sensory nerve fibers (Fig. 1C) (see for a review [37]). Glomus cells are presynaptic-like elements containing neurosecretory vesicles filled with neurotransmitters (ATP, acetylcholine and dopamine, among others) that are released in response to hypoxia to activate the afferent fibers connected with the brainstem respiratory center. The CB is the main responsible for the acute hypoxic ventilatory response (HVR) (Fig. 1A), as this reflex is practically abolished after CB resection [63] or in animal models with CB atrophy (decrease in size and cell number) [39]. At the cellular level, the HVR is represented by the secretory response to hypoxia characteristic of single glomus cells, which can be monitored by amperometry (Fig. 1D). Genetic or pharmacological alterations that result in elimination of glomus cell responsiveness to hypoxia also abolish the HVR ([14]; see below).
CB glomus cells are excitable cells that contain a variety of voltage-gated and background ion channels (see for review [37]). It has been more than two decades since a “membrane model” of glomus cell chemotransduction was established (see [36], [49], [60]). The model is based on the existence in glomus cells of K+ channels reversibly inhibited by the decrease in oxygen tension (PO2), resulting in cell depolarization (see for a recent review [38]). This leads to an influx of extracellular Ca2+, which triggers the release of neurotransmitters from secretory vesicles in order to activate afferent sensory nerve fibers [5], [66]. As a consequence, the respiratory center in the brainstem is activated leading to hyperventilation (Fig. 1E). In addition to hypoxia, CB glomus cells are activated by other chemosensory stimuli, including hypercapnia, decrease in extracellular pH, and hypoglycemia [16], [19], [47], [75]. O2-sensitive ion channels have also been reported in other organs of the “homeostatic acute O2-sensing system”, such as the AM [26], [32], [42], [56], [61], resistance pulmonary arteries [52], [74], neuroepithelial bodies in the lung airway [43], [73], or the ductus arteriosus [40].
Although the “membrane model” explains the basic sensory function of the CB, the underlying molecular mechanisms have remained poorly understood and a subject of debate [30], [37], [38], [50]. This partially results from the small tissue size of the CB, which makes it technically difficult to study, and the fact that the response to hypoxia can be easily lost in apparently healthy CB glomus cells. Although several hypotheses have been proposed to explain how changes in blood PO2 lead to modulation of K+ channel function (mitochondrial energy metabolism, redox signaling, gas transmitters, etc.), none of them has gained broad acceptance due to the lack of consensus among the workers in the field (see for updated reviews [37], [38]). Recently, production of reactive oxygen species (ROS) by mitochondrial complex I (MCI) has been reported to play a critical role in CB acute O2 sensing [14]. In the next section, we discuss the role of MCI signaling in acute O2 sensing, with special attention to the roles of ROS and NADH in the signaling pathway. Although we focus on the CB, many of the concepts and processes discussed may be also applicable to cells in other organs acutely responding to hypoxia.
3. Mitochondria complex I signaling during hypoxia is mediated by ROS and pyridine nucleotides
Mitochondria have long been considered a potential candidate in the signaling pathway of O2 sensing due to the high O2 consumption of the CB [10], [59] and the fact that this organ is highly sensitive to mitochondrial poisons [44], [72]. Similar to hypoxia (see Fig. 1E), glomus cell activation by mitochondrial inhibitors requires extracellular Ca2+ influx [44]; hence hypoxic mitochondria could generate signaling molecules to regulate the function of K+ channels in the plasma membrane. Indeed, it has been proposed that a decrease in cytosolic ATP concentration in glomus cells during hypoxia can modulate membrane K+ channels [67], [72]. Mitochondrial complex IV (MCIV) is the natural O2-dependent site in the electron transport chain (ETC). Therefore, changes in MCIV cytochrome c oxidase activity or its affinity to O2 could also affect the sensibility to hypoxia. Although a low-affinity cytochrome c oxidase has been suggested to exist in the CB, this hypothesis has not been proven experimentally (see below) [13], [41], [6].
Recent interest on the role of mitochondria in acute O2-sensing is based on studies performed using blockers of mitochondrial ETC [19,44]. It was shown that the response to acute hypoxia in rat CB glomus cells is efficiently blocked by MCI inhibitors, such as rotenone or 1-methyl-4-phenylpyridinium (MPP+), acting on the quinone binding site. In contrast, MCI proximal inhibitors (e.g. diphenyliodonium), acting outside the distal ubiquinone-binding site, appeared to have much less effect [44]. Interestingly, mitochondrial inhibitors that abolish responsiveness to hypoxia do not alter sensitivity to hypoglycemia in glomus cells [19]. In agreement with the data obtained in glomus cells, it has been shown that functional mitochondria are required for O2 sensing in immortalized AM chromaffin cells [7]. In addition, the inhibition of sensitivity to hypoxia by rotenone was also observed in ovine [27] and rat [62] chromaffin cells. These findings led to the hypothesis that a rotenone-binding molecule is involved in acute O2 sensing by peripheral chemoreceptors.
Rotenone is known to block MCI by preventing ubiquinone binding to a site that is highly conserved from bacteria to mammals [15], [4], [77], [78]. To test the involvement of MCI in acute O2-sensing, we generated mice with conditional ablation of the Ndufs2 gene. This nuclear gene encodes a 49 kDa protein that contributes to the ubiquinone-binding site, located at the junction between the peripheral and transmembrane arms of MCI near the most distal Fe/S cluster (N2 site) from where electrons are transferred to ubiquinone [15], [25], [4], [77], [78]. In our initial experiments, the Ndufs2 gene was deleted in tyrosine hydroxylase (TH)-positive catecholaminergic cells, such as O2-sensitive CB glomus cells and AM chromaffin cells (TH-NDUFS2 mice; [14]). TH-NDUFS2 mice show a complete abolition of the HVR while maintaining normal responsiveness to hypercapnia (Fig. 2A). In fair correspondence with this observation at the whole animal level, hypoxia-induced catecholamine secretion is also abolished in single Ndufs2-deficient glomus cells, although they show normal responses to hypercapnia (Fig. 2B) and hypoglycemia [14]. The selective loss of responsiveness to hypoxia is also observed in ESR-NDUFS2 mice, in which the floxed Ndufs2 alleles are deleted after the administration of tamoxifen, once the mice have reached adulthood [14]. In addition, AMs from Ndufs2-deficient mice (TH-NDUFS2 and ESR-NDUFS2 models) also fail to respond to hypoxia. Interestingly, CB glomus cells in Ndufs2-null mice appear in normal number and have normal morphology, ATP levels, and density of voltage-dependent ion channels [14]. This suggests that Ndufs2-deficient glomus cells, without MCI function, rely on succinate-dependent mitochondrial complex II (MCII) activity for oxidative phosphorylation. In agreement with this finding, abundant glomus cell loss has been observed in MCII-deficient mice [11], [51].
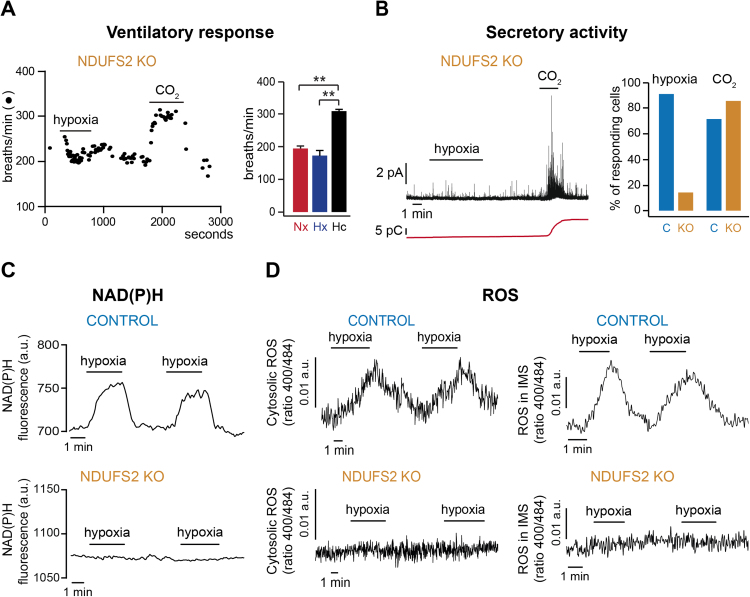
Fig. 2.
Loss of mitochondrial complex I (MCI) signaling during hypoxia in Ndufs2-deficient mice. A. Plethysmographic recording (left) and quantification (right) showing selective loss of hypoxic ventilatory response in Ndufs2 knockout mice. Responsiveness to hypercapnia (Hc, CO2) remained unaltered. Nx, normoxia; Hx, hypoxia. B. Amperometric recording (left) and quantification (right) demonstrating selective loss of secretory response to hypoxia in Ndufs2-deficient glomus cells (pA, picoAmperes; pC, picoCoulombs). C, control; KO, knockout. C. Changes in NAD(P)H auto-fluorescence during hypoxia in control and Ndufs2-deficient carotid body glomus cells. D. Changes in ROS levels in the cytosol (left) and intermembrane mitochondrial space (IMS, right) during hypoxia in control and Ndufs2-deficient glomus cells infected with redox-sensitive fluorescent probes (roGFP). a.u., arbitrary units; **, p<0.01. (Modified from [14]).
MCI catalyzes the oxidation of NADH to NAD+ and the reduction of ubiquinone to ubiquinol (QH2). Therefore, pyridine nucleotides could be potential mitochondria signaling molecules to modulate membrane ion channels. This hypothesis is supported by early reports showing that the level of pyridine nucleotides (NADH and NADPH) in glomus cells, as determined by microfluorimetry, is reversibly increased during hypoxia [13], [6]. In addition, we have shown that increases in NAD(P)H induced by hypoxia are abolished in Ndufs2-deficient glomus cells (Fig. 2C).
Rotenone mimics hypoxia, as it induces external Ca2+-dependent secretion from glomus cells, and is also known to increase ROS production in MCI [44,65]. Therefore, mitochondria ROS production during hypoxia could also play a signaling role. It is long ago that a mitochondrial redox-based sensor was proposed to mediate hypoxic pulmonary artery vasoconstriction [2], [31], [69], although whether cytosolic ROS increases or decreases during hypoxia in pulmonary artery myocytes and other cell types has been a matter of controversy [20], [23], [70], [71]. In the pulmonary artery, the main source of ROS generated during hypoxia has been suggested to be mitochondrial complex III (MCIII) [70]. On the other hand, non-mitochondrial ROS generated in NADPH oxidases have been reported to participate in acute O2 sensing in several tissues [17], [33], [9]. In the CB setting, however, this proposal has not received strong experimental support [22]. In addition, ROS have traditionally been considered not involved in CB chemotransduction [1], [72]. ROS are promiscuous agents and their determination in small cells is a long-standing methodological challenge. In recent years, a ratiometric redox probe, based on a genetically encoded green fluorescent protein (roGFP), has been developed [12], [21], [55]. Using roGFP targeted to either the cytosol or mitochondria intermembrane space we have been able to detect robust reversible increases in ROS in CB glomus cells exposed to hypoxia, which are absent, or drastically reduced in amplitude, in Ndufs2-deficient cells (Fig. 2D). Taken together, these results demonstrate that in Ndufs2-null mice the response to hypoxia is specifically lost and MCI is essential for acute O2 sensing by peripheral chemoreceptors.
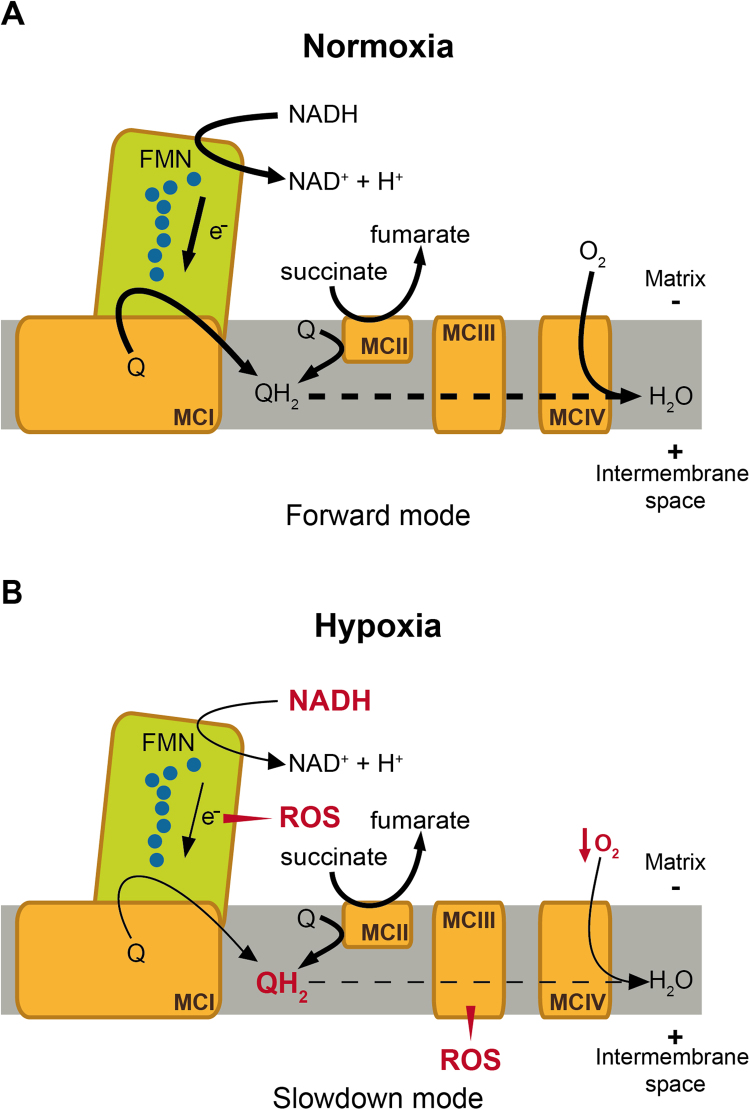
Based on the data obtained from Ndufs2-null mice, we have proposed a mechanistic model of O2 sensing in arterial chemoreceptor, which relies on the generation of MCI signaling molecules (ROS and NADH) during hypoxia capable of modulating membrane ion channels (see [14], [38]). We suggest the existence in glomus cell mitochondria of a special form of MCIV whose enzymatic activity is decreased during hypoxia. This would slow down ETC and lead to accumulation of reduced quinone (QH2), which in turn would cause an increase in NADH and in ROS production due to backlog accumulation of electrons in the iron/sulfur clusters of MCI (Fig. 3). When QH2 reaches sufficiently high value, reversal of MCI (a state favored by high succinate-dependent quinone reduction [53], [68], [8] may potentiate the generation of ROS and NADH [14], [38]. Recent data have shown that glomus cells express atypical mitochondrial subunits (Ndufa4l2 and Cox4i2), which could account for the high sensitivity of CB cells to hypoxia ([76]; our unpublished data). Cox4i2 is induced by low PO2 in some cells to optimize O2 consumption during hypoxia [18]. However, the role of Cox4i2 may change in different tissues (see [24]) and in the CB it may be its co-expression with Ndufa4l2, which confers upon MCIV special sensitivity to PO2. Indeed, Ndufa4 (the predominantly expressed isoform of Ndufa4l2) is expressed in close association with MCIV subunits [3,24]. In addition, we have shown that succinate concentration is higher in the normoxic CB than in other neural (central and peripheral) tissues studied [14], thus supporting the continuous production of QH2. This proposal is also compatible with the fact, mentioned before, that glomus cell survival strongly depends on succinate dehydrogenase (MCII) activity [11], [51]. Although the model is compatible with the concomitant production of ROS in MCI and MCIII, the experimental data suggest that in glomus cells ROS produced outside MCI do not efficiently signal the plasma membrane to produce rise of cytosolic Ca2+ or cell secretion. Blockade of glomus cell sensitivity to hypoxia by rotenone is not reverted by incubation of the cells with membrane permeable methyl succinate to favor respiration through the MCII-III-IV pathway [44]. Similarly, although methyl succinate can support respiration (O2 consumption) in cells from TH-NDUFS2 mice, this treatment fails to rescue hypoxia sensitivity in Ndufs2-deficient glomus cells [14].
Fig. 3.
Model of mitochondrial complex I signaling during hypoxia in carotid body glomus cells mediated by ROS and pyridine nucleotides (NADH). See text for description. Q, ubiquinone; QH2, ubiquinol; MCI, MCII, MCIII, MCIV, mitochondrial complex I, II, III, IV, respectively. (Modified from [38]).
4. Intracellular redox regulation of ionic currents in glomus cells
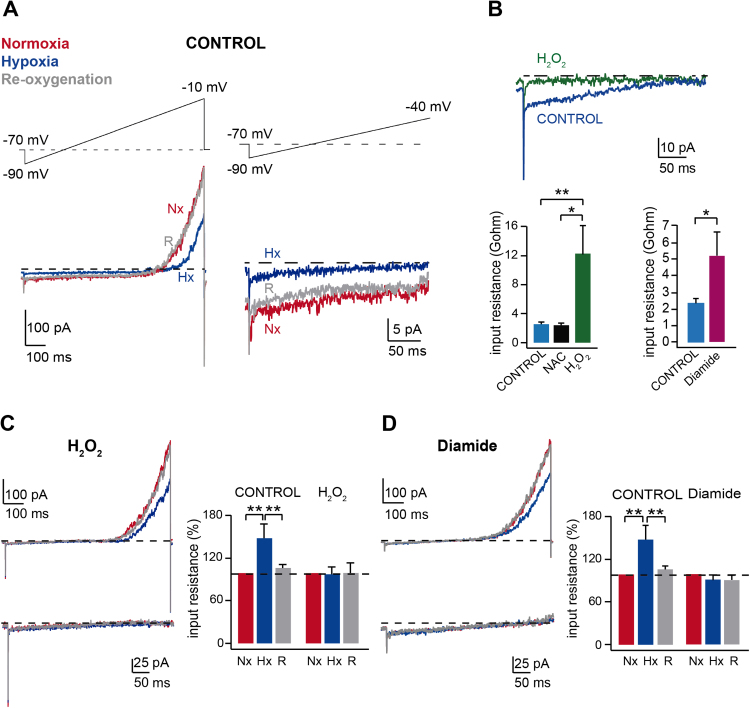
The primary effect of hypoxia in glomus cells is to inhibit membrane K+ channels to produce depolarization. CB glomus cells express a variety of O2-sensitive K+ channels, including voltage-dependent K+ channels, maxi-K channels, and background K+ channels ([45], see for reviews [37], [38]). Therefore, it seems that rather than a specific K+ channel type, hypoxia regulates numerous K+ channels and other ion channels over a broad range of membrane potentials [35], [58]. As shown in Fig. 4A, hypoxia inhibits background channels, causing an increase in input resistance, and voltage-gated K+ channels in CB glomus cells, both effects being reversible upon re-oxygenation. Interestingly, similar to hypoxia, intracellular application of oxidants H2O2 and diamide (a thiol-oxidizing agent) result in an increase in input resistance whereas N-acetylcysteine (a reducing agent) has no effect (Fig. 4B). In the presence of intracellular H2O2 or diamide the inhibitory effect of hypoxia on background channels is blocked, leaving unaltered the modulation of the current mediated by voltage-gated channels (Fig. 4C,D). These results, suggesting that cysteine oxidation inhibits the O2 sensitive K+ current in glomus cells, are compatible with the signaling role of ROS on membrane channels, in particular on background channels, which are the most critical for the regulation of the cell resting potential (see preceding section). Task3 homomers or Task1/Task3 heteromers seem to be the most abundant ion channel subunits contributing to the O2-sensitive background K+ currents in CB glomus cells [28], [64]; although acute responsiveness to hypoxia is maintained in the Task1/Task3 knockout mouse [45]. Interestingly, Task 3 subunits contain several cysteine residues facing the cytosol [29], which is compatible with the effects of sulfhydryl reagents described in Fig. 4. To our knowledge, the modulation of Task 3 channels by internal redox reagents has not been studied in detail, however the closely related TREK2 channels are inhibited by the intracellular application of oxidizing agents [48]. It remains also the possibility that in addition to the pore-forming subunits, accessory subunits modulating ion channel function are susceptible to redox regulation during hypoxia. NADH, the other signaling molecule produced in hypoxic mitochondria, seems to modulate preferentially voltage-dependent K+ channels (see [14]). This concept is, however, preliminary and must be further investigated in future experimental work.
Fig. 4.
Redox regulation of ionic currents in carotid body glomus cells. A. Hypoxia-induced reversible inhibition of background and voltage-dependent K+ currents recorded during depolarizing ramps in patch clamped glomus cells (perforated patch recording). The initial part of the recordings (between −90 and −40 mV) is shown at an expanded amplitude and time base on the right part of the panel. Nx, normoxia, Hx, hypoxia, R, re-oxygenation. B. Top. Inhibition of the background current in glomus cells following intracellular application of H2O2 (15 µM). Bottom. Increase in input resistance (inversely proportional to the slope of the background current) in glomus cells recorded with intracellular solutions containing: H2O2 (15–50 µM), N-acetylcysteine (25 µM), or diamide (50–60 μM). C. and D. Blockage of hypoxia-induced closure of background K+ channels by H2O2 (15 µM) or diamide (50–60 μM). Recordings between −90 and −40 mV are shown at an expanded amplitude and time base *, p<0.05; **, p<0.01. (Modified from [14]).
5. Conclusions
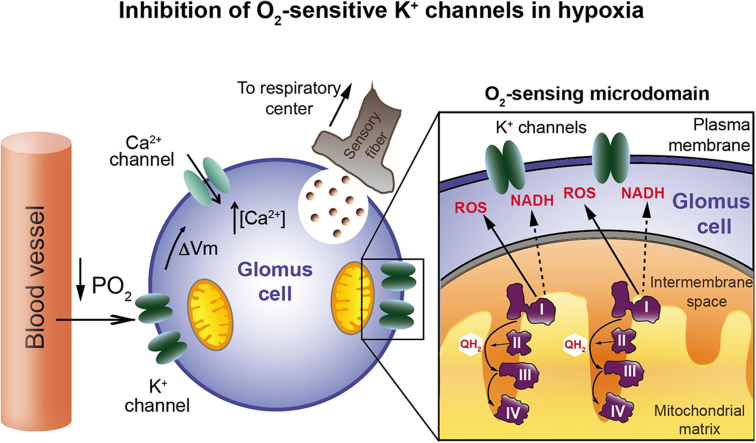
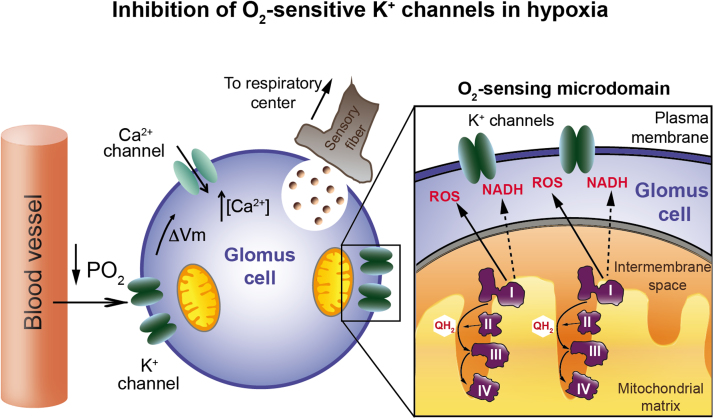
We have proposed a model of acute O2 sensing by peripheral chemoreceptors, which combines the standard membrane model based on the modulation of O2-sensitive K+ channels [36], and the production of signaling molecules (ROS and NADH) in mitochondria [14]. CB glomus cells contain numerous mitochondria located near the plasma membrane [14], [51], therefore it is conceivable that they form with neighboring K+ channels “O2-sensing microdomains” (Fig. 5). However, specialized morphological structures supporting this concept have not yet been described. Glomus cells contain several subtypes of “O2-sensitive” K+ channels, hence it is logical that chemosensory transduction relies on relatively promiscuous signals (such as ROS or NADH) rather than on an O2 sensor molecule selectively associated with a specific K+ channel class (see [45], [14]).
Fig. 5.
Model of mitochondrial complex I-mediated acute O2 sensing by arterial chemoreceptor cells. I, II, III, IV, mitochondrial complex I, II, III, IV, respectively; PO2, oxygen tension; QH2, ubiquinol; ΔVm, increase in membrane potential (depolarization). See text for details (Modified from [14]).
Although fundamental advances have recently been made regarding the mechanism underlying acute O2 sensing by peripheral chemoreceptors, several questions remain to be clarified by further research. In the TH-NDUFS2 mice, failure to form MCI poses an abnormal challenge to cell intermediary metabolism [14]. A mouse model defective in ubiquinone binding without affecting the MCI formation and activity, if available, could critically improve our understanding of the relationship between MCI and O2 sensing. ROS and NADH/NAD+ metabolism, oxidative phosphorylation, and energy production are closely linked in mitochondria. Therefore analytical experimental tools that could separate these processes would be also of great value to discern their specific role in acute O2 sensing. Superoxide and H2O2 react with different molecules and cause distinct molecular modifications. The specific ROS implicated in acute O2 sensing and their direct molecular targets are not known. In this regard, thiol redox proteomics in CB tissue could eventually help to identify the targets of hypoxia-induced ROS. In addition, it remains to be determined whether during hypoxia ROS are generated only in MCI or if MCIII also participates in acute O2 sensing by CB cells, as it seems to do in pulmonary arteries [70]. Finally, it cannot be ruled out the possibility that in CB glomus cells, as well as in other cells of the homeostatic acute O2 sensing system [71], multiple O2-sensing mechanisms work in coordination to ensure a fast response to hypoxia. Advances in these questions will also help us understanding the role of the CB in hypoxia-mediated diseases.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Botín Foundation, Spanish Ministry of Economy, Industry, and Competitiveness (SAF2012-39343; PIE 13/0004), and the European Research Council (PRJ201502629).
Contributor Information
Lin Gao, Email: lgao-ibis@us.es.
José López-Barneo, Email: lbarneo@us.es.
References
- 1.Agapito M.T., Sanz-Alfayate G., Gomez-Nino A., Gonzalez C., Obeso A. General redox environment and carotid body chemoreceptor function. Am. J. Physiol. Cell Physiol. 2009;296:C620–C631. doi: 10.1152/ajpcell.00542.2008. [DOI] [PubMed] [Google Scholar]
- 2.Archer S.L., Huang J., Henry T., Peterson D., Weir E.K. A redox-based O2 sensor in rat pulmonary vasculature. Circ. Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 3.Balsa E., Marco R., Perales-Clemente E., Szklarczyk R., Calvo E., Landazuri M.O., Enriquez J.A. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 2012;16:378–386. doi: 10.1016/j.cmet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Baradaran R., Berrisford J.M., Minhas G.S., Sazanov L.A. Crystal structure of the entire respiratory complex I. Nature. 2013;494:443–448. doi: 10.1038/nature11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckler K.J., Vaughan-Jones R.D. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J. Physiol. 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckler K.J., Turner P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J. Physiol. 2013;591:3549–3563. doi: 10.1113/jphysiol.2013.257741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buttigieg J., Brown S.T., Lowe M., Zhang M., Nurse C.A. Functional mitochondria are required for O2 but not CO2 sensing in immortalized adrenomedullary chromaffin cells. Am. J. Physiol. Cell Physiol. 2008;294:C945–C956. doi: 10.1152/ajpcell.00495.2007. [DOI] [PubMed] [Google Scholar]
- 8.Chouchani E.T. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross A.R., Henderson L., Jones O.T., Delpiano M.A., Hentschel J., Acker H. Involvement of an NAD(P)H oxidase as a PO2 sensor protein in the rat carotid body. Biochem. J. 1990;272:743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly Md.B., Lambertsen C.J., Schweitzer A. Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J. Physiol. 1954;125:67–89. doi: 10.1113/jphysiol.1954.sp005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Castro B., Pintado C.O., Garcia-Flores P., Lopez-Barneo J., Piruat J.I. Differential impairment of catecholaminergic cell maturation and survival by genetic mitochondrial complex II dysfunction. Mol. Cell Biol. 2012;32:3347–3357. doi: 10.1128/MCB.00128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dooley C.T., Dore T.M., Hanson G.T., Jackson W.C., Remington S.J., Tsien R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 13.Duchen M.R., Biscoe T.J. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J. Physiol. 1992;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Aguera M.C., Gao L., Gonzalez-Rodriguez P., Pintado C.O., Arias-Mayenco I., Garcia-Flores P., Garcia-Perganeda A., Pascual A., Ortega-Saenz P., Lopez-Barneo J. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 2015;22:825–837. doi: 10.1016/j.cmet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Fiedorczuk K., Letts J.A., Degliesposti G., Kaszuba K., Skehel M., Sazanov L.A. Atomic structure of the entire mammalian mitochondrial complex I. Nature. 2016;538:406–410. doi: 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald R.S., Shirahata M., Chang I., Kostuk E. The impact of hypoxia and low glucose on the release of acetylcholine and ATP from the incubated cat carotid body. Brain Res. 2009;1270:39–44. doi: 10.1016/j.brainres.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 17.Fu X.W., Wang D., Nurse C.A., Dinauer M.C., Cutz E. NADPH oxidase is an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild-type and oxidase-deficient mice. Proc. Natl. Acad. Sci. USA. 2000;97:4374–4379. doi: 10.1073/pnas.97.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda R., Zhang H., Kim J.W., Shimoda L., Dang C.V., Semenza G.L. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Fernandez M., Ortega-Saenz P., Castellano A., Lopez-Barneo J. Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes. 2007;56:2893–2900. doi: 10.2337/db07-0122. [DOI] [PubMed] [Google Scholar]
- 20.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr. Opin. Cell Biol. 2009;21:894–899. doi: 10.1016/j.ceb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson G.T., Aggeler R., Oglesbee D., Cannon M., Capaldi R.A., Tsien R.Y., Remington S.J. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 22.He L., Chen J., Dinger B., Sanders K., Sundar K., Hoidal J., Fidone S. Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am. J. Physiol. Cell Physiol. 2002;282:C27–C33. doi: 10.1152/ajpcell.2002.282.1.C27. [DOI] [PubMed] [Google Scholar]
- 23.Hernansanz-Agustin P., Izquierdo-Alvarez A., Sanchez-Gomez F.J., Ramos E., Villa-Pina T., Lamas S., Bogdanova A., Martinez-Ruiz A. Acute hypoxia produces a superoxide burst in cells. Free Radic. Biol. Med. 2014;71:146–156. doi: 10.1016/j.freeradbiomed.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Kadenbach B., Huttemann M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion. 2015;24:64–76. doi: 10.1016/j.mito.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Kashani-Poor N., Zwicker K., Kerscher S., Brandt U. A central functional role for the 49-kDa subunit within the catalytic core of mitochondrial complex I. J. Biol. Chem. 2001;276:24082–24087. doi: 10.1074/jbc.M102296200. [DOI] [PubMed] [Google Scholar]
- 26.Keating D.J., Rychkov G.Y., Roberts M.L. Oxygen sensitivity in the sheep adrenal medulla: role of SK channels. Am. J. Physiol. Cell Physiol. 2001;281:C1434–C1441. doi: 10.1152/ajpcell.2001.281.5.C1434. [DOI] [PubMed] [Google Scholar]
- 27.Keating D.J., Rychkov G.Y., Giacomin P., Roberts M.L. Oxygen-sensing pathway for SK channels in the ovine adrenal medulla. Clin. Exp. Pharmacol. Physiol. 2005;32:882–887. doi: 10.1111/j.1440-1681.2010.04279.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim D., Cavanaugh E.J., Kim I., Carroll J.L. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J. Physiol. 2009;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y., Bang H., Kim D. TASK-3, a new member of the tandem pore K(+) channel family. J. Biol. Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P., Prabhakar N.R. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr. Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leach R.M., Hill H.M., Snetkov V.A., Robertson T.P., Ward J.P. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J. Physiol. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J., Lim W., Eun S.Y., Kim S.J., Kim J. Inhibition of apamin-sensitive K+ current by hypoxia in adult rat adrenal chromaffin cells. Pflug. Arch. 2000;439:700–704. doi: 10.1007/s004249900228. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y.M., Kim B.J., Chun Y.S., So I., Choi H., Kim M.S., Park J.W. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal. 2006;18:499–507. doi: 10.1016/j.cellsig.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Barneo J. Oxygen and glucose sensing by carotid body glomus cells. Curr. Opin. Neurobiol. 2003;13:493–499. doi: 10.1016/s0959-4388(03)00093-x. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Barneo J., Pardal R., Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev. Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Barneo J., Lopez-Lopez J.R., Urena J., Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Barneo J., Gonzalez-Rodriguez P., Gao L., Fernandez-Aguera M.C., Pardal R., Ortega-Saenz P. Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am. J. Physiol. Cell Physiol. 2016;310:C629–C642. doi: 10.1152/ajpcell.00265.2015. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Barneo J., Ortega-Saenz P., Gonzalez-Rodriguez P., Fernandez-Aguera M.C., Macias D., Pardal R., Gao L. Oxygen-sensing by arterial chemoreceptors: mechanisms and medical translation. Mol. Asp. Med. 2016;47–48:90–108. doi: 10.1016/j.mam.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Macias D., Fernandez-Aguera M.C., Bonilla-Henao V., Lopez-Barneo J. Deletion of the von Hippel-Lindau gene causes sympathoadrenal cell death and impairs chemoreceptor-mediated adaptation to hypoxia. EMBO Mol. Med. 2014;6:1577–1592. doi: 10.15252/emmm.201404153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michelakis E.D., Rebeyka I., Wu X., Nsair A., Thebaud B., Hashimoto K., Dyck J.R., Haromy A., Harry G., Barr A., Archer S.L. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ. Res. 2002;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 41.Mills E., Jobsis F.F. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J. Neurophysiol. 1972;35:405–428. doi: 10.1152/jn.1972.35.4.405. [DOI] [PubMed] [Google Scholar]
- 42.Mochizuki-Oda N., Takeuchi Y., Matsumura K., Oosawa Y., Watanabe Y. Hypoxia-induced catecholamine release and intracellular Ca2+ increase via suppression of K+ channels in cultured rat adrenal chromaffin cells. J. Neurochem. 1997;69:377–387. doi: 10.1046/j.1471-4159.1997.69010377.x. [DOI] [PubMed] [Google Scholar]
- 43.O'Kelly I., Stephens R.H., Peers C., Kemp P.J. Potential identification of the O2-sensitive K+ current in a human neuroepithelial body-derived cell line. Am. J. Physiol. 1999;276:L96–L104. doi: 10.1152/ajplung.1999.276.1.L96. [DOI] [PubMed] [Google Scholar]
- 44.Ortega-Saenz P., Pardal R., Garcia-Fernandez M., Lopez-Barneo J. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J. Physiol. 2003;548:789–800. doi: 10.1113/jphysiol.2003.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortega-Saenz P., Levitsky K.L., Marcos-Almaraz M.T., Bonilla-Henao V., Pascual A., Lopez-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J. Gen. Physiol. 2010;135:379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortega-Saenz P., Pardal R., Levitsky K., Villadiego J., Munoz-Manchado A.B., Duran R., Bonilla-Henao V., Arias-Mayenco I., Sobrino V., Ordonez A., Oliver M., Toledo-Aral J.J., Lopez-Barneo J. Cellular properties and chemosensory responses of the human carotid body. J. Physiol. 2013;591:6157–6173. doi: 10.1113/jphysiol.2013.263657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardal R., Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat. Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- 48.Park K.S., Bang H., Shin E.Y., Kim C.H., Kim Y. The Inhibition of TREK2 channel by an oxidizing agent, 5,5'-dithio-bis (2-nitrobenzoic acid), via interaction with the C-terminus distal to the 353rd amino acid. Korean J. Physiol. Pharmacol. 2008;12:211–216. doi: 10.4196/kjpp.2008.12.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci. Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- 50.Peers C. Acute oxygen sensing--Inching ever closer to an elusive mechanism. Cell Metab. 2015;22:753–754. doi: 10.1016/j.cmet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Platero-Luengo A., Gonzalez-Granero S., Duran R., Diaz-Castro B., Piruat J.I., Garcia-Verdugo J.M., Pardal R., Lopez-Barneo J. An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell. 2014;156:291–303. doi: 10.1016/j.cell.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Post J.M., Hume J.R., Archer S.L., Weir E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am. J. Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 53.Pryde K.R., Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem. 2011;286:18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ratcliffe P.J. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J. Physiol. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remington S.J. Fluorescent proteins: maturation, photochemistry and photophysics. Curr. Opin. Struct. Biol. 2006;16:714–721. doi: 10.1016/j.sbi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Rychkov G.Y., Adams M.B., McMillen I.C., Roberts M.L. Oxygen-sensing mechanisms are present in the chromaffin cells of the sheep adrenal medulla before birth. J. Physiol. 1998;509(Pt 3):887–893. doi: 10.1111/j.1469-7793.1998.887bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semenza G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 58.Shimoda L.A., Polak J. Hypoxia. 4. Hypoxia and ion channel function. Am. J. Physiol. Cell Physiol. 2011;300:C951–C967. doi: 10.1152/ajpcell.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starlinger H., Lubbers D.W. Oxygen consumption of the isolated carotid body tissue (cat) Pflug. Arch. 1976;366:61–66. doi: 10.1007/BF02486561. [DOI] [PubMed] [Google Scholar]
- 60.Stea A., Nurse C.A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflug. Arch. 1991;418:93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- 61.Thompson R.J., Jackson A., Nurse C.A. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J. Physiol. 1997;498(Pt 2):503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson R.J., Buttigieg J., Zhang M., Nurse C.A. A rotenone-sensitive site and H2O2 are key components of hypoxia-sensing in neonatal rat adrenomedullary chromaffin cells. Neuroscience. 2007;145:130–141. doi: 10.1016/j.neuroscience.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 63.Timmers H.J., Karemaker J.M., Wieling W., Marres H.A., Folgering H.T., Lenders J.W. Baroreflex and chemoreflex function after bilateral carotid body tumor resection. J. Hypertens. 2003;21:591–599. doi: 10.1097/00004872-200303000-00026. [DOI] [PubMed] [Google Scholar]
- 64.Turner P.J., Buckler K.J. Oxygen and mitochondrial inhibitors modulate both monomeric and heteromeric TASK-1 and TASK-3 channels in mouse carotid body type-1 cells. J. Physiol. 2013;591:5977–5998. doi: 10.1113/jphysiol.2013.262022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turrens J.F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urena J., Fernandez-Chacon R., Benot A.R., Alvarez de Toledo G.A., Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc. Natl. Acad. Sci. USA. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varas R., Wyatt C.N., Buckler K.J. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J. Physiol. 2007;583:521–536. doi: 10.1113/jphysiol.2007.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Votyakova T.V., Reynolds I.J. DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 69.Waypa G.B., Chandel N.S., Schumacker P.T. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ. Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 70.Waypa G.B., Marks J.D., Guzy R.D., Mungai P.T., Schriewer J.M., Dokic D., Ball M.K., Schumacker P.T. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am. J. Respir. Crit. Care Med. 2013;187:424–432. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weir E.K., Lopez-Barneo J., Buckler K.J., Archer S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyatt C.N., Buckler K.J. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J. Physiol. 2004;556:175–191. doi: 10.1113/jphysiol.2003.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youngson C., Nurse C., Yeger H., Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]
- 74.Yuan X.J., Goldman W.F., Tod M.L., Rubin L.J., Blaustein M.P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am. J. Physiol. 1993;264:L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]
- 75.Zhang M., Buttigieg J., Nurse C.A. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J. Physiol. 2007;578:735–750. doi: 10.1113/jphysiol.2006.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou T., Chien M.S., Kaleem S., Matsunami H. Single cell transcriptome analysis of mouse carotid body glomus cells. J. Physiol. 2016;594:4225–4251. doi: 10.1113/JP271936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu J., Vinothkumar K.R., Hirst J. Structure of mammalian respiratory complex I. Nature. 2016;536:354–358. doi: 10.1038/nature19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zickermann V., Wirth C., Nasiri H., Siegmund K., Schwalbe H., Hunte C., Brandt U. Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science. 2015;347:44–49. doi: 10.1126/science.1259859. [DOI] [PubMed] [Google Scholar]